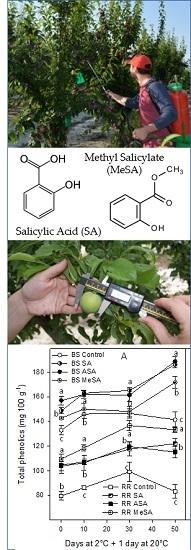
Enhancement of Antioxidant Systems and Storability of Two Plum Cultivars by Preharvest Treatments with Salicylates
Abstract
:1. Introduction
2. Results
2.1. Fruit Quality Parameters
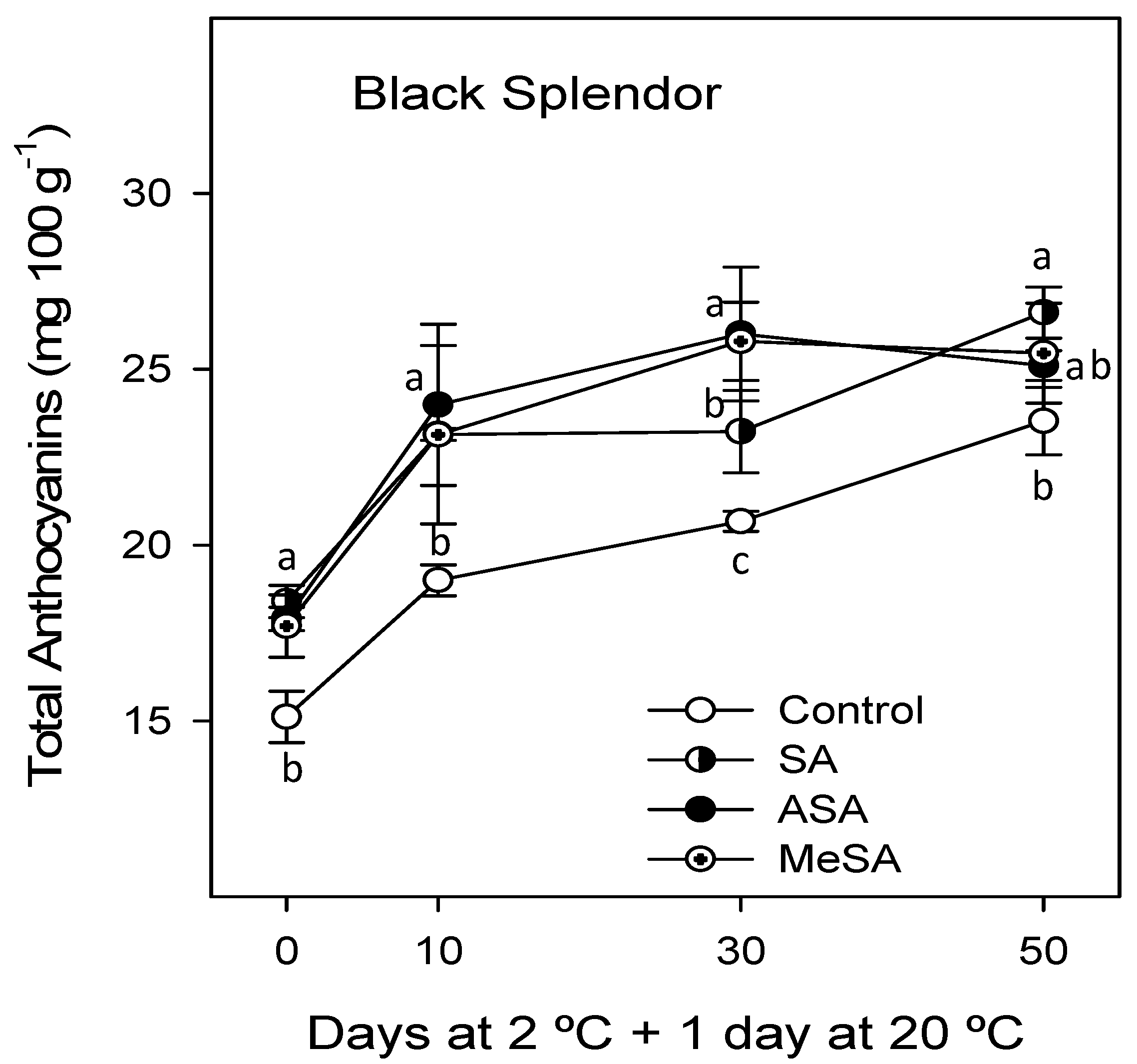
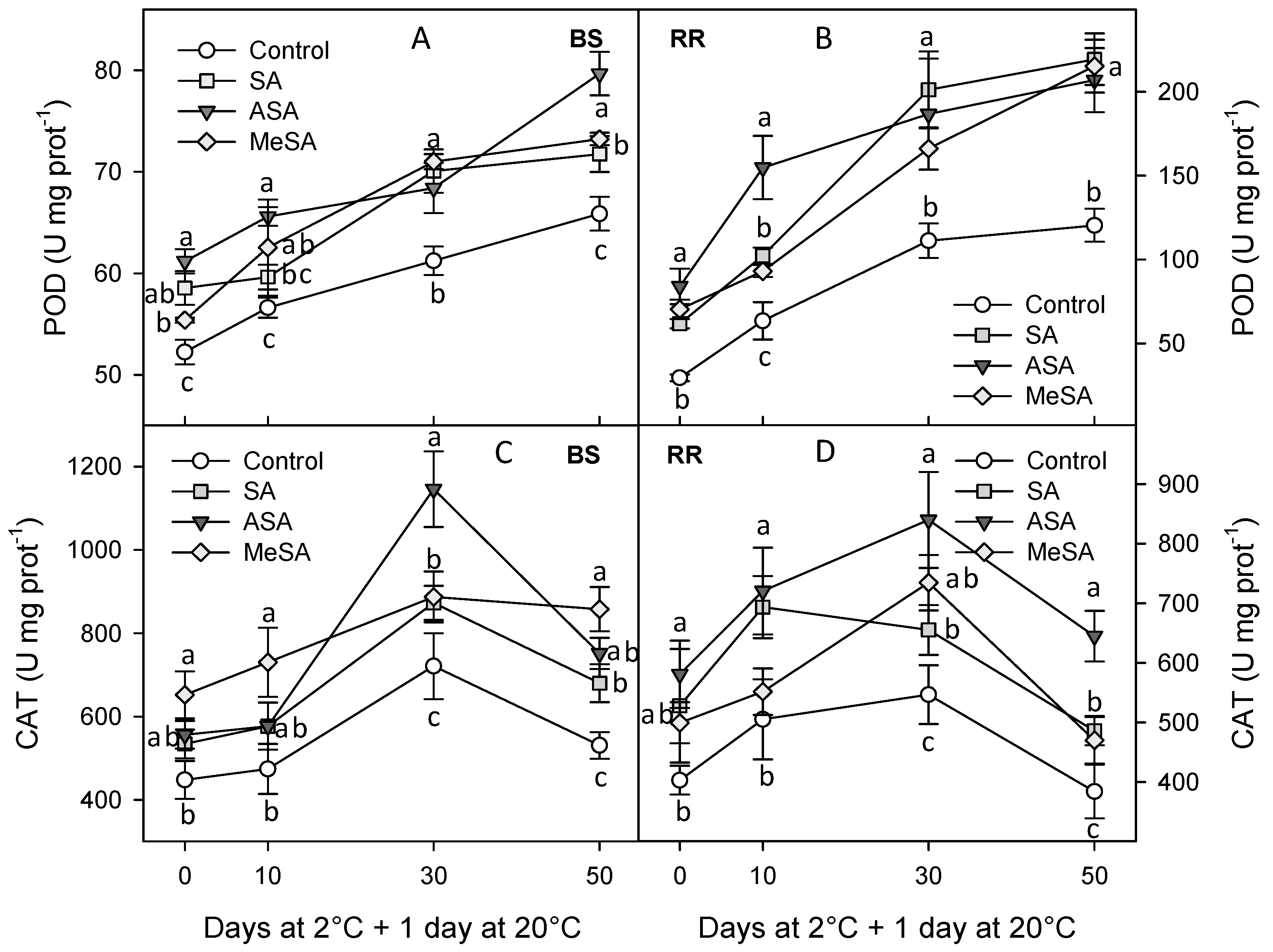
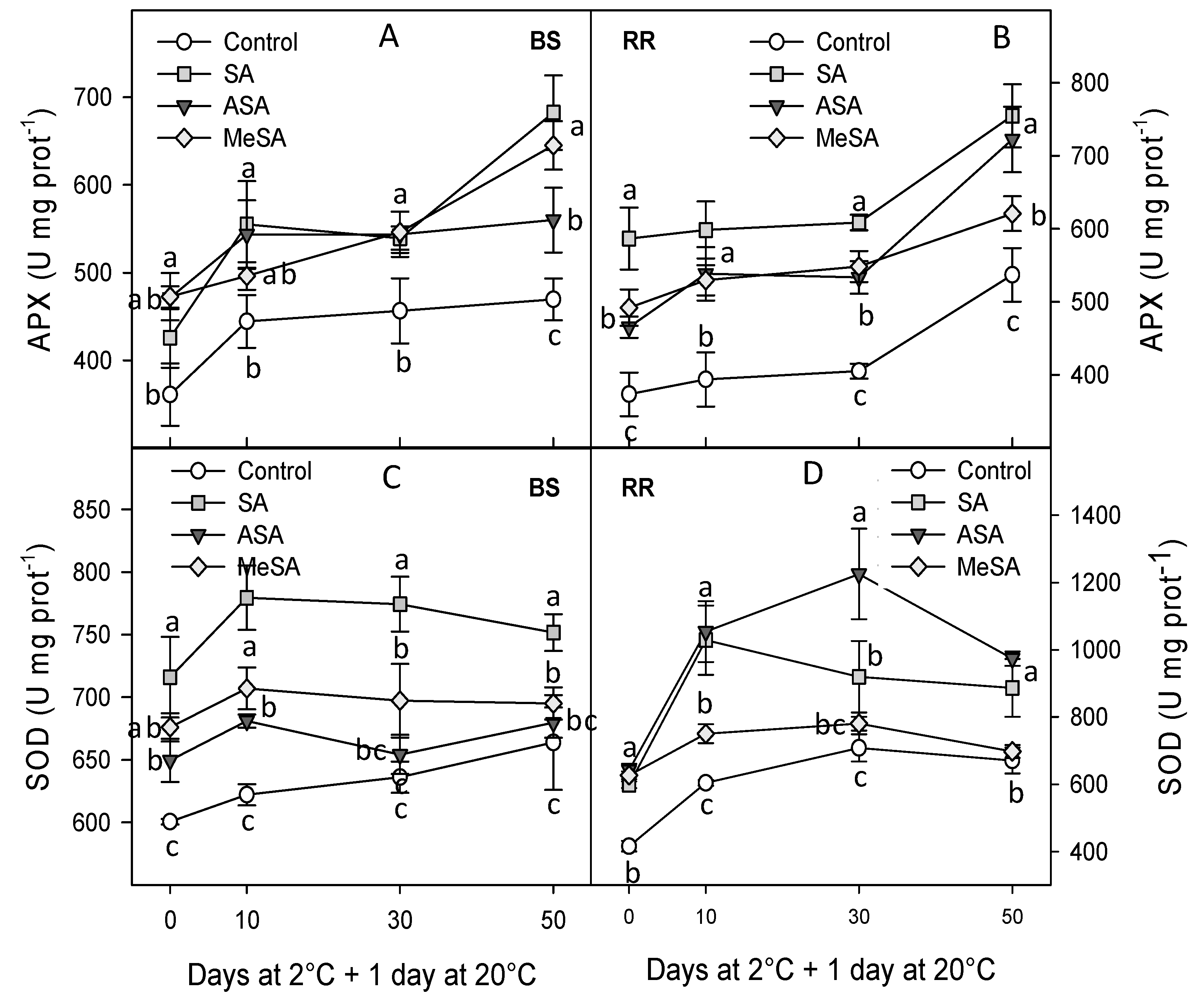
2.2. Bioactive Compounds and Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Weight Loss, Firmness, Color, Total Soluble Solids (TSS), and Total Acidity (TA)
4.3. Bioactive Compounds and Antioxidant Activity
4.4. Antioxidant Enzymes
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; Lester, G.E.; Munro, K.D.; Toivonen, P.M.A. Oxidative stress: Importance for postharvest quality. HortScience 2004, 39, 924–929. [Google Scholar]
- Kumar, D. Salicylic acid signalling in disease resistance. Plant Sci. 2014, 22, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Asghari, M.; Aghdam, M.S. Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends Food Sci. Technol. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Glowacz, M.; Ree, D. Using jasmonates and salicylates to reduce losses within the fruit supply chain. Eur. Food Res. Technol. 2015, 242, 143–156. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, L.; Zhang, X.; Xu, L.; Cao, J.; Jiang, W. The effect of exogenous salicylic acid on antioxidant activity, bioactive compounds and antioxidant system in apricot fruit. Sci. Hortic. 2015, 181, 113–120. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Martínez, R.D.; Guillén, F.; Serrano, M.; Valero, D. Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem. 2011a, 124, 964–970. [Google Scholar] [CrossRef]
- Sayyari, M.; Castillo, S.; Valero, D.; Díaz-Mula, H.M.; Serrano, M. Acetylsalicylic acid alleviates chilling injury and maintains nutritive and bioactive compounds and antioxidant activity during postharvest storage of pomegranates. Postharvest Biol. Technol. 2011b, 60, 136–142. