Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis
Abstract
:1. Iron and Its Homeostasis
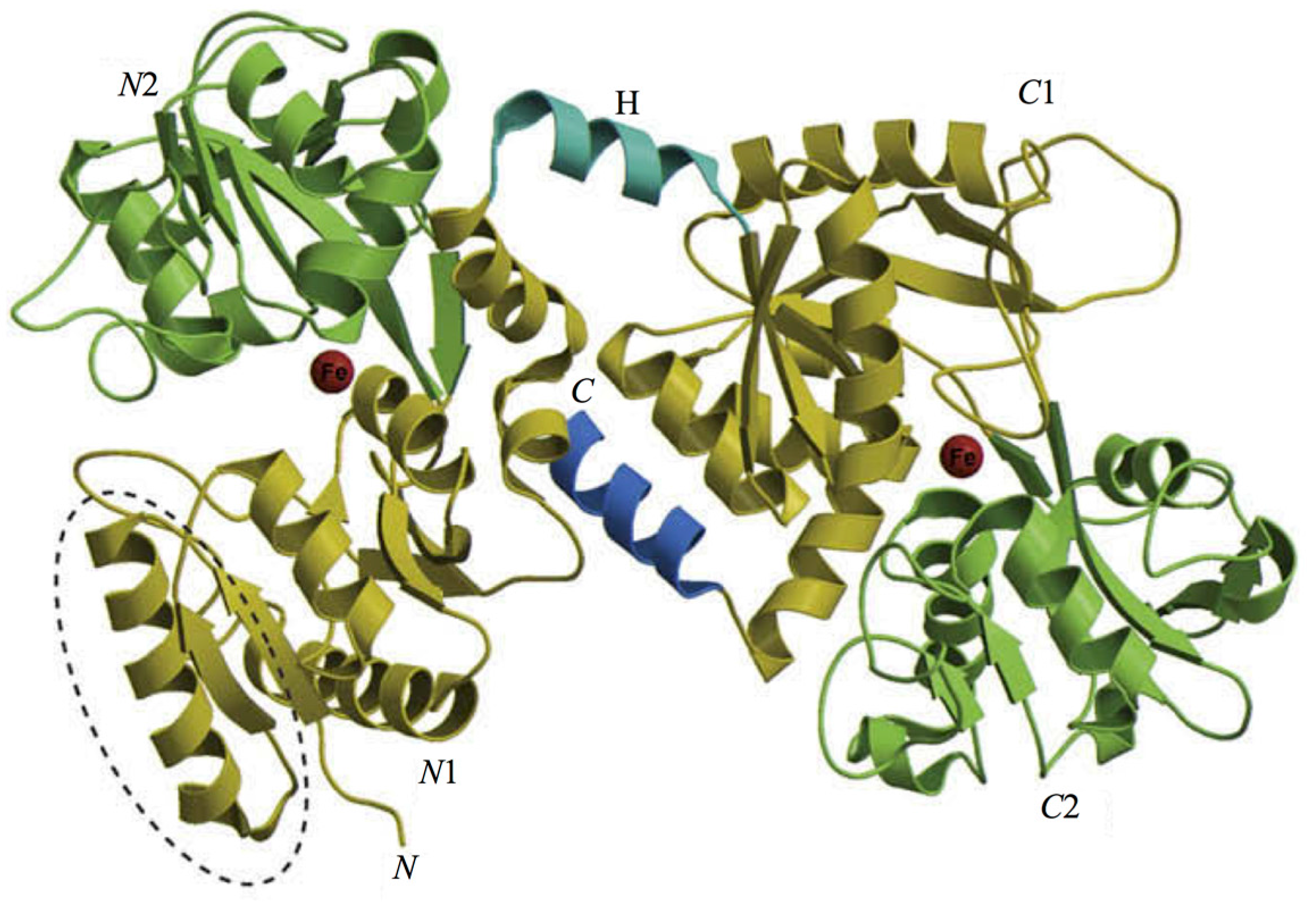
2. Lactoferrin
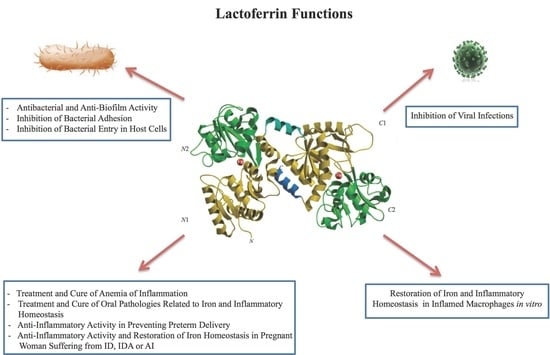
2.1. Lactoferrin Functions
2.1.1. Antibacterial and Anti-Biofilm Activity Dependent on Lf Iron-Binding Ability
2.1.2. Antibacterial Activity Independent of Lf Iron-Binding Ability
2.1.3. Inhibition of Bacterial Adhesion on Abiotic and Cell Surfaces
2.1.4. Inhibition of Bacterial Entry into Host Cells
2.1.5. Inhibition of Viral Infections
2.1.6. Anti-Inflammatory Activity of Lf in Infected and Inflamed Host Cells
3. Lf and Anemia of Inflammation
4. Anti-Inflammatory Activity of bLf in Preventing Preterm Delivery
5. Lf in Oral Pathologies: Surprising Poor Oral Health Related to Iron and Inflammatory Homeostasis Disorders in Athletes Participating in the Olympic Games
6. Conclusions
- -
- that antimicrobial Lf activity is influenced by the different microbial genera tested;
- -
- that anti-adhesive Lf activity, independent from Lf iron saturation and from its binding to cell GAG and HS, is dependent on the different abiotic or cellular structures studied;
- -
- that anti-invasive Lf activity, independent from Lf iron saturation, but dependent on its binding to cell GAG and HS, is strictly influenced by the different cellular monolayers tested and from the different invasion mechanisms carried out by facultative or obligate intracellular pathogens;
- -
- that antiviral Lf activity, against enveloped or naked viruses, can be or not related to its anti-inflammatory activity;
- -
- that anti-inflammatory Lf activity is dependent on the experimental model chosen.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andrews, N.C. Disorders of iron metabolism. N. Engl. J. Med. 2000, 341, 1986–1995, Erratum in 2000, 342, 364. [Google Scholar] [CrossRef] [PubMed]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Musci, G.; Polticelli, F.; Bonaccorsi di Patti, M.C. The ceruloplasmin—Ferroportin system of iron traffic in vertebrates. World J. Biol. Chem. 2014, 26, 204–215. [Google Scholar] [CrossRef]
- Cutone, A.; Frioni, A.; Berlutti, F.; Valenti, P.; Musci, G.; Bonaccorsi di Patti, M.C. Lactoferrin prevents LPS-induced decrease of the iron exporter ferroportin in human monocytes/macrophages. Biometals 2014, 27, 807–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutone, A.; Rosa, L.; Lepanto, M.S.; Scotti, M.J.; Berlutti, F.; Bonaccorsi di Patti, M.C.; Musci, G.; Valenti, P. Lactoferrin efficiently counteracts the inflammation-induced changes of the iron homeostasis system in macrophages. Front. Immunol. 2017, 15, 705. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Sugianto, P.; Fung, E.; Del-Castillo-Rueda, A.; Moran-Jimenez, M.J.; Ganz, T.; Nemeth, E. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012, 15, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a urinaryantimicrobial peptide synthesized in the liver. J. Biol. Chem. 2001, 276, 7806–7810. [Google Scholar] [CrossRef] [PubMed]
- Hunter, H.N.; Fulton, D.B.; Ganz, T.; Vogel, H.J. The solution structure of human hepcidin, a peptide hormone with antimicrobial activity that is involved in iron uptake and hereditary hemochromatosis. J. Biol. Chem. 2002, 277, 37597–37603. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.; Neitz, S.; Magert, H.J.; Schulz, A.; Forssmann, W.G.; Schulz-Knappe, P.; Adermann, K. LEAP-1, a novel highly disulfidebonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000, 480, 147–150. [Google Scholar] [CrossRef]
- Coffey, R.; Ganz, T. Iron homeostasis—An anthropocentric perspective. J. Biol. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zumerle, S.; Mathieu, J.R.; Delga, S.; Heinis, M.; Viatte, L.; Vaulont, S.; Peyssonnaux, C. Targeted disruption of hepcidin in the liver recapitulates the hemochromatotic phenotype. Blood 2014, 123, 3646–3650. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef] [PubMed]
- Verga Falzacappa, M.V.; Vujic Spasic, M.; Kessler, R.; Stolte, J.; Hentze, M.W.; Muckenthaler, M.U. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood 2007, 109, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Peng, H.; Gelbart, T.; Wang, L.; Beutler, E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc. Natl. Acad. Sci. USA 2005, 102, 1906–1910. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C. Iron metabolism: Iron deficiency and iron overload. Annu. Rev. Genom. Hum. Genet. 2000, 1, 75–98. [Google Scholar] [CrossRef] [PubMed]
- Frazer, D.M.; Anderson, G.J. The orchestration of body iron intake: How and where do enterocytes receive their cues? Blood Cells Mol. Dis. 2003, 30, 288–297. [Google Scholar] [CrossRef]
