Endolysosomal Degradation of Allergenic Ole e 1-Like Proteins: Analysis of Proteolytic Cleavage Sites Revealing T Cell Epitope-Containing Peptides
Abstract
:1. Introduction
2. Results
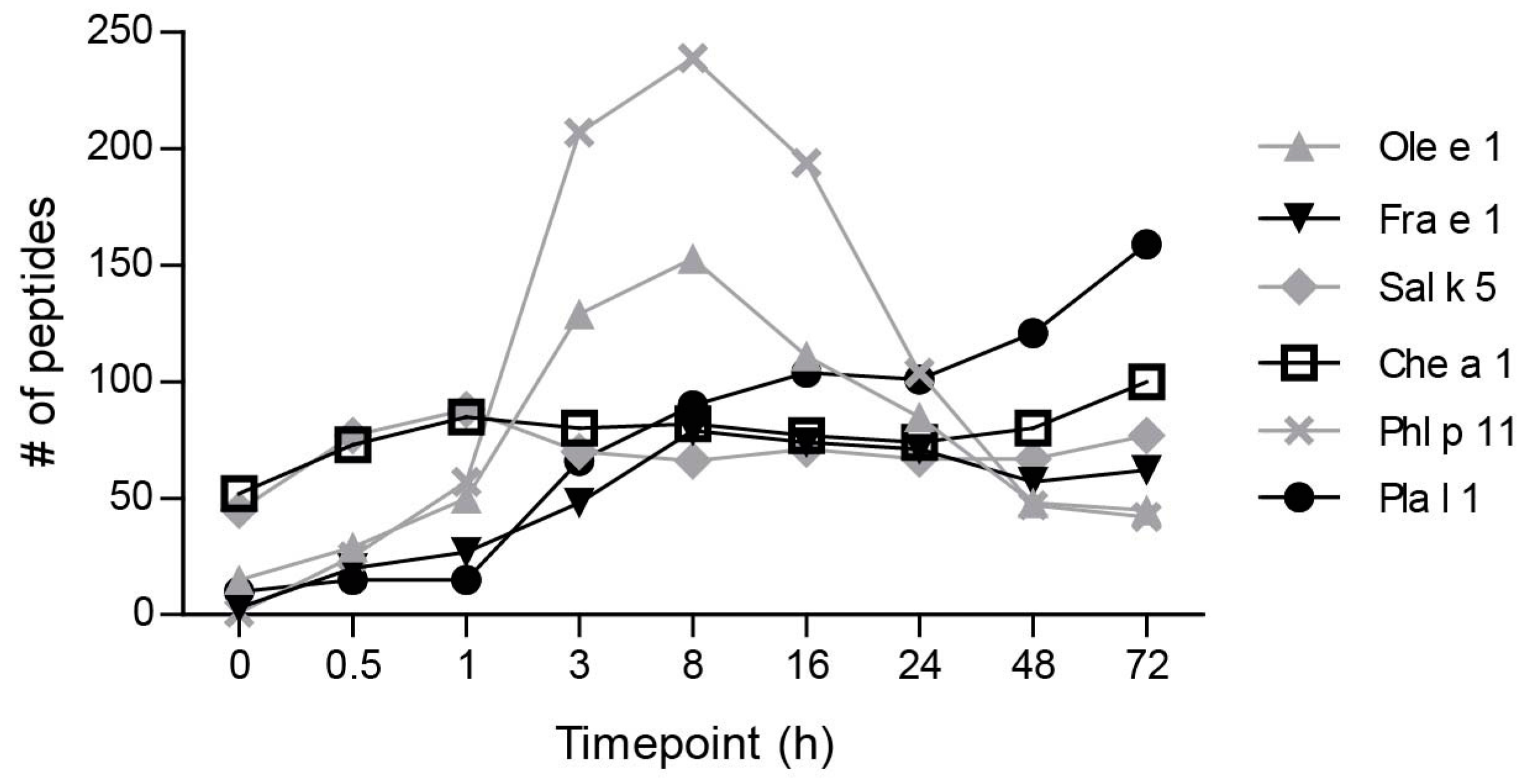