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P J.; Castillo, S.; Guillén, F.; Martínez, R.D.; Serrano, M. Postharvest treatments with salicylic acid, acetylsalicylic acid or oxalic acid delayed ripening and enhanced bioactive compounds and antioxidant capacity in sweet cherry. J. Agric. Food Chem. 2011, 59, 5483–5489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Zhang, S.; Ferguson, I. The role of salicylic acid in postharvest ripening of kiwifruit. Postharvest Biol. Technol. 2003, 28, 67–74. [Google Scholar] [CrossRef]
- Ding, Z.S.; Tian, S.P.; Zheng, X.L.; Zhou, Z.W.; Xu, Y. Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiol. Plantarum 2007, 30, 112–121. [Google Scholar] [CrossRef]
- Mo, Y.; Gong, D.; Liang, G.; Han, R.; Xie, J.; Li, W. Enhanced preservation effects of sugar apple fruits by salicylic acid treatment during post-harvest storage. J. Sci. Food Agric. 2008, 88, 2693–2699. [Google Scholar] [CrossRef]
- Tareen, M.J.; Abbasi, N.A.; Hafiz, I.A. Postharvest application of salicylic acid enhanced antioxidant enzyme activity and maintained quality of peach cv. “Flordaking” fruit during storage. Sci. Hortic. 2012, 142, 221–228. [Google Scholar] [CrossRef]
- Davarynejad, G.H.; Zarei, M.; Nasrabadi, M.E.; Ardakani, E. Effects of salicylic acid and putrescine on storability, quality attributes and antioxidant activity of plum cv. “Santa Rosa”. J. Food Sci. Tech. 2015, 52, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, R.R. Impact of staggered treatments of novel molecules and ethylene absorbents on postharvest fruit physiology and enzyme activity of “Santa Rosa” plums. Sci. Hortic. 2016, 198, 242–248. [Google Scholar] [CrossRef]
- Majeed, R.; Jawandha, S.K. Enzymatic changes in plum (Prunus salicina Lindl.) subjected to some chemical treatments and cold storage. J. Food Sci. Technol. 2016, 53, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Chen, C.; Xie, J. Effect of salicylic acid treatment on alleviating postharvest chilling injury of “Qingnai” plum fruit. Postharvest Biol. Technol. 2011, 62, 115–120. [Google Scholar] [CrossRef]
- Babalar, M.; Asghari, M.; Talaei, A.; Khosroshahi, A. Effect of pre- and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chem. 2007, 105, 449–453. [Google Scholar] [CrossRef]
