NAFLD: Is There Anything New under the Sun?
1. Introduction
2. Epidemiology
3. Diagnosis
4. Genetics, Epigenetics, Pathophysiology, and Molecular Pathogenesis
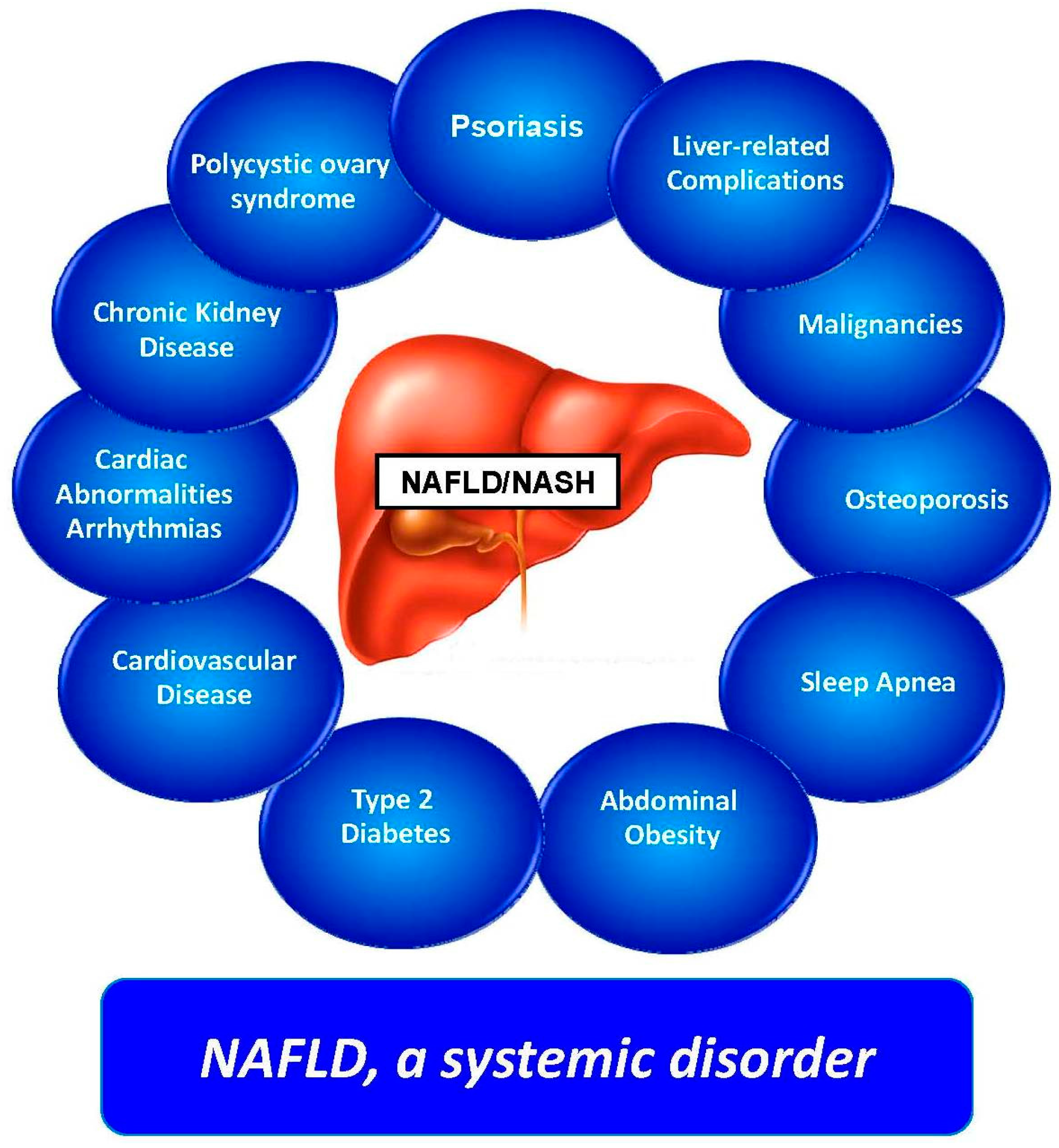
5. Clinical Features and Comorbidities
6. Clinical Course and Natural History
7. Pediatric Nonalcoholic Fatty Liver Disease
8. NAFLD and Hepatitis C Virus
9. Management
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMPK | AMP-Activated Protein Kinase |
| CRN | Clinical Research Network |
| EVs | Extracellular Vesicles |
| FLIP | Fatty Liver Inhibition of Progression |
| HCC | Hepatocellular Carcinoma |
| Hh | Hedgehog |
| LAL | Lysosomal Acid Lipase |
| NAFLD | Nonalcoholic Fatty Liver Disease |
| NASH | Nonalcoholic Steatohepatitis |
| PNPLA3 | Patatin-Like Phospholipase Domain Containing 3 |
| TM6SF2 | Trans-Membrane 6 Superfamily Member 2 |
References
- Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig. Liver Dis. 2017, 49, 471–483. [Google Scholar]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Zona, S.; Targher, G.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Roverato, A.; Guaraldi, G.; Lonardo, A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Nascimbeni, F.; Romagnoli, D.; Lonardo, A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features. Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol. Res. 2016, 46, 1074–1087. [Google Scholar] [PubMed]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Loria, P.; Argo, C.; Caldwell, S. Perspectives on cellular dysfunction in nonalcoholic steatohepatitis: A case of “multiorganelle failure”? Proceedings of a virtual workshop on nonalcoholic steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Farrell, G.C.; Wong, V.W.; Chitturi, S. NAFLD in Asia—As common and important as in the West. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Blissett, D.; Blissett, R.; Henry, L.; Stepanova, M.; Younossi, Y.; Racila, A.; Hunt, S.; Beckerman, R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016, 64, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Bellentani, S.; Argo, C.K.; Ballestri, S.; Byrne, C.D.; Caldwell, S.H.; Cortez-Pinto, H.; Grieco, A.; Machado, M.V.; Miele, L.; et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig. Liver Dis. 2015, 47, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar]