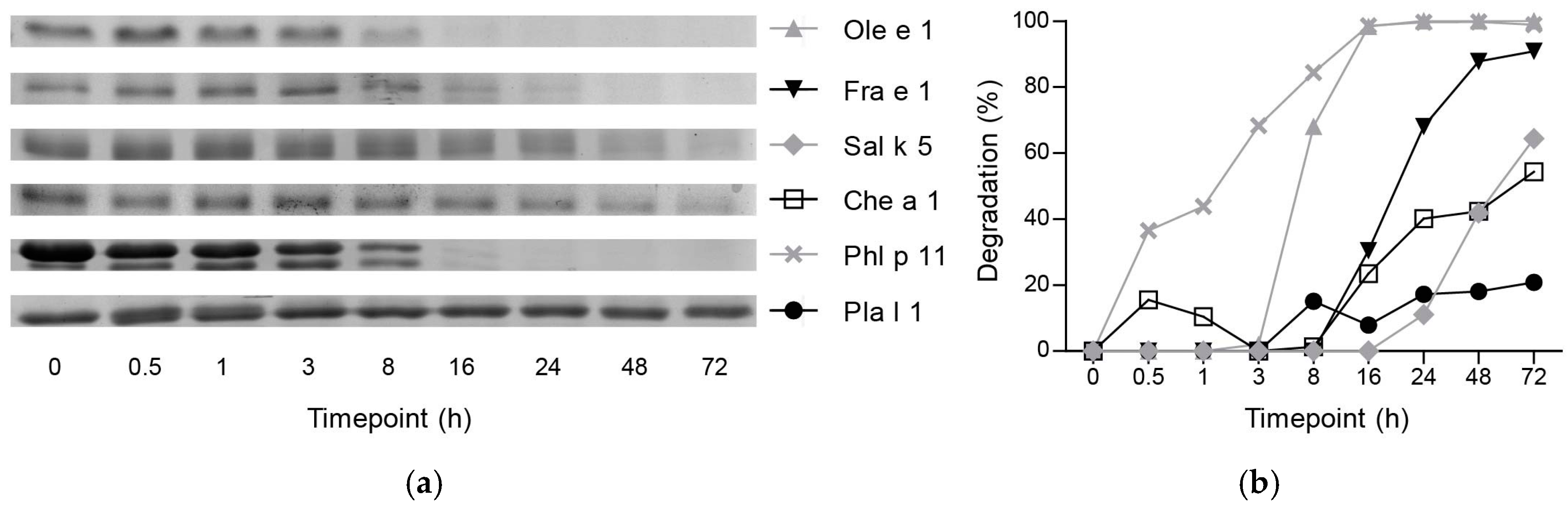
2.1. Ole e 1-Like Allergens Showed Different Susceptibility to Endolysosomal