- Cao, J.K.; Yan, J.Q.; Zhao, Y.M.; Jiang, W.B. Effects of four pre-harvest foliar sprays with β-aminobutyric acid or salicylic acid on the incidence of post-harvest disease and induced defence responses in jujube (Zizyphus jujuba Mill.) fruit after storage. J. Hortic. Sci. Biotechnol. 2013, 88, 338–344. [Google Scholar] [CrossRef]
- Chan, Z.; Wang, Q.; Xu, X.; Meng, X.; Qin, G.; Li, B.; Tian, S. Functions of defense-related proteins and dehydrogenases in resistance response induced by salicylic acid in sweet cherry fruits at different maturity stages. Proteomics 2008, 8, 4791–4807. [Google Scholar] [CrossRef] [PubMed]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez, R.D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Serrano, M.; Moral, J.; Castillo, S. Methyl salicylate treatments of sweet cherry trees improve fruit quality at harvest and during storage. Sci. Hortic. 2015, 197, 665–673. [Google Scholar] [CrossRef]
- Giménez, M.J.; Serrano, M.; Valverde, J.M.; Martínez, R.D.; Castillo, S.; Valero, D.; Guillén, F. Preharvest salicylic acid and acetylsalicylic acid treatments preserve quality and enhance antioxidant systems during postharvest storage of sweet cherry cultivars. J. Sci. Food Agric. 2017, 97, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Crisosto, G.M.; Crisosto, C.H.; Echeverría, G.; Puy, J. Segregation of plum and pluot cultivars according to their organoleptic characteristics. Postharvest Biol. Technol. 2007, 44, 271–276. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Castillo, S.; Martínez, R.D.; Valero, D.; Serrano, M. Changes in physicochemical and nutritive parameters and bioactive compounds during development and on-tree ripening of eight plum cultivars: A comparative study. J. Sci. Food Agric. 2008, 88, 2499–2507. [Google Scholar] [CrossRef]