- Losekann, A.; Weston, A.; de Mattos, A.; Tovo, C.; de Carli, L.; Espindola, M.; Pioner, S.; Coral, G. Non-alcoholic steatohepatitis (NASH): Risk factors in morbidly obese patients. Int. J. Mol. Sci. 2015, 16, 25552–25559. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Pisano, G.; Fargion, S. Role of serum uric acid and ferritin in the development and progression of NAFLD. Int. J. Mol. Sci. 2016, 17, 548. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; Pastori, D.; Polimeni, L.; Tozzi, G.; Violi, F.; Angelico, F.; del Ben, M. Does Lysosomial acid lipase reduction play a role in adult non-alcoholic fatty liver disease? Int. J. Mol. Sci. 2015, 16, 28014–28021. [Google Scholar] [CrossRef] [PubMed]
- Shteyer, E.; Villenchik, R.; Mahamid, M.; Nator, N.; Safadi, R. Low serum lysosomal acid lipase activity correlates with advanced liver disease. Int. J. Mol. Sci. 2016, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Nonalcoholic steatohepatitis clinical research network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E. Nonalcoholic fatty liver disease: Pros and cons of histologic systems of evaluation. Int. J. Mol. Sci. 2016, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, H.; Shaheen, F.; Kalscheuer, H.; Schmid, S.M.; Oster, H.; Lehnert, H. The telomeric complex and metabolic disease. Genes 2017, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Laish, I.; Mannasse-Green, B.; Hadary, R.; Konikoff, F.M.; Amiel, A.; Kitay-Cohen, Y. Aneuploidy and asynchronous replication in non-alcoholic fatty liver disease and cryptogenic cirrhosis. Gene 2016, 593, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Donati, B.; Valenti, L. Telomeres NAFLD and chronic liver disease. Int. J. Mol. Sci. 2016, 17, 383. [Google Scholar] [CrossRef] [PubMed]
- Ban, L.; Shackel, N.; McLennan, S. Extracellular vesicles: A new frontier in biomarker discovery for non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2016, 17, 376. [Google Scholar] [CrossRef] [PubMed]
- Nuño-Lámbarri, N.; Barbero-Becerra, V.; Uribe, M.; Chávez-Tapia, N. Mitochondrial molecular pathophysiology of nonalcoholic fatty liver disease: A proteomics approach. Int. J. Mol. Sci. 2016, 17, 281. [Google Scholar] [CrossRef] [PubMed]
- Gambino, R.; Bugianesi, E.; Rosso, C.; Mezzabotta, L.; Pinach, S.; Alemanno, N.; Saba, F.; Cassader, M. Different serum free fatty acid profiles in NAFLD subjects and healthy controls after oral fat load. Int. J. Mol. Sci. 2016, 17, 479. [Google Scholar] [CrossRef] [PubMed]