Degradation
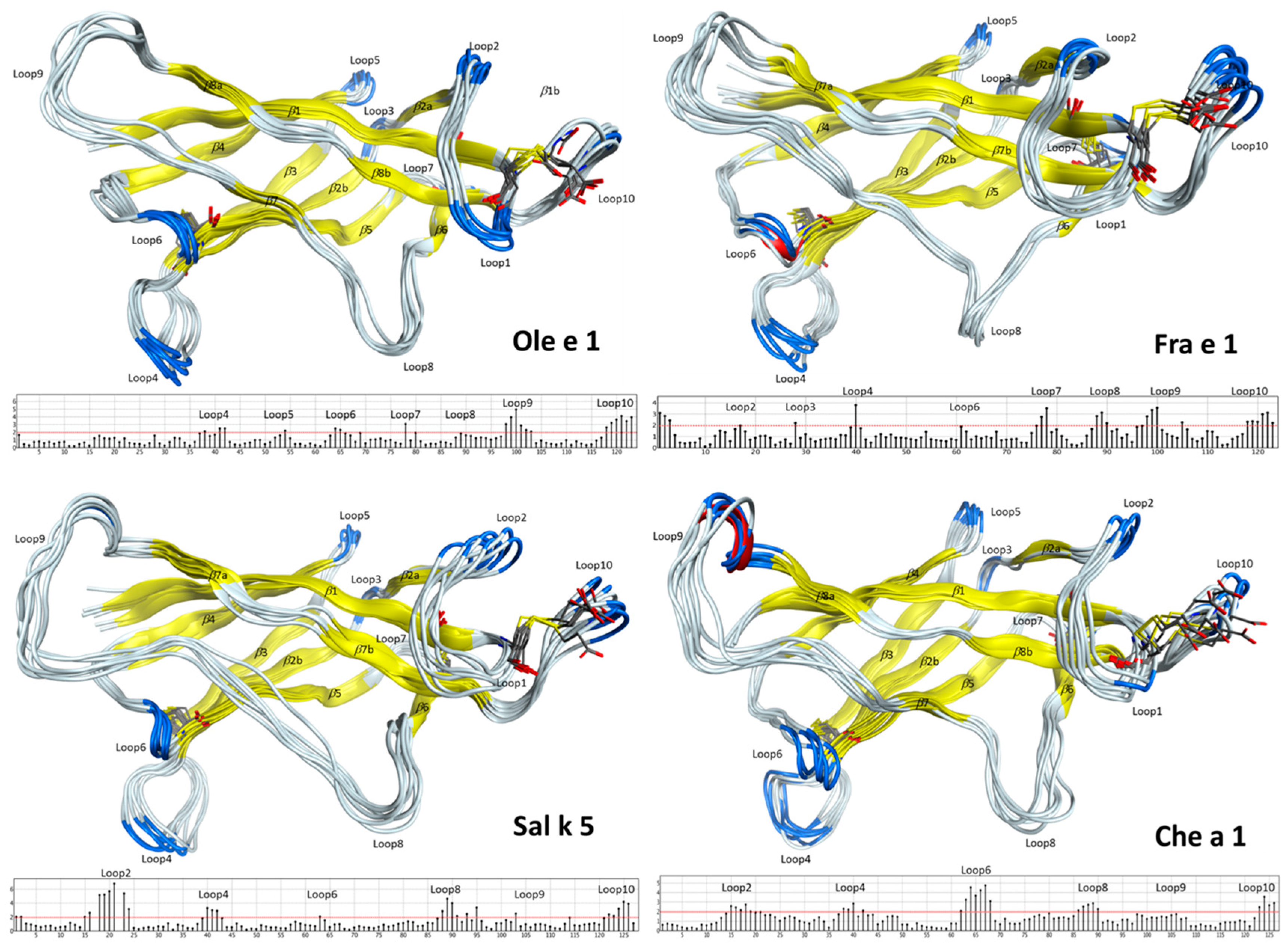
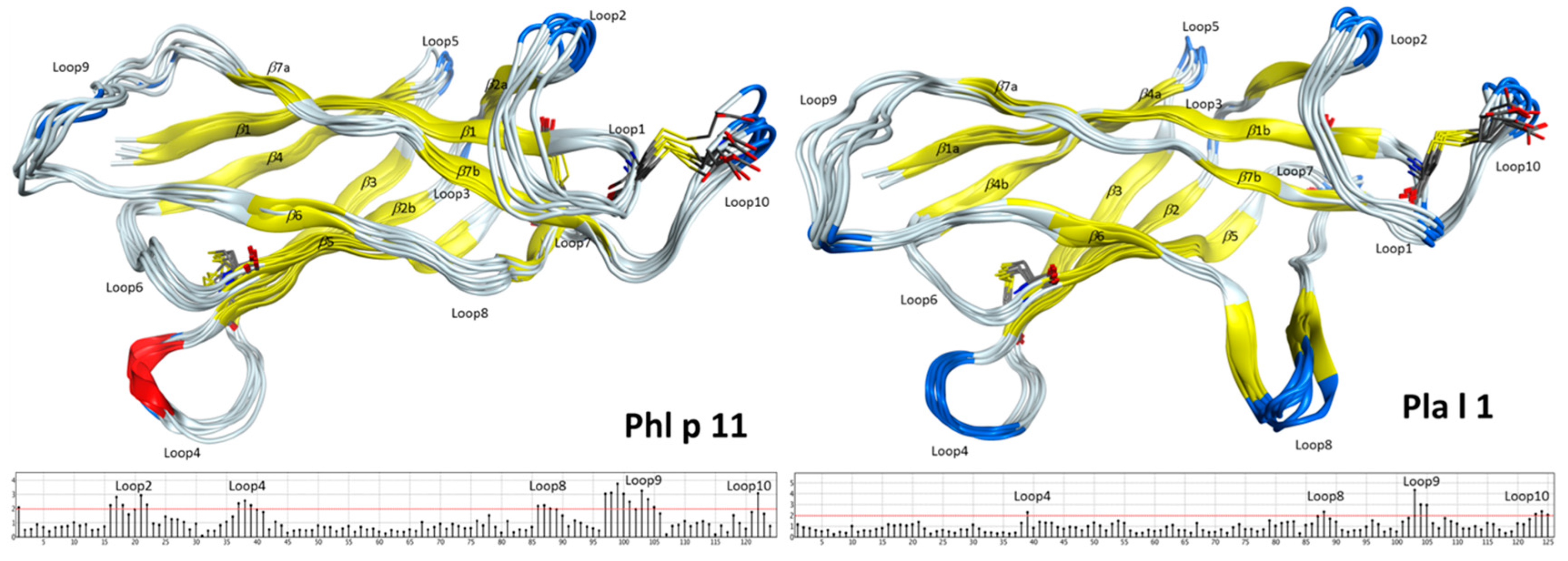