- Guerra, M.; Casquero, P.A. Effect of harvest date on cold storage and postharvest quality of plum cv. Green Gage. Postharvest Biol. Technol. 2008, 47, 325–332. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez, R.D.; Castillo, S.; Serrano, M.; Valero, D. Changes in hydrophilic and lipophilic antioxidant activity and related bioactive compounds during postharvest storage of yellow and purple plum cultivars. Postharvest Biol. Technol. 2009, 51, 354–363. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press: Boca Raton, FL, USA, 2010; pp. 7–68. [Google Scholar]
- Champa, W.A.H.; Gill, M.I.S.; Mahajan, B.V.C.; Arora, N.K. Preharvest salicylic acid treatments to improve quality and postharvest life of table grapes (Vitis vinifera L.) cv. Flame Seedless. J. Food Sci. Tech. 2015, 52, 3607–3616. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.E. Interactive effect of salicylic acid on some physiological features and antioxidant enzymes activity in ginger (Zingiber officinale Roscoe). Molecules 2013, 18, 5965–5979. [Google Scholar] [CrossRef] [PubMed]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity and seed yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez, R.D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Yin, X.R.; Zhang, Y.; Zhang, B.; Yang, S.L.; Shi, Y.N.; Ferguson, I.B.; Chen, K.S. Effects of acetylsalicylic acid on kiwifruit ethylene biosynthesis and signalling components. Postharvest Biol. Technol. 2013, 83, 27–33. [Google Scholar] [CrossRef]
- Kazemi, M.; Aran, M.; Zamani, S. Effect of salicylic acid treatments on quality characteristics of apple fruits during storage. Am. J. Plant Physiol. 2011, 6, 113–119. [Google Scholar] [CrossRef]
- Igwe, E.O.; Charlton, K.E.A. Systematic review on the health effects of plums (Prunus domestica and Prunus salicina). Phytother. Res. 2016, 30, 701–731. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.C.; Gupta, S.C. Phytochemicals and antioxidants: An evaluation in understanding the human lifeline. Curr. Nutr. Food Sci. 2013, 9, 298–309. [Google Scholar] [CrossRef]