- Miller, J.L. Iron deficiency anemia: A common and curable disease. Cold Spring Harb. Perspect. Med. 2012, 3, a011866. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Torcia, F.; Berlutti, F.; Pacifici, E.; Ebano, V.; Moscarini, M.; Valenti, P. Oral administration of lactoferrin increases hemoglobin and total serum iron in pregnant women. Biochem. Cell Biol. 2006, 84, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S. The management of iron deficiency in menometrorrhagia. Gynecol. Endocrinol. 2011, 27, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Schoen, R.E. Supplementation with oral vs. intravenous iron for anemia with IBD or gastrointestinal bleeding: Is oral iron getting a bad rap? Am. J. Gastroenterol. 2011, 106, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Toblli, J.E.; Romero, J.D.; Monterrosa, B.; Frer, C.; Macagno, E.; Breymann, C. Efficacy and safety of oral iron(III) polymaltose complex versus ferrous sulfate in pregnant women with iron-deficiency anemia: A multicenter, randomized, controlled study. J. Matern. Fetal Neonatal Med. 2011, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zaim, M.; Piselli, L.; Fioravanti, P.; Kanony-Truc, C. Efficacy and tolerability of a prolonged release ferrous sulphate formulation in iron deficiency anaemia: A non-inferiority controlled trial. Eur. J. Nutr. 2012, 51, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Kadiiska, M.B.; Burkitt, M.J.; Xiang, Q.H.; Mason, R.P. Iron supplementation generates hydroxyl radical in vivo. An ESR spintrapping investigation. J. Clin. Investig. 1995, 96, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Reifen, R.; Matas, Z.; Zeidel, L.; Berkovitch, Z.; Bujanover, Y. Iron supplementation may aggravate inflammatory status of colitis in a rat model. Dig. Dis. Sci. 2000, 45, 394–397. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, I.; Ward, D.M.; Kaplan, J. Regulation of iron acquisition and storage: Consequences for iron-linked disorders. Nat. Rev. Mol. Cell Biol. 2008, 9, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006, 26, 323–342. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, E.L.; Iwasaki, K.; Tsuji, Y. Intracellular iron transport and storage: From molecular mechanisms to health implications. Antioxid. Redox Signal. 2008, 10, 997–1030. [Google Scholar] [CrossRef] [PubMed]
- Ajello, M.; Greco, R.; Giansanti, F.; Massucci, M.T.; Antonini, G.; Valenti, P. Anti-invasive activity of bovine lactoferrin towards group A streptococci. Biochem. Cell Biol. 2002, 80, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Rosa, L.; Cutone, A.; Frioni, A.; Berlutti, F.; Paesano, R.; Valenti, P. Effect of bovine lactoferrin on Chlamydia trachomatis infection and inflammation. Biochem. Cell Biol. 2017, 95, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B. Isolation of an iron-containing red protein from human milk. Acta Chem. Scand. 1960, 14, 510–512. [Google Scholar] [CrossRef]
- Montreuil, J.; Tonnelat, J.; Mullet, S. Preparation and properties of lactosiderophilin (lactotransferrin) of human milk. Biochim. Biophys. Acta 1960, 45, 413–421. [Google Scholar] [CrossRef]
- Groves, M.L. The isolation of a red protein from milk. J. Am. Chem. Soc. 1960, 82, 3345–3350. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F. Lactoferrin in milk from different species. Comp. Biochem. Physiol. 1971, B39, 119–129. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F.; Dive, C. An iron-binding protein common to many external secretions. Clin. Chim. Acta 1966, 14, 735–739. [Google Scholar] [CrossRef]
- Masson, P.L.; Heremans, J.F.; Schonne, E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J. Exp. Med. 1969, 130, 643–658. [Google Scholar] [CrossRef] [PubMed]
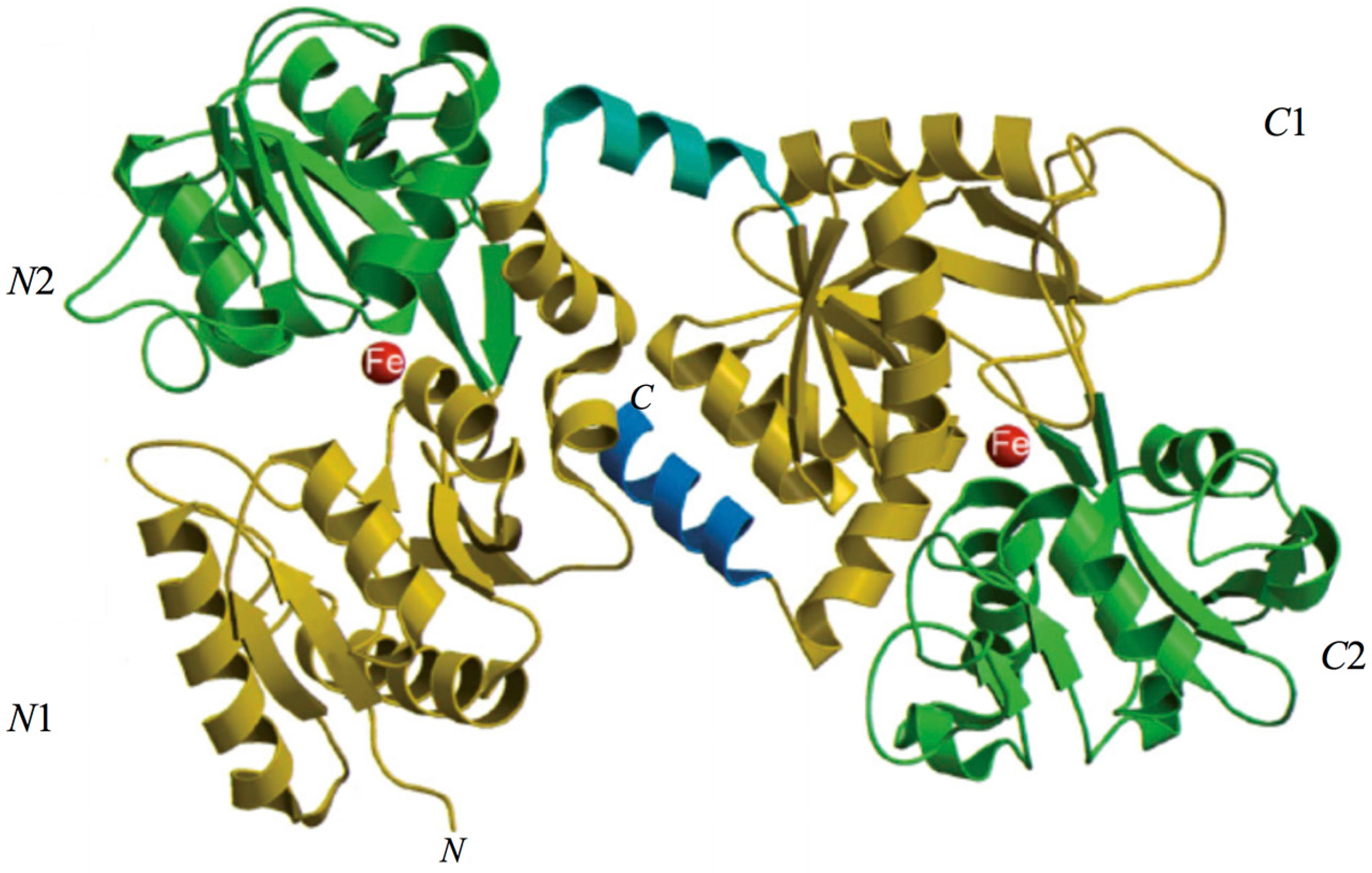
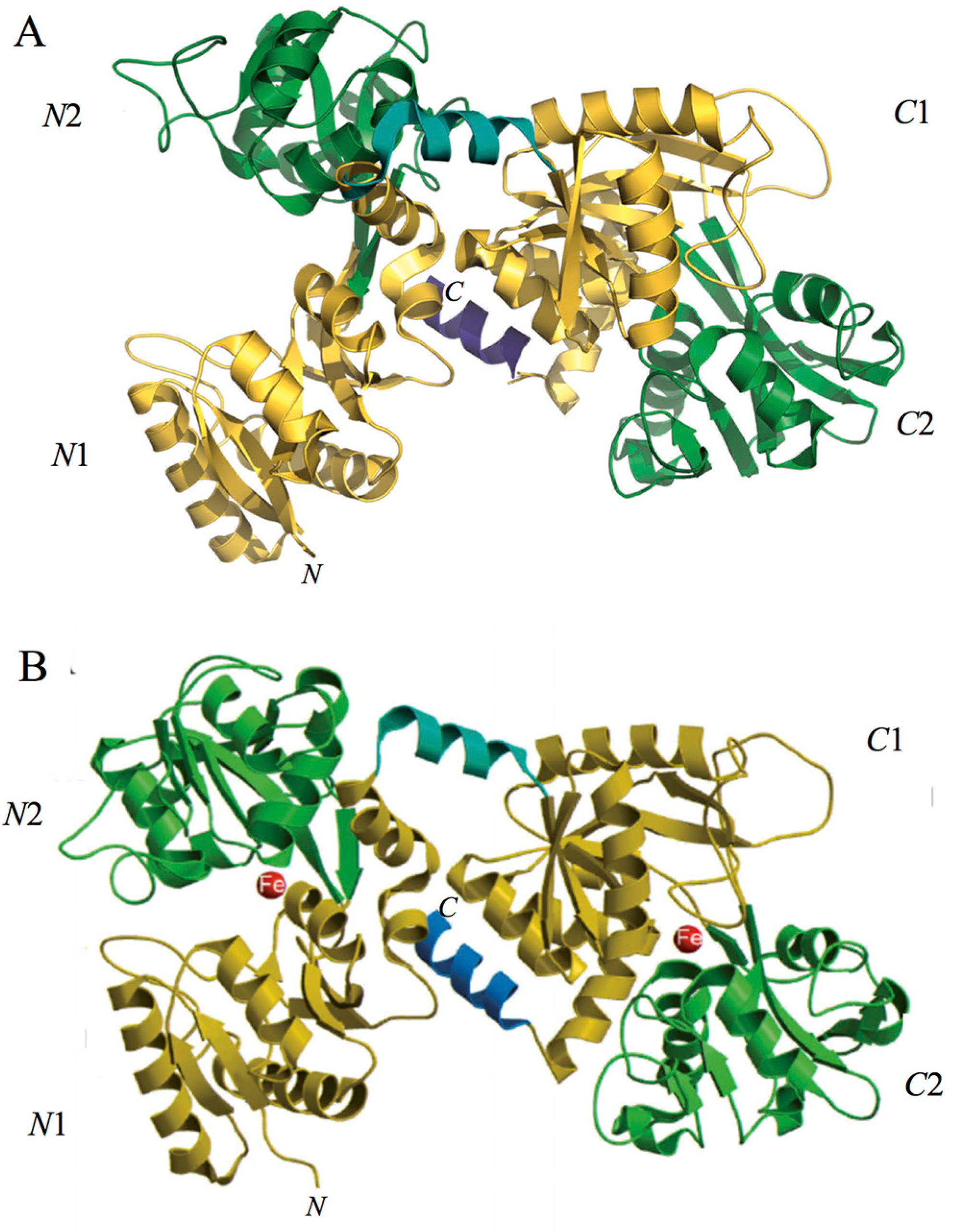
- Anderson, B.F.; Baker, H.M.; Norris, G.E.; Rice, D.W.; Baker, E.N. Structure of human lactoferrin: Crystallographic structure analysis and refinement at 2.8 A resolution. J. Mol. Biol. 1989, 209, 711–734. [Google Scholar] [CrossRef]
- Baker, E.N.; Baker, H.M. Molecular structure, binding properties and dynamics of lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N.; Baker, H.M. A structural framework for understanding the multifunctional character of lactoferrin. Biochimie 2009, 91, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.F.; Baker, H.M.; Dodson, E.J.; Norris, G.E.; Rumball, S.V.; Waters, J.M.; Baker, E.N. Structure of human lactoferrin at 3.2-A resolution. Proc. Natl. Acad. Sci. USA 1987, 84, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N.; Rumball, S.V.; Anderson, B.F. Transferrins: Insights into structure and function from studies on lactoferrin. Trends Biochem. Sci. 1987, 12, 350–353. [Google Scholar] [CrossRef]
- Bruns, C.M.; Nowalk, A.J.; Arvai, A.S.; McTigue, M.A.; Vaughan, K.G.; Mietzner, T.A.; McRee, D.E. Structure of Haemophilus influenzae Fe(+3)-binding protein reveals convergent evolution within a superfamily. Nat. Struct. Biol. 1997, 4, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N. Structure and reactivity of transferrins. Adv. Inorg. Chem. 1994, 41, 389–463. [Google Scholar]