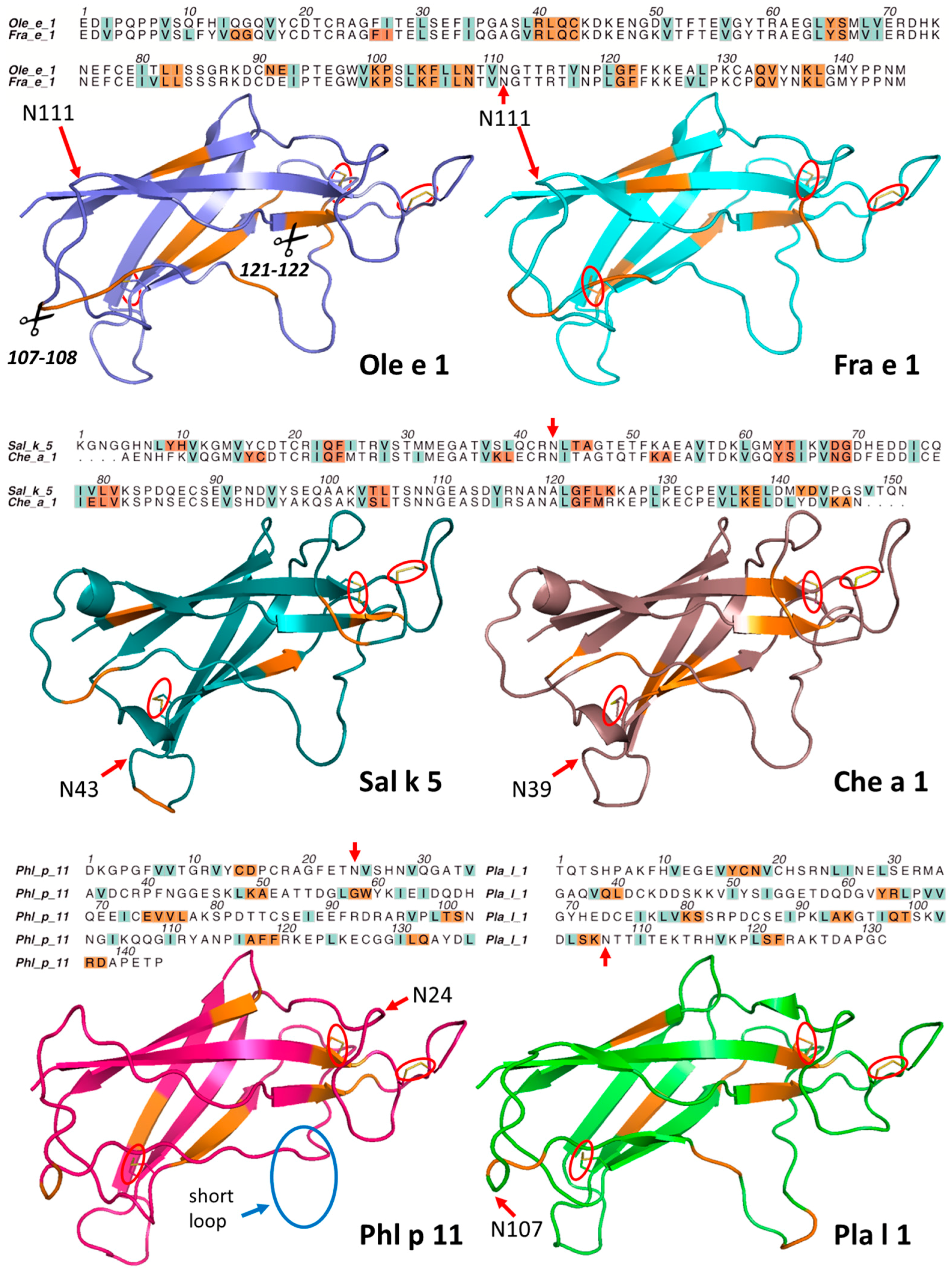
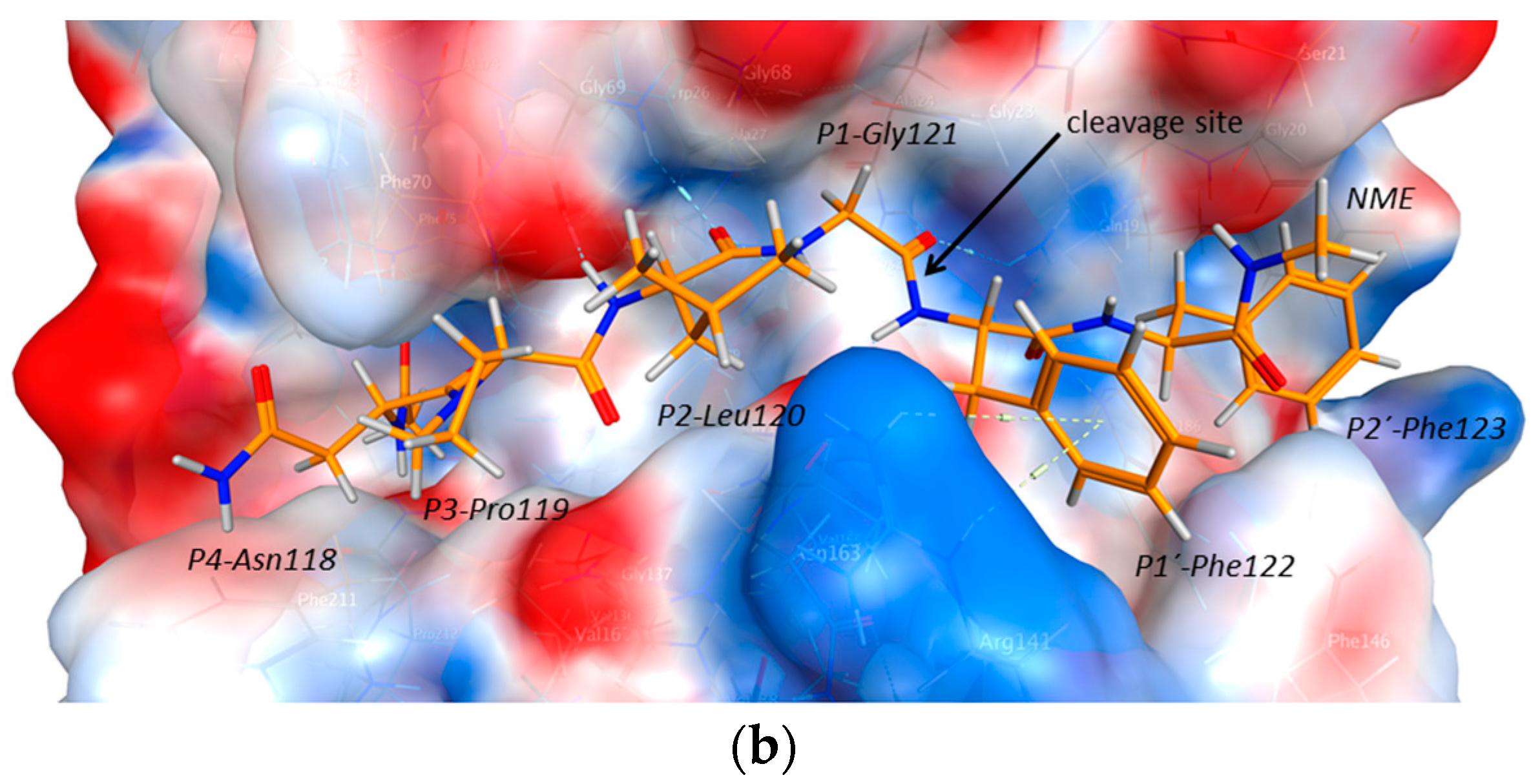
2.2. Structural Modeling and Molecular Dynamics Simulation