- Houghton, D.; Stewart, C.; Day, C.; Trenell, M. Gut microbiota and lifestyle interventions in NAFLD. Int. J. Mol. Sci. 2016, 17, 447. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Cortez-Pinto, H. Diet microbiota obesity and NAFLD: A dangerous quartet. Int. J. Mol. Sci. 2016, 17, 481. [Google Scholar] [CrossRef] [PubMed]
- Aragonès, G.; Auguet, T.; Armengol, S.; Berlanga, A.; Guiu-Jurado, E.; Aguilar, C.; Martínez, S.; Sabench, F.; Porras, J.; Ruiz, M.; et al. PNPLA3 expression is related to liver steatosis in morbidly obese women with non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2016, 17, 630. [Google Scholar] [CrossRef] [PubMed]
- Petäjä, E.; Yki-Järvinen, H. Definitions of normal liver fat and the association of insulin sensitivity with acquired and genetic NAFLD—A systematic review. Int. J. Mol. Sci. 2016, 17, 633. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Ballestri, S.; Targher, G. “Not all forms of NAFLD were created equal”. Do metabolic syndrome-related NAFLD and PNPLA3-related NAFLD exert a variable impact on the risk of early carotid atherosclerosis? Atherosclerosis 2017, 257, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Pisano, G.; Lombardi, R.; Fracanzani, A. Vascular damage in patients with nonalcoholic fatty liver disease: Possible role of iron and ferritin. Int. J. Mol. Sci. 2016, 17, 675. [Google Scholar] [CrossRef] [PubMed]
- Verdelho Machado, M.; Diehl, A. Role of hedgehog signaling pathway in NASH. Int. J. Mol. Sci. 2016, 17, 857. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, A.; Gentilini, A.; Marra, F. Molecular pathogenesis of NASH. Int. J. Mol. Sci. 2016, 17, 1575. [Google Scholar] [CrossRef] [PubMed]
- France, M.; Kwok, S.; Soran, H.; Williams, S.; Ho, J.; Adam, S.; Canoy, D.; Liu, Y.; Durrington, P. Liver fat measured by MR spectroscopy: Estimate of imprecision and relationship with serum glycerol, caeruloplasmin and non-esterified fatty acids. Int. J. Mol. Sci. 2016, 17, 1089. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Bellini, M.; Tondelli, E.; Frazzoni, M.; Grisendi, A.; Pulvirenti, M.; Della Casa, G. Nonalcoholic steatohepatitis and the “bright liver syndrome”: Should a recently expanded clinical entity be further expanded? Am. J. Gastroenterol. 1995, 90, 2072–2074. [Google Scholar] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62 (Suppl. 