- Vizzotto, M.; Porter, W.; Byrne, D.; Cisneros, Z.L. Polyphenols of selected peach and plum genotypes reduce cell viability and inhibit proliferation of breast cancer cells while not affecting normal cells. Food Chem. 2014, 164, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Sahamishirazi, S.; Moehring, J.; Claupein, W.; Graeff, H.S. Quality assessment of 178 cultivars of plum regarding phenolic, anthocyanin and sugar content. Food Chem. 2017, 2014, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Valverde, J.M.; Giménez, M.J.; Guillén, F.; Valero, D.; Martínez, R.D.; Serrano, M. Methyl salicylate treatments of sweet cherry trees increase antioxidant systems in fruit at harvest and during storage. Postharvest Biol. Technol. 2015, 109, 106–113. [Google Scholar] [CrossRef]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Zapata, S.; Castillo, C.; Serrano, M. Postharvest methyl salicylate treatments delay ripening and maintain quality attributes and antioxidant compounds of “Early Lory” sweet cherry. Postharvest Biol. Technol. 2016, 117, 102–109. [Google Scholar] [CrossRef]
- Dokhanieh, A.Y.; Aghdam, M.S.; Fard, J.R.; Hassanpour, H. Postharvest salicylic acid treatment enhances antioxidant potential of cornelian cherry fruit. Sci. Hortic. 2013, 154, 31–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Yang, H. Postharvest chitosan-g-salicylic acid application alleviates chilling injury and preserves cucumber fruit quality during cold storage. Food Chem. 2015, 174, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Mondal, K.; Malhotra, S.P.; Jain, V.; Singh, R. Oxidative stress and antioxidant systems in guava (Psidium guajava L.) fruits during ripening. Physiol. Mol. Biol. Plants 2009, 15, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yadav, P.; Jain, V.; Malhotra, S.P. Isozymes of antioxidative enzymes during ripening and storage of ber (Ziziphus mauritiana Lamk.). J. Food Sci. Technol. 2014, 51, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.A.; Zapata, P.J.; Castillo, S.; Guillén, F.; Martínez, R.D.; Valero, D.; Serrano, M. Preharvest application of methyl jasmonate (MeJA) in two plum cultivars. 1. Improvement of fruit growth and quality attributes at harvest. Postharvest Biol. Technol. 2014, 98, 98–105. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- García, V.C.; Zafrilla, P.; Romero, F.; Abellá, P.; Artés, F.; Tomás-Barberán, F.A. Color stability of strawberry jam as affected by cultivar and storage temperature. J. Food Sci. 1999, 64, 243–247. [Google Scholar] [CrossRef]
