The Lactate/Albumin Ratio: A Valuable Tool for Risk Stratification in Septic Patients Admitted to ICU
Abstract
:1. Introduction
2. Results
2.1. Study Population
2.2. Survival Data
2.3. Matched-Control Analysis
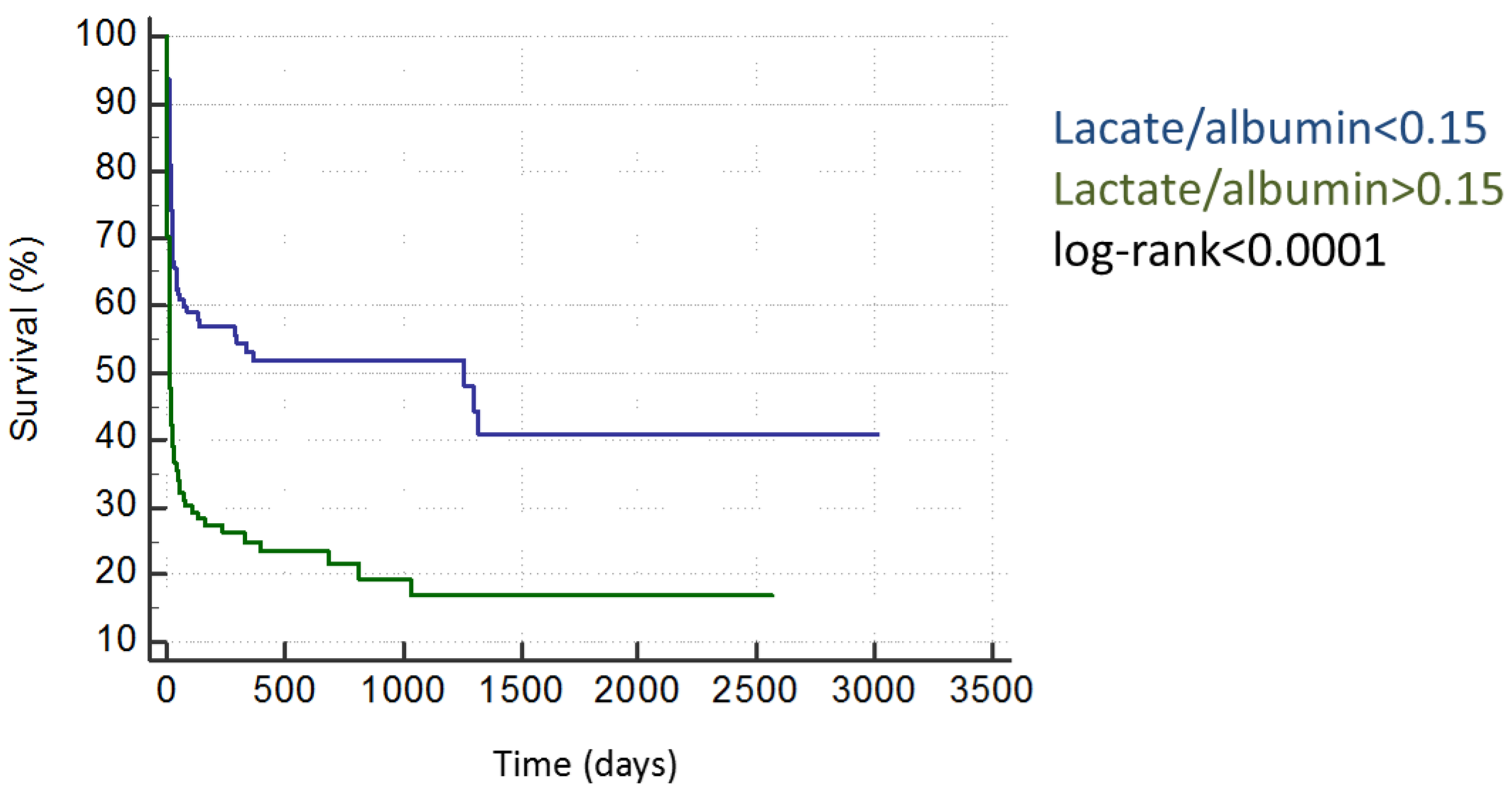
3. Discussion
4. Methods
4.1. Study Subjects
4.2. Statistical Analysis
4.3. Calculation of SAPS2 and APACHE score
5. Limitations
6. Conclusions
Acknowledgments
Author contributions
Conflicts of interest
References
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the united states: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Osborn, T.M.; Nguyen, H.B.; Rivers, E.P. Emergency medicine and the surviving sepsis campaign: An international approach to managing severe sepsis and septic shock. Ann. Emerg. Med. 2005, 46, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, M.M.; Dellinger, R.P.; Townsend, S.R.; Linde-Zwirble, W.T.; Marshall, J.C.; Bion, J.; Schorr, C.; Artigas, A.; Ramsay, G.; Beale, R.; et al. The surviving sepsis campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Crit. Care Med. 2010, 38, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Kumar, N.; Taneja, A.; Kaleekal, T.; Tarima, S.; McGinley, E.; Jimenez, E.; Mohan, A.; Khan, R.A.; Whittle, J.; et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest 2011, 140, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.; Dantes, R.; Magill, S.; Fiore, A. Varying estimates of sepsis mortality using death certificates and administrative codes—United States, 1999–2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Walkey, A.J.; Wiener, R.S.; Lindenauer, P.K. Utilization patterns and outcomes associated with central venous catheter in septic shock: A population-based study. Crit. Care Med. 2013, 41, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Kadri, S.S.; Rhee, C.; Strich, J.R.; Morales, M.K.; Hohmann, S.; Menchaca, J.; Suffredini, A.F.; Danner, R.L.; Klompas, M. Estimating ten-year trends in septic shock incidence and mortality in united states academic medical centers using clinical data. Chest 2017, 151, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the united states from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Kaukonen, K.M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality related to severe sepsis and septic shock among critically ill patients in australia and new zealand, 2000–2012. JAMA 2014, 311, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Lagu, T.; Rothberg, M.B.; Shieh, M.S.; Pekow, P.S.; Steingrub, J.S.; Lindenauer, P.K. Hospitalizations, costs, and outcomes of severe sepsis in the united states 2003 to 2007. Crit. Care Med. 2012, 40, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.R., III; Dong, L.; Nelson, N.C.; Brown, S.M.; Kuttler, K.G.; Probst, D.R.; Allen, T.L.; Clemmer, T.P. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am. J. Respir. Crit. Care Med. 2013, 188, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Peterson, E.; Tomlanovich, M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Y.; Chen, Q.H.; Liu, S.Q.; Pan, C.; Xu, X.P.; Han, J.B.; Xie, J.F.; Huang, Y.Z.; Guo, F.M.; Yang, Y.; et al. The effect of early goal-directed therapy on outcome in adult severe sepsis and septic shock patients: A meta-analysis of randomized clinical trials. Anesth. Analg. 2016, 123, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, V.; Angus, D.C. Surviving intensive care. Crit. Care Med. 2002, 30, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Hofhuis, J.G.; Spronk, P.E.; van Stel, H.F.; Schrijvers, A.J.; Rommes, J.H.; Bakker, J. The impact of severe sepsis on health-related quality of life: A long-term follow-up study. Anesth. Analg. 2008, 107, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Osterholzer, J.J.; Langa, K.M.; Angus, D.C.; Iwashyna, T.J. Late mortality after sepsis: Propensity matched cohort study. BMJ 2016, 353, i2375. [Google Scholar] [CrossRef] [PubMed]
- Kuzniewicz, M.W.; Vasilevskis, E.E.; Lane, R.; Dean, M.L.; Trivedi, N.G.; Rennie, D.J.; Clay, T.; Kotler, P.L.; Dudley, R.A. Variation in icu risk-adjusted mortality: Impact of methods of assessment and potential confounders. Chest 2008, 133, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Minne, L.; Abu-Hanna, A.; de Jonge, E. Evaluation of sofa-based models for predicting mortality in the icu: A systematic review. Crit. Care 2008, 12, R161. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R. Clinical review: Scoring systems in the critically ill. Crit. Care 2010, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Wagner, D.P. Selection bias and the relationship between apache ii and mortality. Crit. Care Med. 1990, 18, 793–795. [Google Scholar] [PubMed]
- Gibot, S. On the origins of lactate during sepsis. Crit. Care 2012, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Nijsten, M.W.; Jansen, T.C. Clinical use of lactate monitoring in critically ill patients. Ann. Intensive Care 2013, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, J.H.; Luchette, F.A.; McCarter, F.D.; Fischer, J.E. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet 1999, 354, 505–508. [Google Scholar] [CrossRef]
- McCarter, F.D.; Nierman, S.R.; James, J.H.; Wang, L.; King, J.K.; Friend, L.A.; Fischer, J.E. Role of skeletal muscle Na+-K+ ATPase activity in increased lactate production in sub-acute sepsis. Life Sci. 2002, 70, 1875–1888. [Google Scholar] [CrossRef]