1), S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Valenti, L.; Bugianesi, E.; Targher, G.; Bellentani, S.; Bonino, F.; Special Interest Group on Personalised Hepatology of the Italian Association for the Study of the Liver (AISF). A “systems medicine” approach to the study of non-alcoholic fatty liver disease. Dig. Liver Dis. 2016, 48, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D. Non-alcoholic fatty liver disease: An emerging driving force in chronic kidney disease. Nat. Rev. Nephrol. 2017, 13, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Loria, P.; Carulli, N. Concurrent non-alcoholic steatohepatitis and psoriasis. Report of three cases from the POLI. ST. ENA study. Dig. Liver Dis. 2001, 33, 86–87. [Google Scholar] [CrossRef]
- Mantovani, A.; Gisondi, P.; Lonardo, A.; Targher, G. Relationship between non-alcoholic fatty liver disease and psoriasis: A novel hepato-dermal axis? Int. J. Mol. Sci. 2016, 17, 217. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, P.; Byrne, C. Bidirectional relationships and disconnects between NAFLD and features of the metabolic syndrome. Int. J. Mol. Sci. 2016, 17, 367. [Google Scholar] [CrossRef] [PubMed]
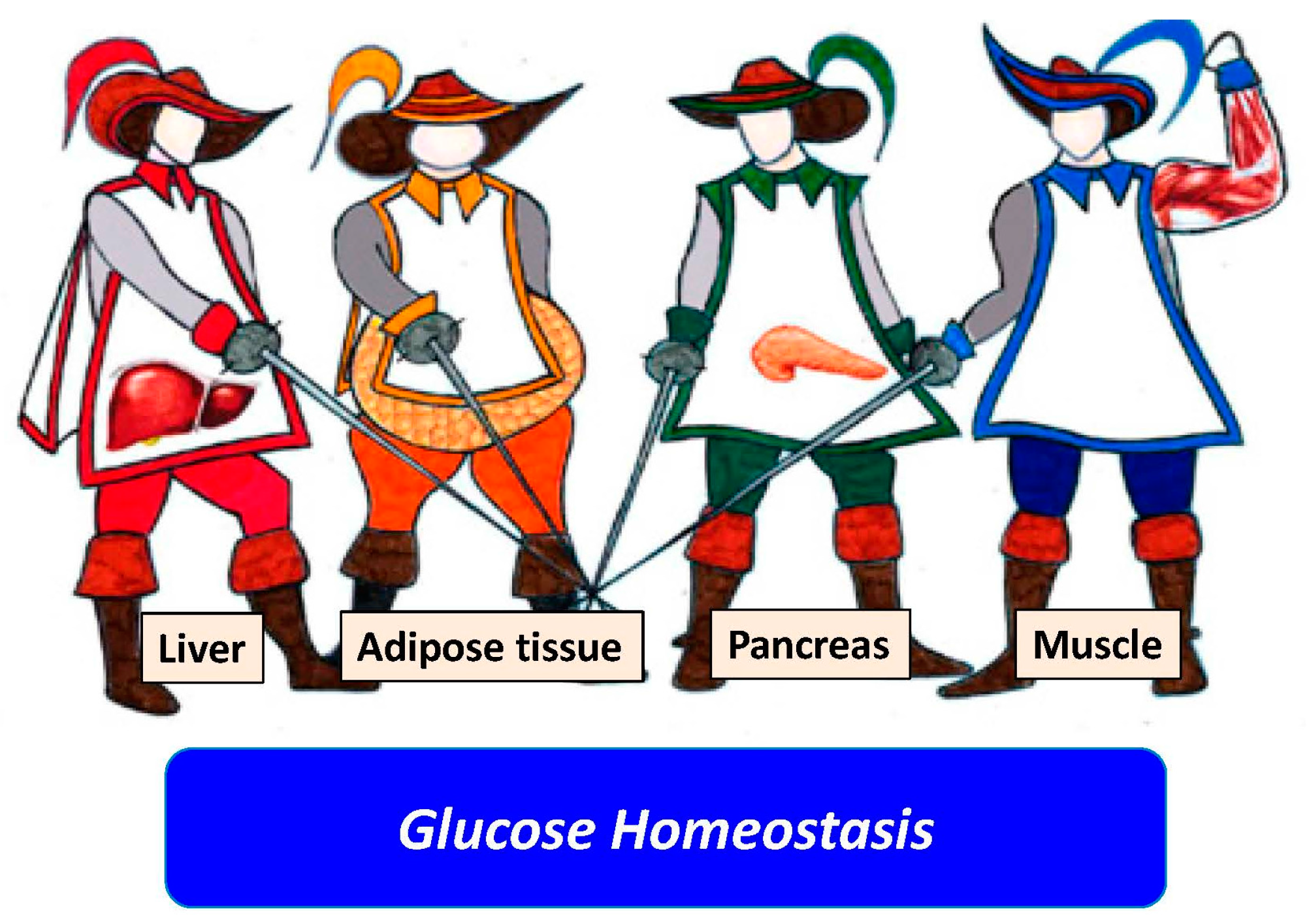
- Ballestri, S.; Nascimbeni, F.; Romagnoli, D.; Baldelli, E.; Targher, G.; Lonardo, A. Type 2 Diabetes in non-alcoholic fatty liver disease and hepatitis C virus infection—Liver: The “Musketeer” in the spotlight. Int. J. Mol. Sci. 2016, 17, 355. [Google Scholar] [CrossRef] [PubMed]
- Perticone, M.; Cimellaro, A.; Maio, R.; Caroleo, B.; Sciacqua, A.; Sesti, G.; Perticone, F. Additive effect of non-alcoholic fatty liver disease on metabolic syndrome-related endothelial dysfunction in hypertensive patients. Int. J. Mol. Sci. 2016, 17, 456. [Google Scholar] [CrossRef] [PubMed]
- Marcuccilli, M.; Chonchol, M. NAFLD and chronic kidney disease. Int. J. Mol. Sci. 2016, 17, 562. [Google Scholar] [CrossRef] [PubMed]
- Villela-Nogueira, C.; Leite, N.; Cardoso, C.; Salles, G. NAFLD and increased aortic stiffness: Parallel or common physiopathological mechanisms? Int. J. Mol. Sci. 2016, 17, 460. [Google Scholar] [CrossRef] [PubMed]
- Sanna, C.; Rosso, C.; Marietti, M.; Bugianesi, E. Non-alcoholic fatty liver disease and extra-hepatic cancers. Int. J. Mol. Sci. 2016, 17, 717. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Nascimbeni, F.; Romagnoli, D.; Baldelli, E.; Lonardo, A. The role of nuclear receptors in the pathophysiology, natural course, and drug treatment of NAFLD in humans. Adv. Ther. 2016, 33, 291–319. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Sookoian, S.; Chonchol, M.; Loria, P.; Targher, G. Cardiovascular and systemic risk in nonalcoholic fatty liver disease—Atherosclerosis as a major player in the natural course of NAFLD. Curr. Pharm. Des. 2013, 19, 5177–5192. [Google Scholar] [CrossRef] [PubMed]