- Serrano, M.; Guillén, F.; Martínez, R.D.; Castillo, S.; Valero, D. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, D.J.; Rao, J. Antioxidant systems of ripening avocado (Persea americana Mill.) fruit following treatment at the preclimacteric stage with aqueous 1-methylcyclopropene. Postharvest Biol. Technol. 2013, 76, 58–64. [Google Scholar] [CrossRef]
- Zapata, P.J.; Martínez, E.A.; Guillén, F.; Díaz-Mula, H.M.; Martínez, R.D.; Serrano, M.; Valero, D. Preharvest application of methyl jasmonate (MeJA) in two plum cultivars. 2. Improvement of fruit quality and antioxidant systems during postharvest storage. Postharvest Biol. Technol. 2014, 98, 115–122. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]


| Parameter | Days | Control | SA | ASA | MeSA |
|---|---|---|---|---|---|
| Weight (g) | |||||
| BS | Day 0 | 92.2 ± 2.3 c | 114.9 ± 4.3 a | 102.1 ± 3.4 b | 99.6 ± 2.6 b |
| RR | Day 0 | 68.6 ± 1.8 b | 76.5 ± 1.9 a | 77.5 ± 1.6 a | 76.5 ± 1.8 a |
| Yield (kg/tree) | |||||
| BS | Day 0 | 33.18 ± 1.42 c | 39.25 ± 1.23 a | 35.81 ± 1.62 b | 36.91 ± 1.71 b |
| RR | Day 0 | 28.01 ± 1.39 b | 34.07 ± 1.71 a | 32.58 ± 2.09 a | 31.42 ± 1.70 a |
| TSS (g/100 g) | |||||
| BS | Day 0 | 12.73 ± 0.21 A,a | 12.40 ± 0.15 A,a | 12.49 ± 0.21 A,a | 12.30 ± 0.11 A,a |
| Day 50 | 13.32 ± 0.12 B,a | 12.70 ± 0.13 A,b | 12.75 ± 0.10 A,b | 12.73 ± 0.19 A,b | |
| RR | Day 0 | 11.97 ± 0.11 A,a | 11.50 ± 0.11 A,a | 11.65 ± 0.24 A,a | 11.78 ± 0.15 A,a |
| Day 50 | 12.85 ± 0.31 B,a | 11.85 ± 0.20 A,b | 11.93 ± 0.22 A,b | 12.03 ± 0.11 A,b | |
| TA (g/100 g) | |||||
| BS | Day 0 | 1.67 ± 0.04 A,a | 1.77 ± 0.03 A,a | 1.64 ± 0.04 A,a | 1.76 ± 0.08 A,a |
| Day 50 | 0.93 ± 0.02 B,c | 1.34 ± 0.02 B,b | 1.30 ± 0.03 B,b | 1.56 ± 0.02 B,a | |
| RR | Day 0 | 0.75 ± 0.03 A,a | 0.79 ± 0.02 A,a | 0.74 ± 0.02 A,a | 0.76 ± 0.03 A,a |
| Day 50 | 0.41 ± 0.02 B,b | 0.64 ± 0.02 B,a | 0.59 ± 0.02 B,a | 0.63 ± 0.02 B,a | |