- Peretz, D.I.; Scott, H.M.; Duff, J.; Dossetor, J.B.; MacLean, L.D.; McGregor, M. The significance of lacticacidemia in the shock syndrome. Ann. NY Acad. Sci. 1965, 119, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Weil, M.H.; Afifi, A.A. Experimental and clinical studies on lactate and pyruvate as indicators of the severity of acute circulatory failure (shock). Circulation 1970, 41, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.E.; Miltiades, A.N.; Gaieski, D.F.; Goyal, M.; Fuchs, B.D.; Shah, C.V.; Bellamy, S.L.; Christie, J.D. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit. Care Med. 2009, 37, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Dominguez de Villota, E.; Mosquera, J.M.; Rubio, J.J.; Galdos, P.; Diez Balda, V.; de la Serna, J.L.; Tomas, M.I. Association of a low serum albumin with infection and increased mortality in critically ill patients. Intensive Care Med. 1980, 7, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Goldwasser, P.; Feldman, J. Association of serum albumin and mortality risk. J. Clin. Epidemiol. 1997, 50, 693–703. [Google Scholar] [CrossRef]
- Artero, A.; Zaragoza, R.; Camarena, J.J.; Sancho, S.; Gonzalez, R.; Nogueira, J.M. Prognostic factors of mortality in patients with community-acquired bloodstream infection with severe sepsis and septic shock. J. Crit. Care 2010, 25, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, B.; Oren Gradel, K.; Gorm Jensen, T.; Kolmos, H.J.; Pedersen, C.; Just Vinholt, P.; Touborg Lassen, A. Association between hypoalbuminaemia and mortality in patients with community-acquired bacteraemia is primarily related to acute disorders. PLoS ONE 2016, 11, e0160466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Chen, G.; Cao, Y.; Xue, J.; Li, J.; Wu, Y. Correlation of lactate/albumin ratio level to organ failure and mortality in severe sepsis and septic shock. J. Crit. Care 2015, 30, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Ha, E.J.; Jhang, W.K.; Park, S.J. Association between the lactate/albumin ratio and mortality in pediatric septic shock patients with underlying chronic disease: Retrospective pilot study. Minerva Pediatr. 2016. [Google Scholar] [PubMed]
- Van Hemelrijck, M.; Harari, D.; Garmo, H.; Hammar, N.; Walldius, G.; Lambe, M.; Jungner, I.; Holmberg, L. Biomarker-based score to predict mortality in persons aged 50 years and older: A new approach in the swedish amoris study. Int. J. Mol. Epidemiol. Genet. 2012, 3, 66–76. [Google Scholar] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.C.; van Bommel, J.; Schoonderbeek, F.J.; Sleeswijk Visser, S.J.; van der Klooster, J.M.; Lima, A.P.; Willemsen, S.P.; Bakker, J. Early lactate-guided therapy in intensive care unit patients: A multicenter, open-label, randomized controlled trial. Am. J. Respir. Crit. Care Med. 2010, 182, 752–761. [Google Scholar] [CrossRef] [PubMed]