- Baker, E.N.; Baker, H.M.; Kidd, R.D. Lactoferrin and transferrin: Functional variations on a common structural framework. Biochem. Cell Biol. 2002, 80, 27–34. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, R.T.A.; Moore, S.A.; Chen, J.; Anderson, B.F.; Baker, H.; Luo, Y.; Bewley, M.; Smith, C.A.; Murphy, M.E.P.; Wang, Y.; et al. Two high-resolution crystal structures of the recombinant N-lobe of human transferrin reveal a structural change implicated in iron release. Biochemistry 1998, 37, 7919–7928. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Anderson, B.F.; Groom, C.R.; Haridas, M.; Baker, E.N. Three-dimensional structure of diferric bovine lactoferrin at 2.8 A resolution. J. Mol. Biol. 1997, 274, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Paramasivam, M.; Srinivasan, A.; Yadav, M.P.; Singh, T.P. Three-dimensional structure of mare diferric lactoferrin at 2.6 A resolution. J. Mol. Biol. 1999, 289, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Paramasivam, M.; Yadav, S.; Srinivasan, A.; Singh, T.P. Structure of buffalo lactoferrin at 2.5 A resolution using crystals grown at 303 K shows different orientations of the N and C lobes. Acta Crystallogr. D Biol. Crystallogr. 1999, 55, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.F.; Baker, H.M.; Norris, G.E.; Rumball, S.V.; Baker, E.N. Apolactoferrin structure demonstrates ligand-induced conformational change in transferrins. Nature 1990, 344, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, M.; Anderson, B.F.; Norris, G.E.; Baker, E.N.; Lesk, A.M.; Chothia, C. Domain closure in lactoferrin. Two hinges produce a see-saw motion between alternative close-packed interfaces. J. Mol. Biol. 1993, 234, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.M.; Baker, E.N. A structural perspective on lactoferrin function. Biochem. Cell Biol. 2012, 90, 320–328. [Google Scholar] [CrossRef]
- Mazurier, J.; Spik, G. Comparative study of the iron-binding properties of human transferrins: I. Complete and sequential iron saturation and desaturation of the lactotransferrin. Biochim. Biophys. Acta 1980, 629, 399–408. [Google Scholar] [CrossRef]
- Smith, C.A.; Anderson, B.F.; Baker, H.M.; Baker, E.N. Metal substitution in transferrins: The crystal structure of human copper-lactoferrin at 2.1-A resolution. Biochemistry 1992, 31, 4527–4533. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Baker, H.M.; Shongwe, M.S.; Anderson, B.F.; Baker, E.N. Crystallographic studies on metal and anion substituted human lactoferrin. Adv. Exp. Med. Biol. 1994, 357, 265–269. [Google Scholar] [PubMed]
- Kumar, H.; Wen, J.; Shaw, J.; Cornish, J.; Bunt, C. Physiochemical characterization of β-glucan and in vitro release of lactoferrin from β-glucan microparticles. Curr. Drug Deliv. 2013, 10, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Løvstad, R.A. A kinetic study on the distribution of Cu(II)-ions between albumin and transferrin. Biometals 2004, 17, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Chahine, J.M.E.H.; Hémadi, M.; Ha-Duong, N.T. Uptake and release of metal ions by transferrin and interaction with receptor 1. Biochim. Biophys. Acta. 2012, 1820, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Antonini, G. Lactoferrin: An important host defence against microbial and viral attack. Cell. Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Puddu, P.; Valenti, P.; Gessani, S. Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie 2009, 91, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Puddu, P.; Latorre, D.; Carollo, M.; Catizone, A.; Ricci, G.; Valenti, P.; Gessani, S. Bovine lactoferrin counteracts Toll-like receptor mediated activation signals in antigen presenting cells. PLoS ONE 2011, 6, e22504. [Google Scholar] [CrossRef] [PubMed]
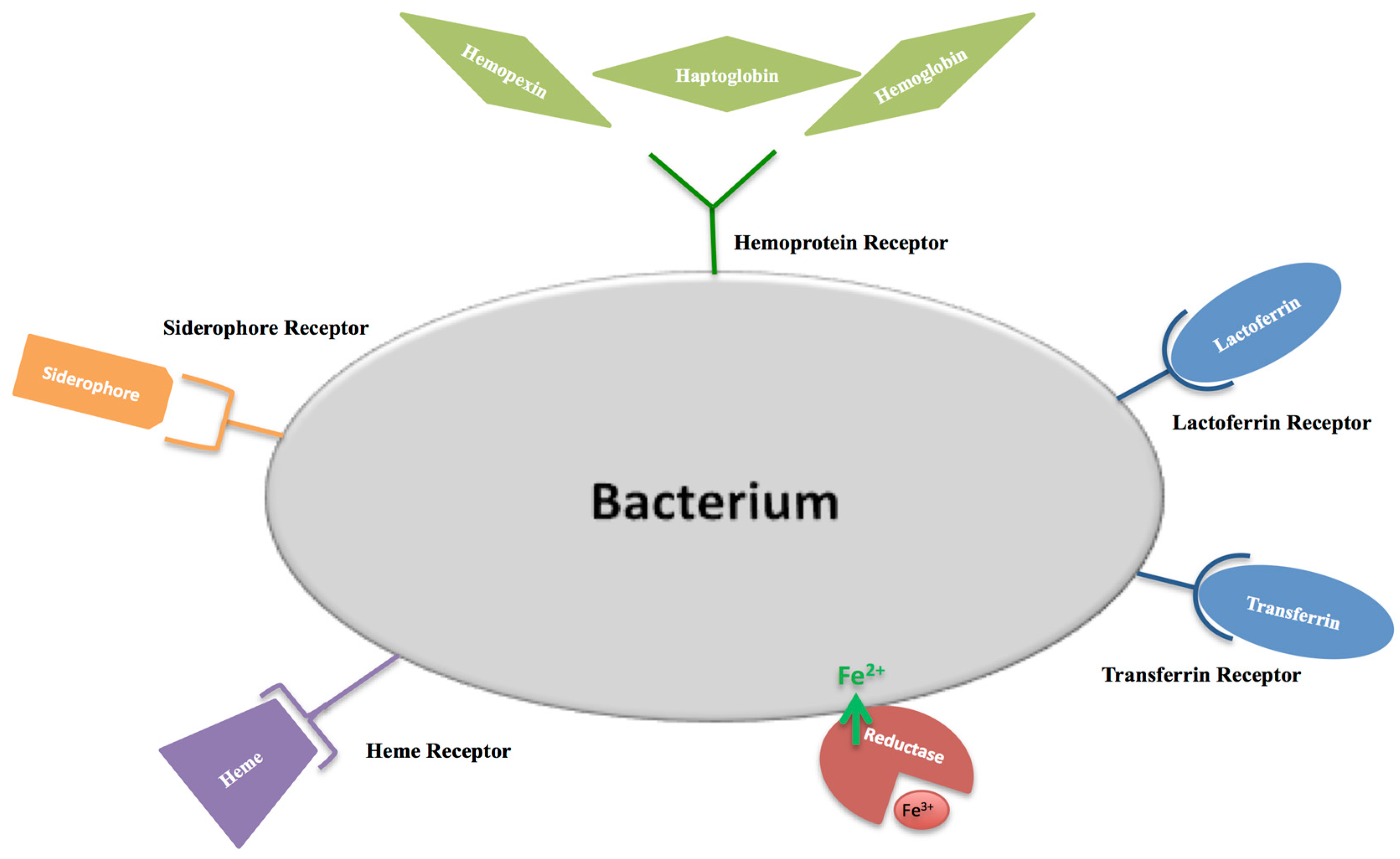
- Weinberg, E.D. The development of awareness of iron-withholding defense. Perspect. Biol. Med. 1993, 36, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.; Zhai, C.; Haas, H.; Decristoforo, C. Siderophores for molecular imaging applications. Clin. Transl. Imaging 2017, 5, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Beddek, A.J.; Schryvers, A.B. The lactoferrin receptor complex in Gram negative bacteria. Biometals 2010, 23, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Pogoutse, A.K.; Moraes, T.F. Iron acquisition through the bacterial transferrin receptor. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Wandersman, C.; Stojiljkovic, I. Bacterial heme sources: The role of heme, hemoprotein receptors and hemophores. Curr. Opin. Microbiol. 2000, 3, 215–220. [Google Scholar] [CrossRef]
- Huang, W.; Wilks, A. Extracellular Heme Uptake and the Challenge of Bacterial Cell Membranes. Annu. Rev. Biochem. 2017, 86, 799–823. [Google Scholar] [CrossRef] [PubMed]
- Pilarczyk-Zurek, M.; Strus, M.; Adamski, P.; Heczko, P.B. The dual role of Escherichia coli in the course of ulcerative colitis. BMC Gastroenterol. 2016, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Parsek, M.R.; Greenberg, E.P.; Welsh, M.J. A component of innate immunity prevents bacterial biofilms development. Nature 2002, 417, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Stites, S.W.; Walters, B.; O’Brien-Ladner, A.R.; Bailey, K.; Wesselius, L.J. Increased iron and ferritin content of sputum from patients with cystic fibrosis or chronic bronchitis. Chest 1998, 114, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Morea, C.; Battistoni, A.; Sarli, S.; Cipriani, P.; Superti, F.; Ammendolia, M.G.; Valenti, P. Iron availability influences aggregation, biofilm, adhesion and invasion of Pseudomonas aeruginosa and Burkholderia cenocepacia. Int. J. Immunopathol. Pharmacol. 2005, 18, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Rogan, M.P.; Taggart, C.C.; Greene, C.M.; Murphy, P.G.; O’Neill, S.J.; McElvaney, N.G. Loss of microbicidal activity and increased formation of biofilm due to decreased lactoferrin activity in patients with cystic fibrosis. J. Infect. Dis. 2004, 190, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.B.; Bohling, T.; Payvandi, F.; Rennard, S.I. Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J. Lab. Clin. Med. 1990, 115, 148–158. [Google Scholar] [PubMed]