- Calzadilla Bertot, L.; Adams, L. The natural course of non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2016, 17, 774. [Google Scholar] [CrossRef] [PubMed]
- Gitto, S.; Villa, E. Non-alcoholic fatty liver disease and metabolic syndrome after liver transplant. Int. J. Mol. Sci. 2016, 17, 490. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Lappe, S.; Feldstein, A.E.; Alkhouri, N. Similarities and differences between pediatric and adult nonalcoholic fatty liver disease. Metabolism 2016, 65, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.; Cordero, P.; Li, J.; Nguyen, V.; Oben, J. A Guide to non-alcoholic fatty liver disease in childhood and adolescence. Int. J. Mol. Sci. 2016, 17, 947. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, L.; Bonci, E.; Andreoli, G.; di Martino, M.; Gallozzi, A.; De Luca, E.; Chiesa, C. The impact of nonalcoholic fatty liver disease on renal function in children with overweight/obesity. Int. J. Mol. Sci. 2016, 17, 1218. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Adinolfi, L.E.; Loria, P.; Carulli, N.; Ruggiero, G.; Day, C.P. Steatosis and hepatitis C virus: Mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology 2004, 126, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Padovani, R.; Rodella, S.; Arcaro, G.; Day, C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J. Hepatol. 2007, 46, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Adinolfi, L.E.; Restivo, L.; Ballestri, S.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Loria, P. Pathogenesis and significance of hepatitis C virus steatosis: An update on survival strategy of a successful pathogen. World J. Gastroenterol. 2014, 20, 7089–7103. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Nascimbeni, F.; Giordano, M.; Masetti, C.; Guerrera, B.; Amelia, A.; Fascione, M.C.; Ballestri, S.; Romagnoli, D.; Zampino, R.; et al. Clinical features and natural history of cryptogenic cirrhosis compared to hepatitis C virus-related cirrhosis. World J. Gastroenterol. 2017, 23, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, L.; Rinaldi, L.; Guerrera, B.; Restivo, L.; Marrone, A.; Giordano, M.; Zampino, R. NAFLD and NASH in HCV infection: Prevalence and significance in hepatic and extrahepatic manifestations. Int. J. Mol. Sci. 2016, 17, 803. [Google Scholar] [CrossRef] [PubMed]