| RI (TSS/TA) | |||||
| BS | Day 0 | 7.26 ± 0.15 A,a | 7.56 ± 0.11 A,a | 7.05 ± 0.31 A,a | 6.99 ± 0.24 A,a |
| Day 50 | 14.32 ± 0.55 B,a | 9.76 ± 0.14 B,b | 9.51 ± 0.22 B,b | 8.16 ± 0.22 B,c | |
| RR | Day 0 | 15.96 ± 0.41 A,a | 15.75 ± 0.36 A,a | 15.74 ± 0.57 A,a | 15.50 ± 0.33 A,b |
| Day 50 | 31.34 ± 0.55 B,a | 18.52 ± 0.35 B,c | 20.22 ± 0.33 B,b | 19.09 ± 0.55 B,b | |
| Colour (Hue) | |||||
| BS | Day 0 | 16.52 ± 0.56 A,a | 17.09 ± 0.78 A,a | 16.40 ± 0.26 A,a | 16.81 ± 0.46 A,a |
| Day 50 | 10.49 ± 0.64 B,c | 12.42 ± 0.91 B,b | 12.51 ± 0.33 B,b | 14.28 ± 0.23 B,a | |
| RR | Day 0 | 25.26 ± 0.21 A,a | 25.21 ± 0.47 A,a | 26.56 ± 0.22 A,a | 26.79 ± 0.22 A,a |
| Day 50 | 18.55 ± 0.14 B,c | 23.14 ± 0.67 B,b | 23.98 ± 0.24 B,b | 25.97 ± 0.21 B,a | |
| Firmness (N·mm−1) | |||||
| BS | Day 0 | 9.47 ± 0.37 A,a | 9.96 ± 0.41 A,a | 9.87 ± 0.0.30 A,a | 9.56 ± 0.39 A,a |
| Day 50 | 3.34 ± 0.14 B,b | 4.62 ± 0.0.16 B,a | 4.69 ± 0.36 B,a | 4.37 ± 0.17 B,a | |
| RR | Day 0 | 5.86 ± 0.29 A,a | 7.72 ± 0.23 A,a | 5.92 ± 0.26 A,a | 6.05 ± 0.32 A,a |
| Day 50 | 2.82 ± 0.16 B,b | 3.92 ± 0.23 B,a | 4.06 ± 0.20 B,a | 3.95 ± 0.15 B,a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Esplá, A.; Serrano, M.; Valero, D.; Martínez-Romero, D.; Castillo, S.; Zapata, P.J. Enhancement of Antioxidant Systems and Storability of Two Plum Cultivars by Preharvest Treatments with Salicylates. Int. J. Mol. Sci. 2017, 18, 1911. https://doi.org/10.3390/ijms18091911
Martínez-Esplá A, Serrano M, Valero D, Martínez-Romero D, Castillo S, Zapata PJ. Enhancement of Antioxidant Systems and Storability of Two Plum Cultivars by Preharvest Treatments with Salicylates. International Journal of Molecular Sciences. 2017; 18(9):1911. https://doi.org/10.3390/ijms18091911
Chicago/Turabian StyleMartínez-Esplá, Alejandra, María Serrano, Daniel Valero, Domingo Martínez-Romero, Salvador Castillo, and Pedro J. Zapata. 2017. "Enhancement of Antioxidant Systems and Storability of Two Plum Cultivars by Preharvest Treatments with Salicylates" International Journal of Molecular Sciences 18, no. 9: 1911. https://doi.org/10.3390/ijms18091911