- Appelmelk, B.J.; An, Y.Q.; Geerts, M.; Thijs, B.G.; de Boer, H.A.; MacLaren, D.M.; de Graaff, J.; Nuijens, J.H. Lactoferrin is a lipid A-binding protein. Infect. Immun. 1994, 62, 2628–2632. [Google Scholar] [PubMed]
- Brandenburg, K.; Jurgens, G.; Muller, M.; Fukuoka, S.; Koch, M.H.J. Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin. Biol. Chem. 2001, 382, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, K.; Kavase, K. Potent antibacterial peptides generated by pepsin of bovine lactoferrin. J. Dairy Sci. 1991, 74, 4137–4142. [Google Scholar] [CrossRef]
- Bellamy, W.; Takase, M.; Wakabayashi, H.; Kavase, K.; Tomita, M. Antibacterial spectrum of lactoferricin B, a potent bactericide peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 1992, 73, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Giansanti, F.; Boffi, A.; Ajello, M.; Valenti, P.; Chiancone, E.; Antonini, G. Ca2+ binding to bovine lactoferrin enhances protein stability and influences the release of bacterial lipopolysaccharide. Biochem. Cell Biol. 2002, 80, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Visca, P.; Berlutti, F.; Vittorioso, P.; Dalmastri, C.; Thaller, M.C.; Valenti, P. Growth and adsorption of Streptococcus mutans 6715–13 to hydroxyapatite in the presence of lactoferrin. Med. Microbiol. Immunol. 1989, 178, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Oho, T.; Mitoma, M.; Koga, T. Functional domain of bovine milk lactoferrin which inhibits the adherence of Streptococcus mutans cells to a salivary film. Infect. Immun. 2002, 70, 5279–5282. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.J.; Schneider, R.P.; Willcox, M.D. The effect of protein-coated contact lenses on the adhesion and viability of gram negative bacteria. Curr. Eye Res. 2003, 27, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Longhi, C.; Conte, M.P.; Seganti, L.; Polidoro, M.; Alfsen, A.; Valenti, P. Influence of lactoferrin on the entry process of Escherichia coli HB101 (pRI203) in HeLa cells. Med. Microbiol. Immunol. 1993, 182, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Tazume, S.; Shimizu, K.; Matsuzawa, H.; Dosako, S.; Isoda, H.; Tsukiji, M.; Fujimura, R.; Muranaka, Y.; Isihida, H. Inhibitory effects of bovine lactoferrin on the adherence of enterotoxigenic Escherichia coli to host cells. Biosci. Biotechnol. Biochem. 2000, 64, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.N.; Giugliano, L.G. Lactoferrin and free secretory component of human milk inhibit the adhesion of enteropathogenic Escherichia coli to HeLa cells. BMC Microbiol. 2001, 1, 25. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Brown, E.L.; Guion, C.E.; Chen, J.Z.; McMahon, R.J.; Cleary, T.G. Effect of lactoferrin on enteroaggregative E. coli (EAEC) 1. Biochem. Cell Biol. 2006, 84, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Superti, F.; Nicoletti, M.; Morea, C.; Frioni, A.; Ammendolia, M.G.; Battistoni, A.; Valenti, P. Bovine lactoferrin inhibits the efficiency of invasion of respiratory A549 cells of different iron-regulated morphological forms of Pseudomonas aeruginosa and Burkholderia cenocepacia. Int. J. Immunopathol. Pharmacol. 2008, 21, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, M.G.; Bertuccini, L.; Iosi, F.; Minelli, F.; Berlutti, F.; Valenti, P.; Superti, F. Bovine lactoferrin interacts with cable pili of Burkholderia cenocepacia. Biometals 2010, 23, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Frioni, A.; Conte, M.P.; Cutone, A.; Longhi, C.; Musci, G.; di Patti, M.C.; Natalizi, T.; Marazzato, M.; Lepanto, M.S.; Puddu, P.; et al. Lactoferrin differently modulates the inflammatory response in epithelial models mimicking human inflammatory and infectious diseases. Biometals 2014, 27, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.N.; Giugliano, L.G. Human milk fractions inhibit the adherence of diffusely adherent Escherichia coli (DAEC) and enteroaggregative E. coli (EAEC) to HeLa cells. FEMS Microbiol. Lett. 2000, 184, 91–94. [Google Scholar] [CrossRef]
- Dalmastri, C.; Valenti, P.; Visca, P.; Vittorioso, P.; Orsi, N. Enhanced antimicrobial activity of lactoferrin by binding to the bacterial surface. Microbiologica 1988, 11, 225–230. [Google Scholar] [PubMed]
- Wu, H.F.; Monroe, D.M.; Church, F.C. Characterization of the glycosaminoglycan-binding region of Lactoferrin. Arch. Biochem. Biophys. 1995, 317, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, K.; Tazume, T.; Uji, K.; Tanaka, M.; Kumura, H.; Mikawa, K.; Shimo-Oka, T. Properties of a heparin-binding peptide derived from bovine lactoferrin. J. Dairy Sci. 1998, 81, 2841–2849. [Google Scholar] [CrossRef]