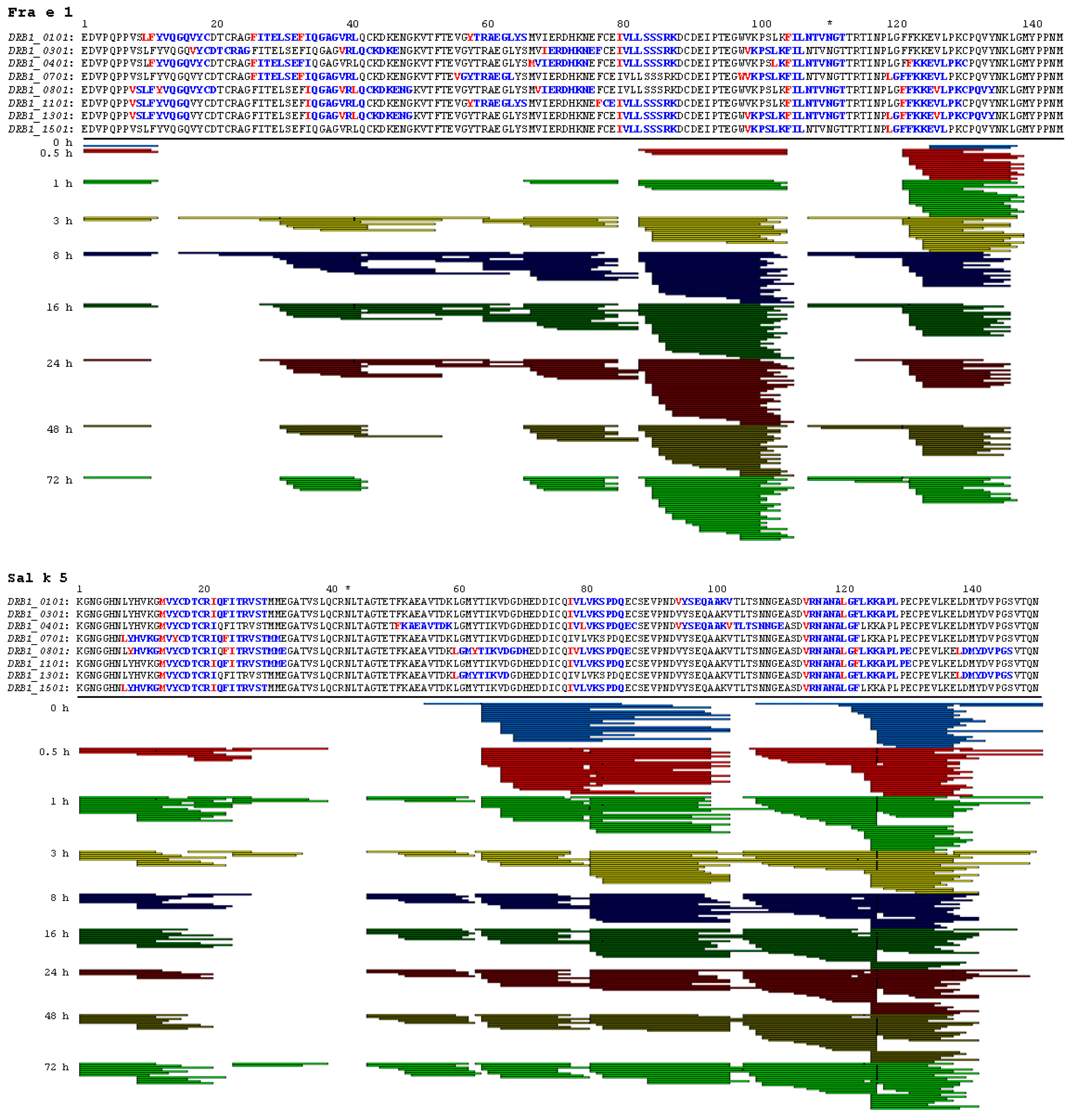
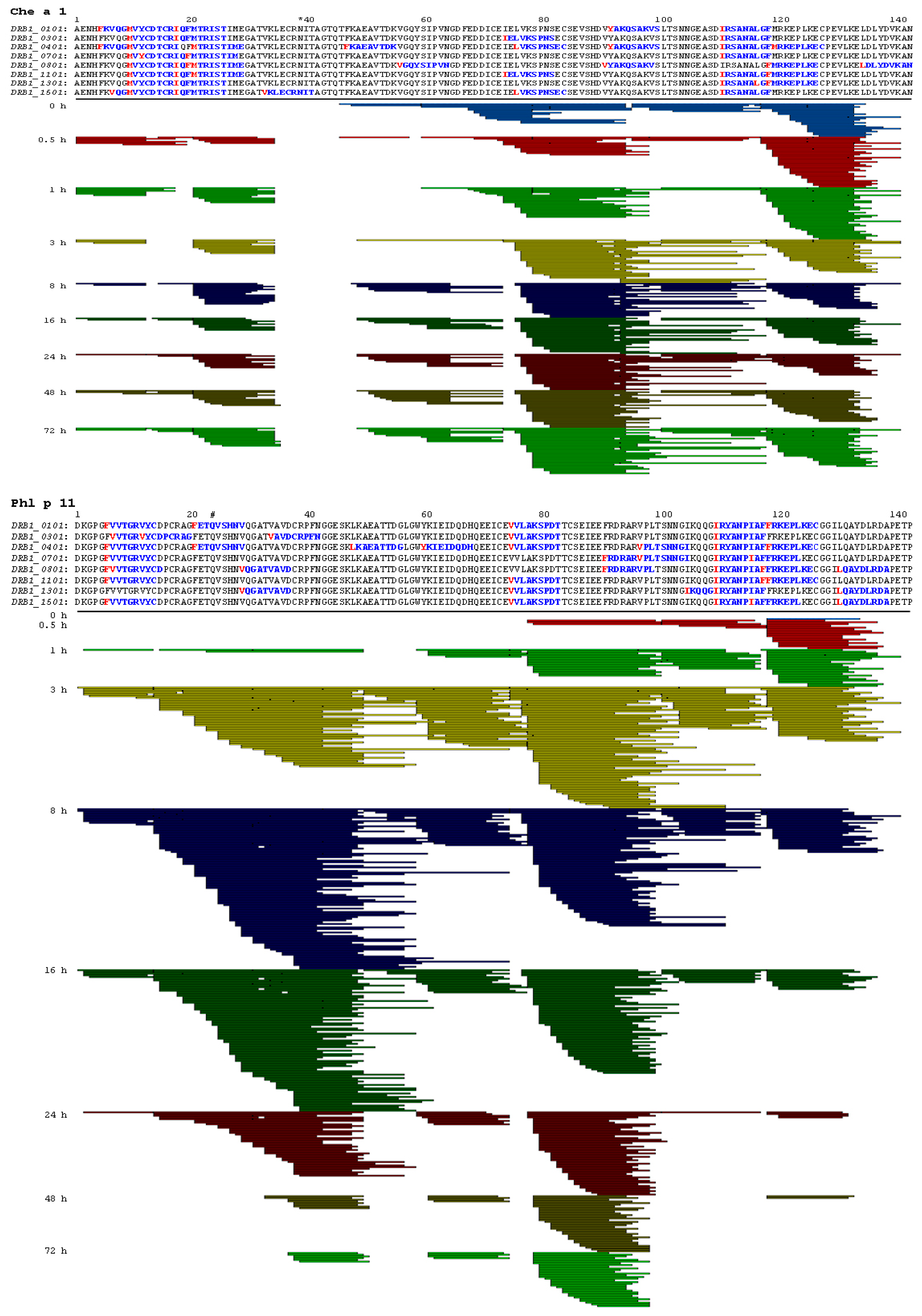