- Shigefuku, R.; Takahashi, H.; Nakano, H.; Watanabe, T.; Matsunaga, K.; Matsumoto, N.; Kato, M.; Morita, R.; Michikawa, Y.; Tamura, T.; et al. Correlations of hepatic hemodynamics liver function and fibrosis markers in nonalcoholic fatty liver disease: Comparison with chronic hepatitis related to hepatitis C virus. Int. J. Mol. Sci. 2016, 17, 1545. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Ballestri, S.; Targher, G.; Loria, P. Diagnosis and management of cardiovascular risk in nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 629–650. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Ryterska, K.; Maciejewska, D.; Banaszczak, M.; Milkiewicz, P.; Milkiewicz, M.; Gutowska, I.; Ossowski, P.; Kaczorowska, M.; Jamioł-Milc, D.; et al. Nutritional strategies for the individualized treatment of non-alcoholic fatty liver disease (NAFLD) based on the nutrient-induced insulin output ratio (NIOR). Int. J. Mol. Sci. 2016, 17, 1192. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rodas, M.; Valenzuela, R.; Videla, L. Relevant aspects of nutritional and dietary interventions in non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2015, 16, 25168–25198. [Google Scholar] [CrossRef] [PubMed]
- Walenbergh, S.; Houben, T.; Hendrikx, T.; Jeurissen, M.; van Gorp, P.; Vaes, N.; Damink, S.; Verheyen, F.; Koek, G.; Lütjohann, D.; et al. Weekly treatment of 2-hydroxypropyl-β-cyclodextrin improves intracellular cholesterol levels in LDL receptor knockout mice. Int. J. Mol. Sci. 2015, 1, 21056–21069. [Google Scholar] [CrossRef] [PubMed]
- Ideta, T.; Shirakami, Y.; Miyazaki, T.; Kochi, T.; Sakai, H.; Moriwaki, H.; Shimizu, M. The DIPEPTIDYL peptidase-4 Inhibitor teneligliptin attenuates hepatic lipogenesis via AMPK activation in non-alcoholic fatty liver disease model mice. Int. J. Mol. Sci. 2015, 16, 29207–29218. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lonardo, A.; Targher, G. NAFLD: Is There Anything New under the Sun? Int. J. Mol. Sci. 2017, 18, 1955. https://doi.org/10.3390/ijms18091955
Lonardo A, Targher G. NAFLD: Is There Anything New under the Sun? International Journal of Molecular Sciences. 2017; 18(9):1955. https://doi.org/10.3390/ijms18091955
Chicago/Turabian StyleLonardo, Amedeo, and Giovanni Targher. 2017. "NAFLD: Is There Anything New under the Sun?" International Journal of Molecular Sciences 18, no. 9: 1955. https://doi.org/10.3390/ijms18091955