- Willer Eda, M.; Lima Rde, L.; Giugliano, L.G. In vitro adhesion and invasion inhibition of Shigella dysenteriae, Shigella flexneri and Shigella sonnei clinical strains by human milk proteins. BMC Microbiol. 2004, 4, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Gomez, H.F.; Ochoa, T.J.; Carlin, L.G.; Cleary, T.G. Human lactoferrin impairs virulence of Shigella flexneri. J. Infect. Dis. 2003, 187, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Dial, E.J.; Lichtenberger, L.M. Effect of lactoferrin on Helicobacter felis induced gastritis. Biochem. Cell Biol. 2002, 80, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Hendrixson, D.R.; Baker, E.N.; Murphy, T.F.; St Geme, J.W.; Plaut, A.G. Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc. Natl. Acad. Sci. USA 1998, 95, 12641–12646. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Noguera-Obenza, M.; Ebel, F.; Guzman, C.A.; Gomez, H.F.; Cleary, T.G. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect. Immun. 2003, 71, 5149–5155. [Google Scholar] [CrossRef] [PubMed]
- Plaut, A.G.; Qiu, J.; St. Geme, J.W. Human lactoferrin proteolytic activity: Analysis of the cleaved region in the IgA protease of Haemophilus influenzae. Vaccine 2001, 19, 148–152. [Google Scholar] [CrossRef]
- Hendrixson, D.R.; Qiu, J.; Shewry, S.C.; Fink, D.L.; Petty, S.; Baker, E.N.; Plaut, A.G.; St. Geme, J.W. Human milk lactoferrin is a serine protease that cleaves Haemophilus surface proteins at arginine-rich sites. Mol. Microbiol. 2003, 47, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.E.; Meyer, D.H.; Fives-Taylor, P.M. Aae, an autotransporter involved in adhesion of Actinobacillus actinomycetemcomitans to epithelial cells. Infect. Immun. 2003, 71, 2384–2393. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Schippa, S.; Morea, C.; Sarli, S.; Perfetto, B.; Donnarumma, G.; Valenti, P. Lactoferrin downregulates pro-inflammatory cytokines up-expressed in intestinal epithelial cells infected with invasive or noninvasive Escherichia coli strains. Biochem. Cell Biol. 2006, 84, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Armstead, A.L.; Li, B. Nanomedicine as an emerging approach against intracellular pathogens. Int. J. Nanomed. 2011, 6, 3281–3293. [Google Scholar] [CrossRef]
- Di Biase, A.M.; Tinari, A.; Pietrantoni, A.; Antonini, G.; Valenti, P.; Conte, M.P.; Superti, F. Effect of bovine lactoferricin on enteropathogenic Yersinia adhesion and invasion in HEp-2 cells. J. Med. Microbiol. 2004, 53, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Antonini, G.; Catania, M.R.; Greco, R.; Longhi, C.; Pisciotta, M.G.; Seganti, L.; Valenti, P. Antiinvasive activity of bovine lactoferrin towards Listeria monocytogenes. J. Food Protect. 1997, 1, 60–72. [Google Scholar]
- Diarra, M.S.; Petitclerc, D.; Deschenes, E.; Lessard, N.; Grondin, G.; Talbot, B.G.; Lacasse, P. Lactoferrin against Staphylococcus aureus mastitis. Lactoferrin alone or in combination with penicillin G on bovine polymorphonuclear function and mammary epithelial cells colonisation by Staphylococcus aureus. Vet. Immunol. Immunopathol. 2003, 95, 33–42. [Google Scholar] [CrossRef]
- Berlutti, F.; Catizone, A.; Ricci, G.; Frioni, A.; Natalizi, T.; Valenti, P.; Polimeni, A. Streptococcus mutans and Streptococcus sobrinus are able to adhere and invade human gingival fibroblast cell line. Int. J. Immunopathol. Pharmacol. 2010, 23, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Valenti, P.; Catizone, A.; Pantanella, F.; Frioni, A.; Natalizi, T.; Tendini, M.; Berlutti, F. Lactoferrin decreases inflammatory response by cystic fibrosis bronchial cells invaded with Burkholderia cenocepacia iron-modulated biofilm. Int. J. Immunopathol. Pharmacol. 2011, 24, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Kohlhoff, S.A.; Hammerschlag, M.R. Treatment of Chlamydial infections: 2014 update. Expert Opin. Pharmacother. 2015, 16, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.S.; Mahley, R.W. Lactoferrin binding to heparan sulfate proteoglycans and the LDL receptor-related protein. Further evidence supporting the importance of direct binding of remnant lipoproteins to HSPG. Arterioscler. Thromb. Vasc. Biol. 1994, 14, 2025–2031. [Google Scholar] [CrossRef]
- Stallmann, S.; Hegemann, J.H. The Chlamydia trachomatis Ctad1 invasin exploits the human integrin 1 receptor for host cell entry. Cell. Microbiol. 2015, 18, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Hangoc, G.; Oliff, A.; Chen, L.T.; Shen, R.N.; Broxmeyer, H.E. Protective influence of lactoferrin on mice infected with the polycythemia-inducing strain of Friend virus complex. Cancer Res. 1987, 47, 4184–4188. [Google Scholar] [PubMed]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral properties of lactoferrin—A natural immunity molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, P.J.; Pirhonen, N.; Ikonen, E.; Ronkko, M.; Strengell, S.M.; Makela, M.; Broman, O.J.; Hamming, R.; Hartmann, T.; Ziegler, T.; et al. Pandemic H1N1 2009 influenza A virus induces weak cytokine responses in human macrophages and dendritic cells and is highly sensitive to the antiviral actions of interferons. J. Virol. 