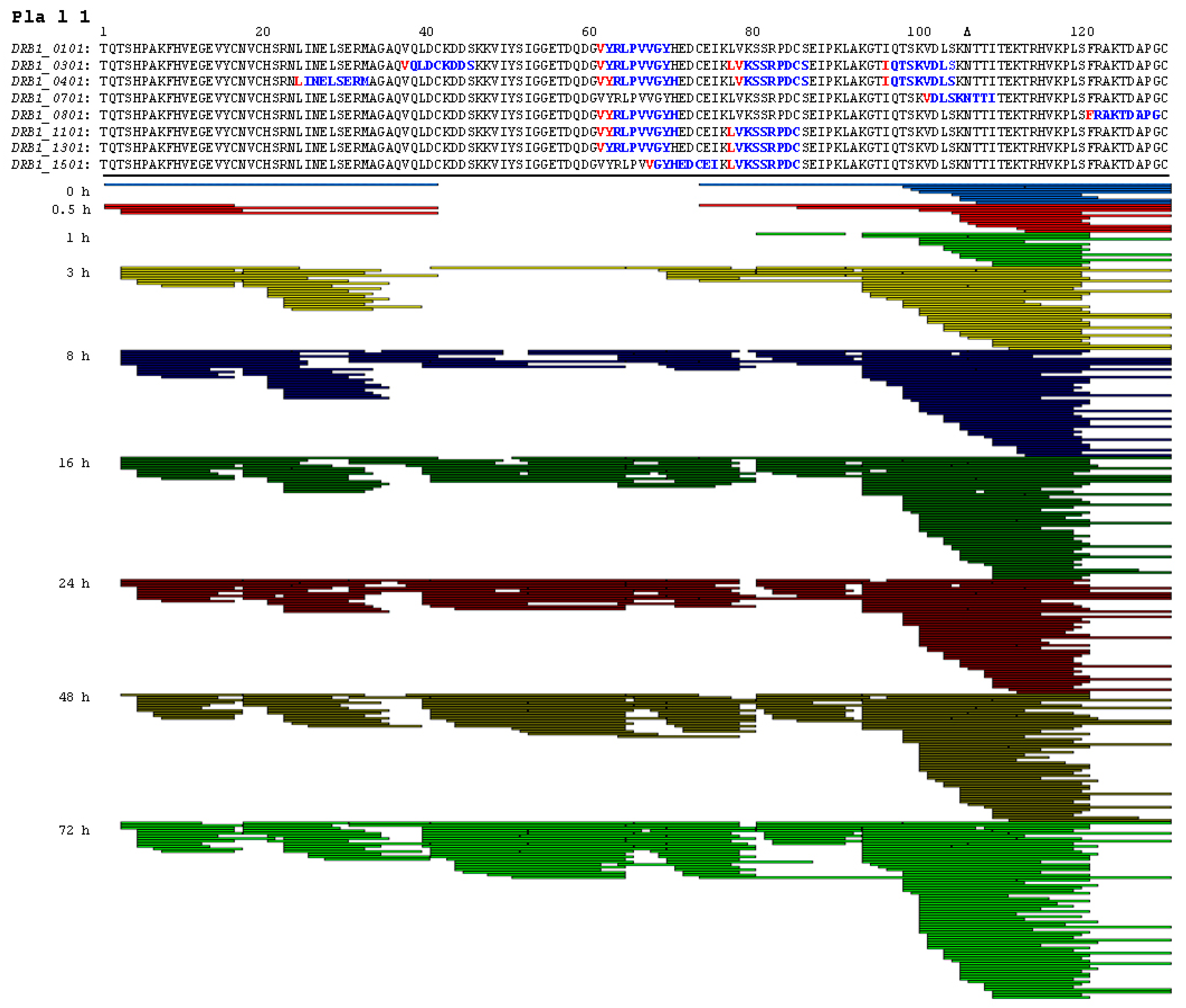
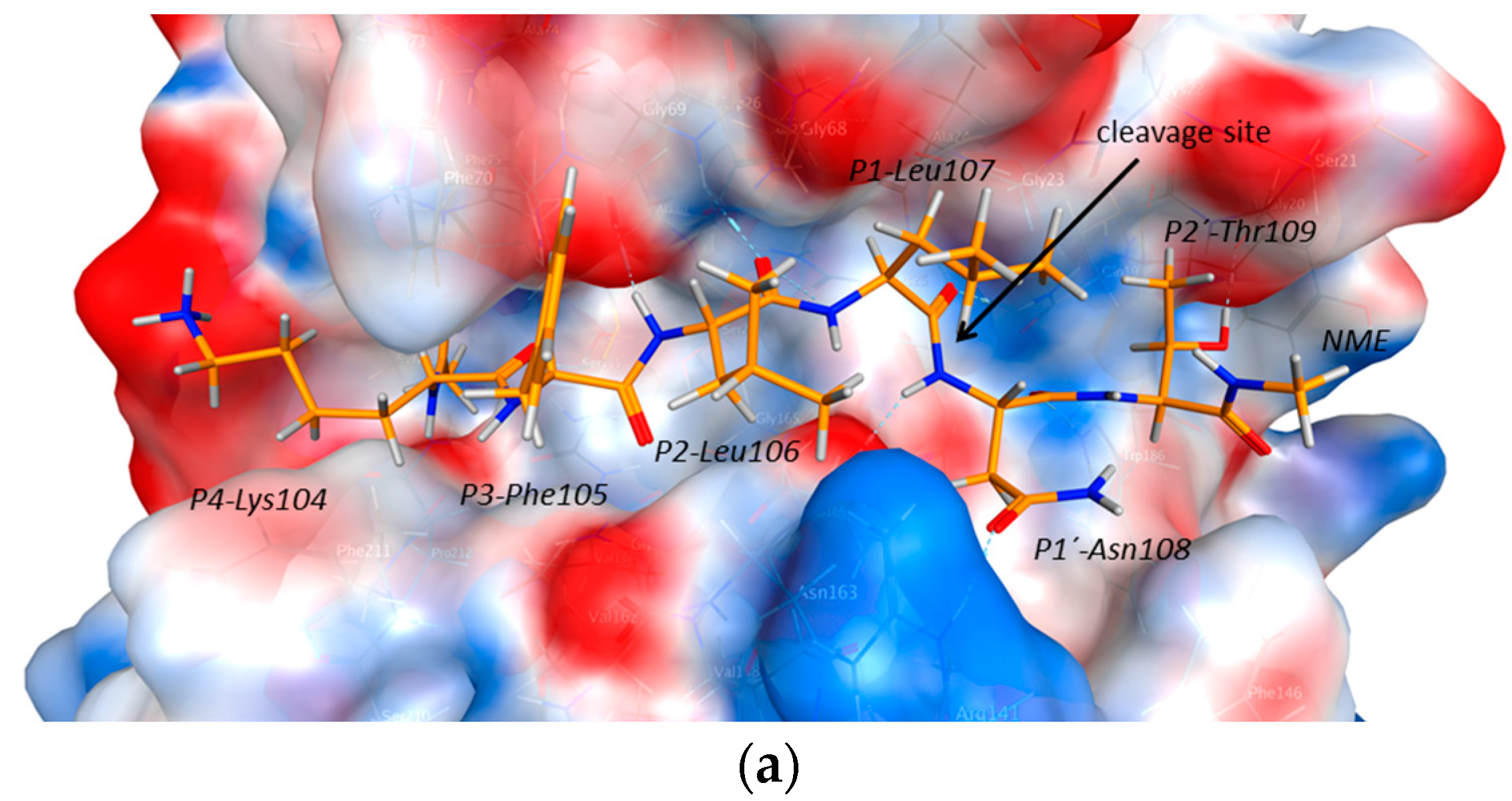
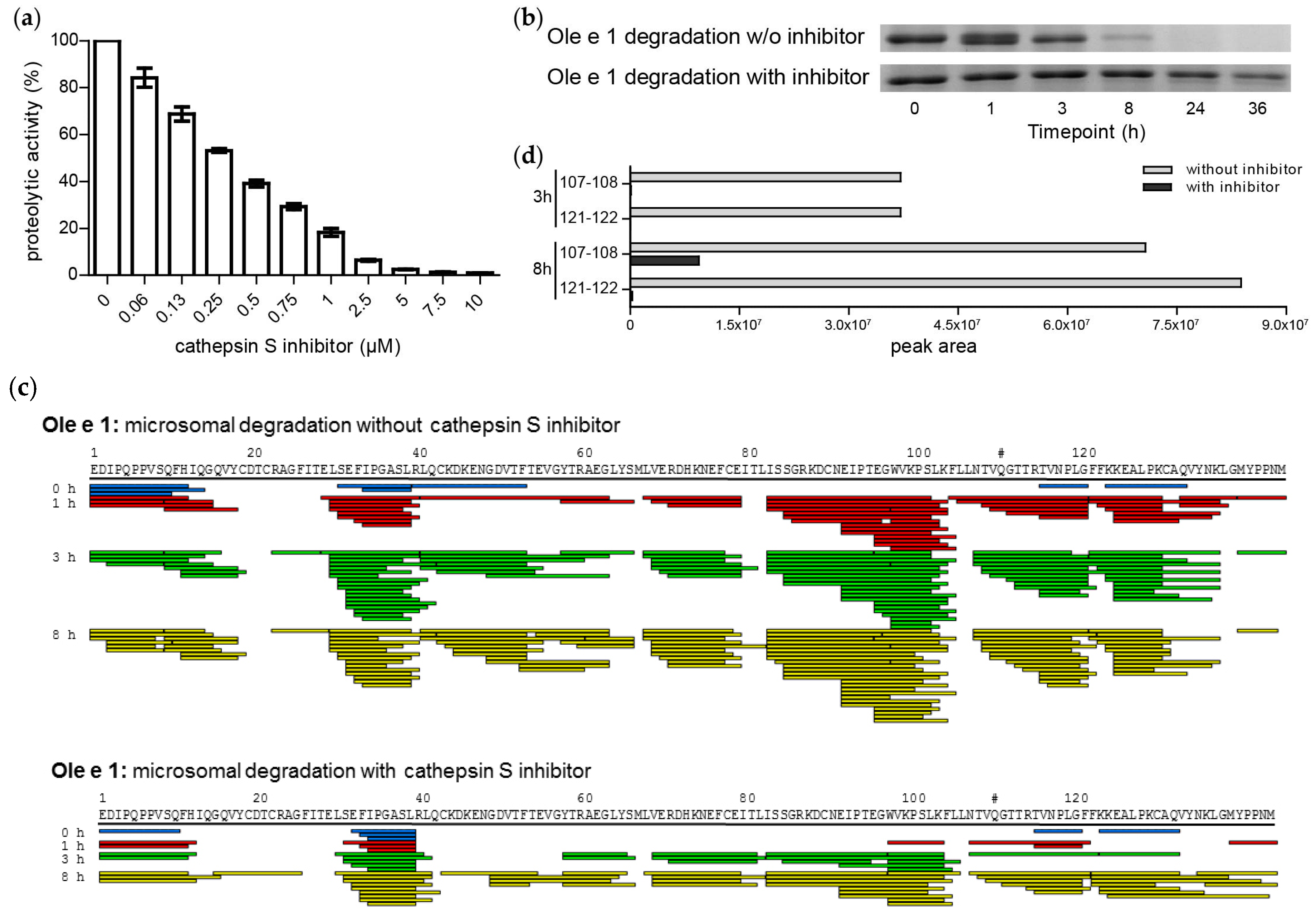