| Clinical Characteristics | Survivors | Non-Survivors | Overall Cohort | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | |||||
| age | 63.67 | 14.71 | 67.67 | 12.02 | 64.97 | 14.00 | 0.01 | |||
| BMI (kg/m2) | 27.06 | 4.80 | 27.63 | 7.83 | 27.24 | 5.95 | 0.47 | |||
| SAPS2 (pts) | 46.43 | 16.70 | 58.57 | 19.37 | 50.58 | 18.55 | <0.001 | |||
| APACHE2 (pts) | 24.50 | 7.81 | 27.84 | 8.44 | 25.64 | 8.17 | 0.001 | |||
| Median | Min. | Max. | Median | Min. | Max. | Median | Min. | Max. | p-Value | |
| lactate (mmol/L) | 2.19 | 0.50 | 20.58 | 3.77 | 0.70 | 22.90 | 2.50 | 0.50 | 22.90 | <0.001 |
| PCT (mmol/L) | 8.82 | 0.08 | 417.00 | 7.82 | 0.07 | 194.00 | 8.18 | 0.07 | 417.00 | 0.98 |
| glucose (mmol/L) | 9.9 | 3.9 | 30.0 | 10.4 | 3.8 | 39.4 | 10.1 | 3.8 | 30.0 | 0.11 |
| hemoglobin (mmol/L) | 6.60 | 2.50 | 10.30 | 6.60 | 4.80 | 8.90 | 6.60 | 2.5 | 10.30 | 0.35 |
| ASAT (μmol/L·s) | 0.85 | 0.20 | 222.27 | 1.07 | 0.20 | 154.50 | 0.89 | 0.20 | 222.27 | 0.02 |
| ALAT (μmol/L·s) | 0.58 | 0.08 | 41.81 | 0.73 | 0.10 | 71.70 | 0.63 | 0.08 | 71.70 | 0.03 |
| γ-GT (μmol) | 1.24 | 0.10 | 15.22 | 1.44 | 0.14 | 14.68 | 1.31 | 0.10 | 15.22 | 0.14 |
| bilirubin (μmol) | 17.0 | 1.8 | 405.0 | 19.0 | 3.0 | 551.0 | 18.0 | 1.8 | 551.0 | 0.28 |
| leucocytes (G/L) | 14.2 | 0.1 | 195.1 | 14.9 | 0.2 | 70.9 | 14.5 | 0.1 | 195.1 | 0.83 |
| BUN (mg/dL) | 12.8 | 1.9 | 59.6 | 19.5 | 0.5 | 80.2 | 14.4 | 0.5 | 80.2 | <0.001 |
| creatinine (mg/dL) | 15.0 | 30.0 | 1609.0 | 212.5 | 23.0 | 952.0 | 175.0 | 23.0 | 1609.0 | <0.001 |
| sodium (mmol/L) | 140 | 119 | 154 | 142 | 131 | 151 | 140 | 119 | 154 | 0.02 |
| potassium (mmol/L) | 4.1 | 2.5 | 9.3 | 4.5 | 3.1 | 10.8 | 4.2 | 2.5 | 10.8 | <0.001 |
| albumin (mg/L) | 20.00 | 10.00 | 57.00 | 18.00 | 10.00 | 27.00 | 19.00 | 10.00 | 57.00 | 0.01 |
| lactate/albumin ratio | 0.09 | 0.02 | 0.15 | 0.31 | 0.15 | 2.00 | 0.14 | 0.02 | 2.00 | <0.001 |
| Clinical Characteristics | Lactate/Albumin <0.15 | Lactate/Albumin >0.15 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||||
| age | 63.70 | 14.75 | 66.23 | 12.69 | 0.09 | ||
| BMI (kg/m2) | 27.33 | 5.12 | 27.14 | 4.82 | 0.77 | ||
| SAPS2 (pts) | 47.81 | 18.03 | 59.01 | 20.82 | <0.001 | ||
| APACHE2 (pts) | 25.07 | 8.38 | 27.98 | 8.24 | <0.001 | ||
| Laboratory Parameters | Median | Min. | Max. | Median | Min. | Max. | p-Value |
| lactate (mmol/L) | 1.7 | 0.5 | 5.9 | 5.9 | 1.8 | 26.0 | <0.001 |
| procalcitonin (mmol/L) | 6.5 | 0.1 | 161.9 | 10.1 | 0.2 | 188.6 | <0.001 |
| glucose (mmol/L) | 9.9 | 5.3 | 25.3 | 9.7 | 3.9 | 30.0 | 0.95 |
| hemoglobin (mmol/L) | 6.6 | 2.5 | 9.3 | 6.7 | 4.9 | 9.8 | 0.36 |
| ASAT (μmol/L·s) | 0.75 | 0.20 | 106.95 | 1.44 | 0.20 | 154.50 | <0.001 |
| ALAT (μmol/L·s) | 0.61 | 0.13 | 78.76 | 0.77 | 0.10 | 71.7 | <0.001 |