2010, 84, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Puddu, P.; Carollo, M.G.; Belardelli, F.; Valenti, P.; Gessani, S. Role of endogenous interferon and LPS in the immunomodulatory effects of bovine lactoferrin in murine peritoneal macrophages. J. Leukoc. Biol. 2007, 82, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Hayworth, J.L.; Kasper, K.J.; Leon-Ponte, M.; Herfst, C.A.; Yue, D.; Brintnell, W.C.; Mazzuca, D.M.; Heinrichs, D.E.; Cairns, E.; Madrenas, J.; et al. Attenuation of massive cytokine response to the staphylococcal enterotoxin B superantigen by the innate immunomodulatory protein lactoferrin. Clin. Exp. Immunol. 2009, 157, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Latorre, D.; Berlutti, F.; Valenti, P.; Gessani, S.; Puddu, P. LF immunomodulatory strategies: Mastering bacterial endotoxin. Biochem. Cell Biol. 2012, 90, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem. Cell Biol. 2012, 90, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Cario, E.; Rosenberg, I.M.; Brandwein, S.L.; Beck, P.L.; Reinecker, H.C.; Podolsky, D.K. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 2000, 164, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Vora, P.; Youdim, A.; Thomas, L.S.; Fukata, M.; Tesfay, S.Y.; Lukasek, K.; Michelsen, K.S.; Wada, A.; Hirayama, T.; Arditi, M.; et al. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J. Immunol. 2004, 173, 5398–5405. [Google Scholar] [CrossRef] [PubMed]
- Ashida, K.; Sasaki, H.; Suzuki, Y.A.; Lönnerdal, B. Cellular internalization of lactoferrin in intestinal epithelial cells. Biometals 2004, 17, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Wong, H.; Ashida, K.Y.; Schryvers, A.B.; Lönnerdal, B. The N1 domain of human lactoferrin is required for internalization by caco-2 cells and targeting to the nucleus. Biochemistry 2008, 47, 10915–10920. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Natalizi, T.; Berlutti, F.; Valenti, P. Body iron delocalization: The serious drawback in iron disorders in both developing and developed countries. Pathog. Glob. Health 2012, 106, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Lee, T.H.; Park, K.H.; Choi, S.Y.; Kim, J. Human lactoferrin suppresses TNF-a-induced intercellular adhesion molecule-1 expression via competition with NF-kB in endothelial cells. FEBS Lett. 2012, 586, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Pietropaoli, M.; Gessani, S.; Valenti, P. The influence of lactoferrin, orally administered, on systemic iron homeostasis in pregnant women suffering of iron deficiency and iron deficiency anaemia. Biochimie 2009, 91, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Lactoferrin: A modulator of immune and inflammatory responses. Cell. Mol. Life Sci. 2005, 62, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.P.; Paz, E.; Conneely, O.M. Multifunctional roles of lactoferrin: A critical overview. Cell. Mol. Life Sci. 2005, 62, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Pacifici, E.; Benedetti, S.; Berlutti, F.; Frioni, A.; Polimeni, A.; Valenti, P. Safety and efficacy of lactoferrin versus ferrous sulphate in curing iron deficiency and iron deficiency anaemia in hereditary thrombophilia pregnant women: An interventional study. Biometals 2014, 27, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Pantanella, F.; Pacifici, E.; Goolsbee, W.; Valenti, P. Lactoferrin efficacy versus ferrous sulfate in curing iron deficiency and iron deficiency anemia in pregnant women. Biometals 2010, 23, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Khalafallah, A.A.; Dennis, A.E. Iron deficiency anaemia in pregnancy and postpartum: Pathophysiology and effect of oral versus intravenous iron therapy. J. Pregnancy 2012, 2012, 630519–630528. [Google Scholar] [CrossRef] [PubMed]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Goolsbee, W.; Pacifici, E.; Valenti, P. Lactoferrin efficacy versus ferrous sulfate in curing iron disorders in pregnant and non-pregnant women. Int. J. Immunopathol. Pharmacol. 2010, 23, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, R.; Schiller, B.; Rao, M.; Coyne, D.; Brenner, L.; Pereira, B.J. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.E.; Wessling-Resnick, M. Iron metabolism and the innate immune response to infection. Microbes Infect. 2012, 14, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Leitch, H.; Bodner-Adler, B.; Brunbauer, M.; Kaider, A.; Egarter, C.; Husslein, P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am. J. Obstet. Gynecol. 2003, 189, 139–147. [Google Scholar] [CrossRef]
- Estrada-Gutierrez, G.; Gomez-Lopez, N.; Zaga-Clavellina, V.; Giono-Cerezo, S.; Espejel-Nunez, A.; Gonzalez-Jimenez, M.A.; Espino y Sosa, S.; Olson, D.M.; Vadillo-Ortega, F. Interaction between pathogenic bacteria and intrauterine leukocytes triggers alternative molecular signaling cascades leading to labor in women. Infect. Immun. 2010, 78, 4792–4799. [Google Scholar] [CrossRef] [PubMed]
- Houben, M.L.; Nikkels, P.G.J.; van Bleek, G.M.; Visser, G.H.A.; Rovers, M.M.; Kessel, H.; de Waal, W.J.; Schuijff, L.; Evers, A.; Kimpen, J.L.; et al. The association between intrauterine inflammation and spontaneous vaginal delivery at term: A cross-sectional study. PLoS ONE 2009, 4, e6572. [Google Scholar] [CrossRef] [PubMed]
- Murtha, A.P.; Greig, P.C.; Jimmerson, C.E.; Herbert, W.N. Maternal serum interleukin-6 concentration as a marker for impending preterm delivery. Obstet. Gynecol. 1998, 91, 161–164. [Google Scholar] [CrossRef]
- Goepfert, A.R.; Goldenberg, R.L.; Andrews, W.W.; Hauth, J.C.; Mercer, B.; Iams, J.; Meis, P.; Moawad, A.; Thom, E.; VanDorsten, J.P.; et al. The preterm prediction study: Association between cervical interleukin 6 concentration and spontaneous preterm birth. Am. J. Obstet. Gynecol. 2001, 184, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, Y.; Romero, R.; Mele, L.; Wapner, R.J.; Iams, J.D.; Dudley, D.J.; Spong, C.Y.; Peaceman, A.M.; Leveno, K.J.; Harper, M.; et al. Maternal serum interleukin-6, c-reactive protein, and matrix metalloproteinase-9 concentrations as risk factors for preterm birth <32 weeks and adverse neonatal outcomes. Am. J. Perinatol. 2010, 27, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Fraser, W.; Luo, Z.C. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: A systematic review. Obstet. Gynecol. 2010, 116, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.D.; Dudley, D.J.; Edwin, S.S.; Schiller, S.L. Interleukin-6 stimulates prostaglandin production by human amnion and decidual cells. Eur. J. Pharmacol. 1991, 192, 189–191. [Google Scholar] [CrossRef]
- Lyon, D.; Cheng, C.Y.; Howland, L.; Rattican, D.; Jallo, N.; Pickler, R.; Brown, L.; McGrath, J. Integrated review of cytokines in maternal, cord, and newborn blood: Part I-associations with preterm birth. Biol. Res. Nurs. 2010, 11, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Gotsch, F.; Romero, R.; Erez, O.; Vaisbuch, E.; Kusanovic, J.P.; Mazaki-Tovi, S.; Kim, S.K.; Hassan, S.; Yeo, L. The preterm parturition syndrome and its implications for understanding the biology, risk assessment, diagnosis, treatment and prevention of preterm birth. J. Matern. Fetal Neonatal Med. 2009, 22, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Lindenmüller, H.; Lambrecht, J.T. Oral care. Curr. Probl. Dermatol. 2011, 40, 107–115. [Google Scholar] [CrossRef]
- Madianos, P.N.; Bobetsis, Y.A.; Kinane, D.F. Generation of inflammatory stimuli: How bacteria setup inflammatory responses in the gingiva. J. Clin. Periodontol. 2005, 32, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Cantley, M.D.; Haynes, D.R. Mechanisms and control of pathologic bone loss in periodontitis. Periodontology 2000 2010, 53, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Thomson, W.M.; Sheiham, A.; Spencer, A.J. Sociobehavioral aspects of periodontal disease. Periodontology 2000 2012, 60, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N. Systemic effects of periodontitis: Lessons learned from research on atherosclerotic vascular disease and adverse pregnancy outcomes. Int. Dent. J. 2015, 65, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Berlutti, F.; Pilloni, A.; Pietropaoli, M.; Polimeni, A.; Valenti, P. Lactoferrin and oral diseases: Current status and perspective in periodontitis. Ann. Stomatol. 2011, 2, 10–18. [Google Scholar]
- Fujita, T.; Ashikaga, A.; Shiba, H.; Kajiya, M.; Kishimoto, A.; Hirata, R.; Tsunekuni, N.; Hirono, C.; Kawaguchi, H.; Shiba, Y.; et al. Irsogladine maleate counters the interleukin-1 beta-induced suppression in gap-junctional intercellular communication but does not affect the interleukin-1 beta-induced zonula occludens protein-1 levels in human gingival epithelial cells. J. Periodontal. Res. 2008, 43, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Hayashida, K.; Shiba, H.; Kishimoto, A.; Matsuda, S.; Takeda, K.; Kawaguchi, H.; Kurihara, H. The expressions of claudin-1 and E-cadherin in junctional epithelium. J. Periodontal. Res. 2010, 45, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Yumoto, H.; Shiba, H.; Ouhara, K.; Miyagawa, T.; Nagahara, T.; Matsuda, S.; Kawaguchi, H.; Matsuo, T.; Murakami, S.; et al. Irsogladine maleate regulates epithelial barrier function in tumor necrosis factor-α-stimulated human gingival epithelial cells. J. Periodontal. Res. 2012, 47, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Yamauchi, K.; Kobayashi, T.; Yaeshima, T.; Iwatsuki, K.; Yoshie, H. Inhibitory Effects of Lactoferrin on Growth and Biofilm Formation of Porphyromonas gingivalis and Prevotella intermedia. Antimicrob. Agents Chemother. 2009, 53, 3308–3316. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T. Susceptibility of Porphyromonas gingivalis in biofilms to amoxicillin, doxycycline and metronidazole. Oral Microbiol. Immunol. 2002, 17, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Ishihara, K.; Kimizuka, R.; Okuda, K.; Kato, T. The effects of tetracycline, minocycline, doxycycline and ofloxacin on Prevotella intermedia biofilm. Oral Microbiol. Immunol. 2006, 21, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Arslan, S.Y.; Leung, K.P.; Wu, C.D. The effect of lactoferrin on oral bacterial attachment. Oral Microbiol. Immunol. 2009, 24, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, T.E. The Management of Inflammation in Periodontal Disease. J. Periodontol. 2008, 79, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Kondo, I.; Kobayashi, T.; Yamauchi, K.; Toida, T.; Iwatsuki, K.; Yoshie, H. Periodontitis, periodontopathic bacteria and lactoferrin. Biometals 2010, 23, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Needleman, I.; Ashley, P.; Petrie, A.; Fortune, F.; Turner, W.; Jones, J.; Niggli, J.; Engebretsen, L.; Budgett, R.; Donos, N.; et al. Oral health and impact on performance of athletes participating in the London 2012 Olympic Games: A cross-sectional study. Br. J. Sports Med. 2013, 47, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.O. Dental condition of Olympic Games contestants-a pilot study, 1968. Dent. Pract. Dent. Rec. 1969, 20, 95–101. [Google Scholar] [PubMed]