| γ-GT (μmol) | 1.29 | 0.1 | 15.22 | 1.3 | 0.14 | 20.33 | 0.92 |
| bilirubin (μmol) | 17.0 | 1.8 | 390.0 | 24.0 | 2.0 | 551.0 | <0.001 |
| leucocytes (G/L) | 12.9 | 0.1 | 50.5 | 15.4 | 0.1 | 96.8 | 0.06 |
| BUN (mg/dL) | 15.0 | 0.5 | 80.2 | 15.8 | 1.9 | 75.4 | 0.34 |
| creatinine (mg/dL) | 174 | 30 | 870 | 192 | 52 | 1332 | 0.34 |
| sodium (mmol/L) | 139 | 124 | 158 | 142 | 126 | 158 | 0.02 |
| potassium (mmol/L) | 4.2 | 2.9 | 7.1 | 4.5 | 2.8 | 12.4 | 0.01 |
| albumin (mg/L) | 21 | 10 | 60 | 17 | 10 | 30 | <0.001 |
| Parameter | HR | 95%CI | p-Value | Non-Survivors Lactat/Albumin >0.15 | vs. | Non-Survivors Lactat/Albumin <0.15 |
|---|---|---|---|---|---|---|
| lactate/albumin >0.15 | 4.27 | 2.42–7.52 | <0.001 | 54% | vs. | 18% |
| Multivariate Analysis | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Model #1 | HR | 95%CI | p-Value | HR | 95%CI | p-Value |
| lactate/albumin >0.15 | 2.5 | 1.85–3.37 | <0.001 | 1.65 | 1.20–2.29 | 0.002 |
| APACHE2 | 1.05 | 1.03–1.07 | <0.001 | 1.05 | 1.03–1.07 | <0.001 |
| Model #2 | ||||||
| lactate/albumin >0.15 | 2.5 | 1.85-3.37 | <0.001 | 1.44 | 1.03–2.00 | 0.03 |
| SAPS2 | 1.03 | 1.02–1.04 | <0.001 | 1.03 | 1.02–1.04 | <0.001 |
| Model #3 | ||||||
| lactate/albumin >0.15 | 2.5 | 1.85–3.37 | <0.001 | 2.94 | 2.39–3.60 | <0.001 |
| glucose (mmol/L) | 1.01 | 0.99–1.03 | 0.41 | |||
| leucocytes (G/L) | 1.003 | 0.993–1.013 | 0.55 | |||
| heart rate (bpm) | 1.009 | 1.005–1.012 | <0.001 | |||
| ASAT (μmol/L·s) | 1.004 | 0.995–1.013 | 0.41 | |||
| ALAT (μmol/L·s) | 1.00 | 0.980–1.019 | 0.97 | |||
| BUN (mg/dL) | 1.034 | 1.025–1.044 | <0.001 | |||
| creatinine (mg/dL) | 0.999 | 0.998–0.999 | <0.001 | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lichtenauer, M.; Wernly, B.; Ohnewein, B.; Franz, M.; Kabisch, B.; Muessig, J.; Masyuk, M.; Lauten, A.; Schulze, P.C.; Hoppe, U.C.; et al. The Lactate/Albumin Ratio: A Valuable Tool for Risk Stratification in Septic Patients Admitted to ICU. Int. J. Mol. Sci. 2017, 18, 1893. https://doi.org/10.3390/ijms18091893
Lichtenauer M, Wernly B, Ohnewein B, Franz M, Kabisch B, Muessig J, Masyuk M, Lauten A, Schulze PC, Hoppe UC, et al. The Lactate/Albumin Ratio: A Valuable Tool for Risk Stratification in Septic Patients Admitted to ICU. International Journal of Molecular Sciences. 2017; 18(9):1893. https://doi.org/10.3390/ijms18091893
Chicago/Turabian StyleLichtenauer, Michael, Bernhard Wernly, Bernhard Ohnewein, Marcus Franz, Bjoern Kabisch, Johanna Muessig, Maryna Masyuk, Alexander Lauten, Paul Christian Schulze, Uta C. Hoppe, and et al. 2017. "The Lactate/Albumin Ratio: A Valuable Tool for Risk Stratification in Septic Patients Admitted to ICU" International Journal of Molecular Sciences 18, no. 9: 1893. https://doi.org/10.3390/ijms18091893