- Soler, B.D.; Batchelor, P.A.; Sheiham, A. The prevalence of oral health problems in participants of the 1992 Olympic Games in Barcelona. Int. Dent. J. 1994, 44, 44–48. [Google Scholar]
- He, C.S.; Tsai, M.L.; Ko, M.H.; Chang, C.K.; Fang, S.H. Relationships among salivary immunoglobulin A, lactoferrin and cortisol in basketball players during a basketball season. Eur. J. Appl. Physiol. 2010, 110, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Mulic, A.; Tveit, A.; Songe, D.; Sivertsen, H.; Skaare, A.B. Dental erosive wear and salivary flow rate in physically active young adults. BMC Oral Health 2012, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, W.H.; Donovan, T.E.; Geissberger, M. Sports drinks and dental erosion. J. Can. Dent. Assoc. 2011, 39, 233–238. [Google Scholar]
- Bryant, S.; McLaughlin, K.; Morgaine, K.; Drummond, B. Elite athletes and oral health. Int. J. Sports Med. 2011, 32, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Rogers, G.; Goodman, C.; Baynes, R.D.; Bothwell, T.H.; Bezwoda, W.R.; Kramer, F.; Hattingh, J. Hematologic, iron-related, and acute-phase protein responses to sustained strenuous exercise. J. Appl. Physiol. 1987, 62, 464–469. [Google Scholar] [PubMed]
- Newlin, M.K.; Williams, S.; McNamara, T.; Tjalsma, H.; Swinkels, D.W.; Haymes, E.M. The effects of acute exercise bouts on hepcidin in women. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Metz-Boutigue, M.H.; Jollés, J.; Mazurier, J.; Schoentgen, F.; Legrand, D.; Spik, G.; Montreuil, J.; Jollès, P. Human lactotransferrin: Amino acid sequence and structural comparisons with other transferrins. Eur. J. Biochem. 1984, 145, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Adlerova, L.; Bartoskova, A.; Faldyna, M. Lactoferrin: A review. Vet. Med. 2008, 53, 457–468. [Google Scholar]
- Karav, S.; German, J.B.; Rouquié, C.; Le Parc, A.; Barile, D. Studying Lactoferrin N-Glycosylation. Int. J. Mol. Sci. 2017, 18, E870. [Google Scholar] [CrossRef] [PubMed]
- Superti, F.; Siciliano, R.; Rega, B.; Giansanti, F.; Valenti, P.; Antonini, G. Involvement of bovine lactoferrin metal saturation, sialic acid and protein fragments in the inhibition of rotavirus infection. Biochim. Biophys. Acta 2001, 1528, 107–115. [Google Scholar] [CrossRef]

| Biological Fluids | Concentration (mg/mL) |
|---|---|
| Colostrum | 8 |
| Milk | 1.5–4 |
| Tears | 2 |
| Saliva | 0.008 |
| Vaginal secretion | 0.008 |
| Seminal fluid | 0.112 |
| Cerebrospinal fluid | Undetectable |
| Plasma | 0.0004 |
| Joint fluid | 0.001 |
| Serum Parameters | Mean Values ± Standard Deviation | |||||
|---|---|---|---|---|---|---|
| RBC 103/mmc | Hb g/dL | TSI µg/dL | sFtn ng/mL | Hematocrit % | IL-6 pg/mL | |
| Before bLf treatment | 3680 ± 216 | 10.8 ± 0.5 | 56 ± 22 | 13 ± 7 | 29 ± 3 | 25 ± 7 |
| After bLf treatment | 4160 ± 286 * | 11.9 ± 0.8 * | 88 ± 16 * | 25 ± 8 * | 42 ± 3 * | 13 ± 6 * |
| Serum Parameters | Mean Values ± Standard Deviation | |||||
|---|---|---|---|---|---|---|
| RBC 103/mmc | Hb g/dL | TSI µg/dL | sFtn ng/mL | Hematocrit % | IL-6 pg/mL | |
| Before bLf treatment | 3860 ± 214 | 10.4 ± 0.8 | 60 ± 18 | 15 ± 7 | 28 ± 4 | 94 ± 7 |
| After bLf treatment | 4150 ± 75 * | 12.5 ± 0.3 * | 94 ± 7 * | 32 ± 4 * | 36 ± 9 | 48 ± 12 * |
| Serum Parameters | Mean Values ± Standard Deviation | |||||
|---|---|---|---|---|---|---|
| RBC 103/mmc | Hb g/dL | TSI µg/dL | sFtn ng/mL | Hematocrit % | IL-6 pg/mL | |
| Before ferrous sulfate treatment | 3705 ± 162 | 10 ± 0.7 | 37 ± 10 | 15 ± 5 | 29 ± 6 | 33 ± 9 |
| After ferrous sulfate treatment | 3745 ± 123 | 11 ± 1.0 | 47 ± 11 | 14 ± 4 | 29 ± 3 | 52 ± 13 |
| Clinical Parameters | Mean Values ± Standard Deviation | |||||
|---|---|---|---|---|---|---|
| PPD | GI | PlI | BOP (%) | CAL (mm) | IL-6 (ng/mL) | |
| Baseline | 2.8 ± 0.3 | 0.80 ± 0.10 | 0.80 ± 0.10 | 32 | 1.50 ± 0.60 | 1.42 ± 0.30 |
| After 4 weeks of bLf treatment | 0.6 ± 0.7 | 0.50 ± 0.10 | 0.40 ± 0.20 | 0 | 0.50 ± 0.30 | 0.55 ± 0.31 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. https://doi.org/10.3390/ijms18091985
Rosa L, Cutone A, Lepanto MS, Paesano R, Valenti P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. International Journal of Molecular Sciences. 2017; 18(9):1985. https://doi.org/10.3390/ijms18091985
Chicago/Turabian StyleRosa, Luigi, Antimo Cutone, Maria Stefania Lepanto, Rosalba Paesano, and Piera Valenti. 2017. "Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis" International Journal of Molecular Sciences 18, no. 9: 1985. https://doi.org/10.3390/ijms18091985