2.3. Endolysosomal Cleavage Sites
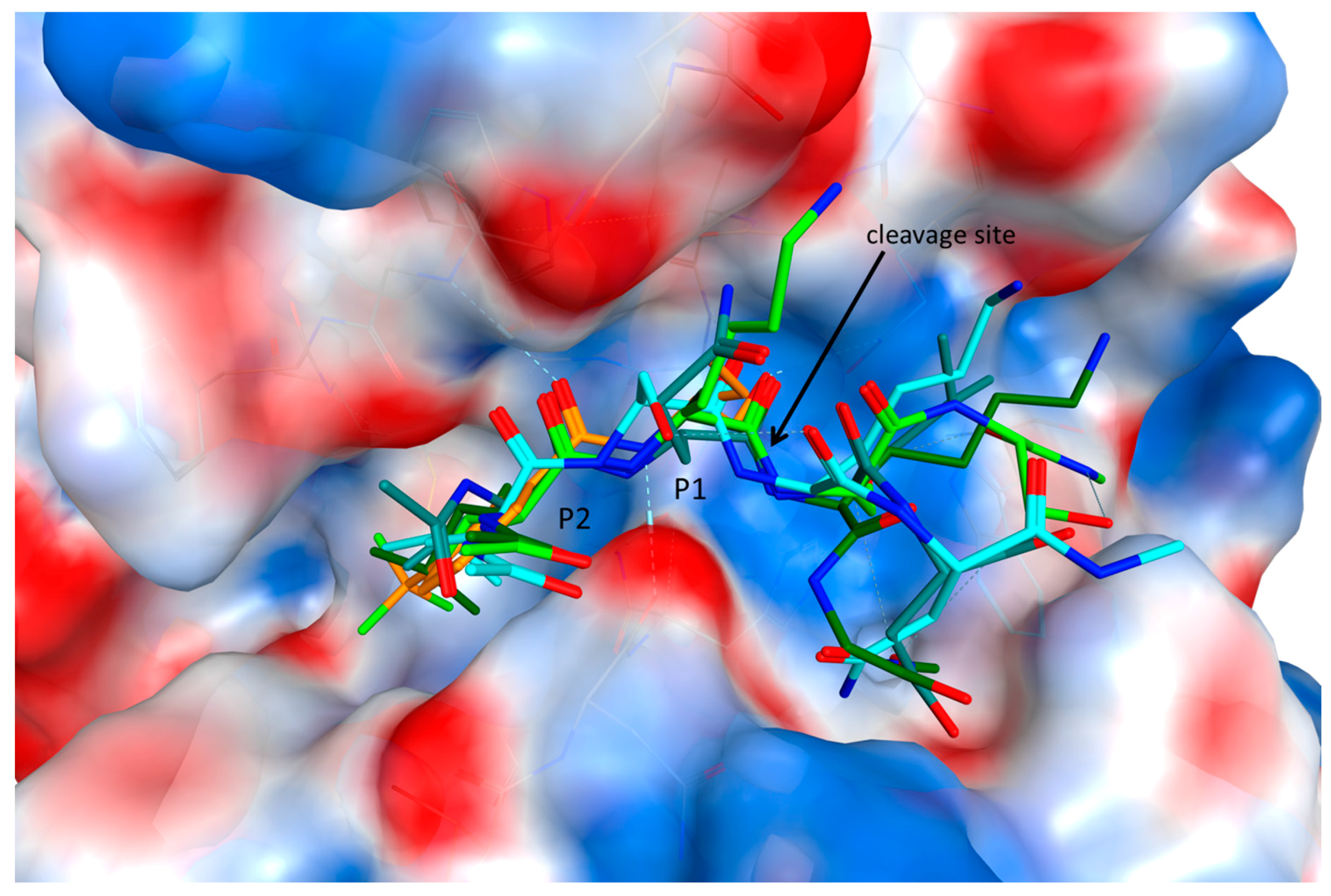
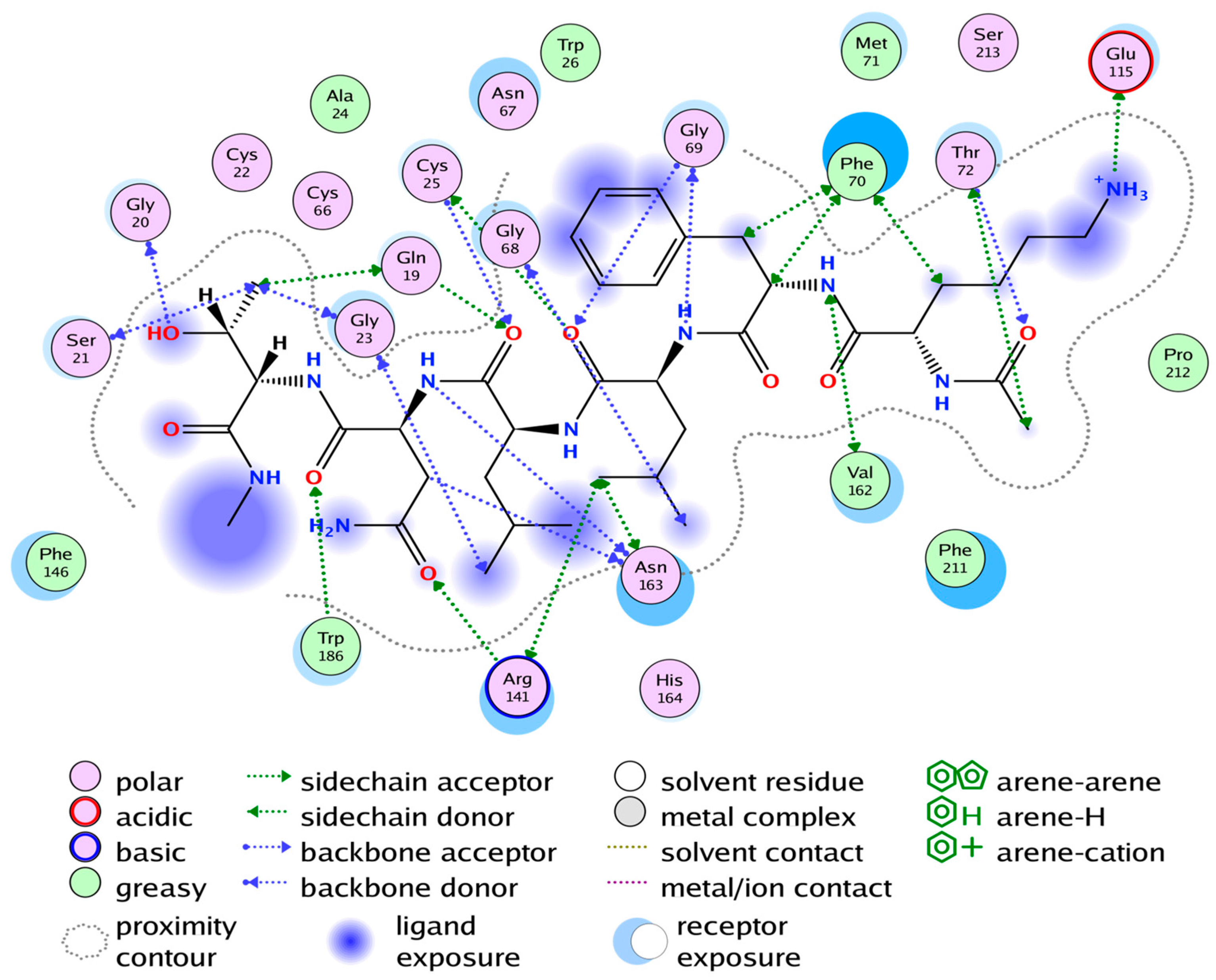
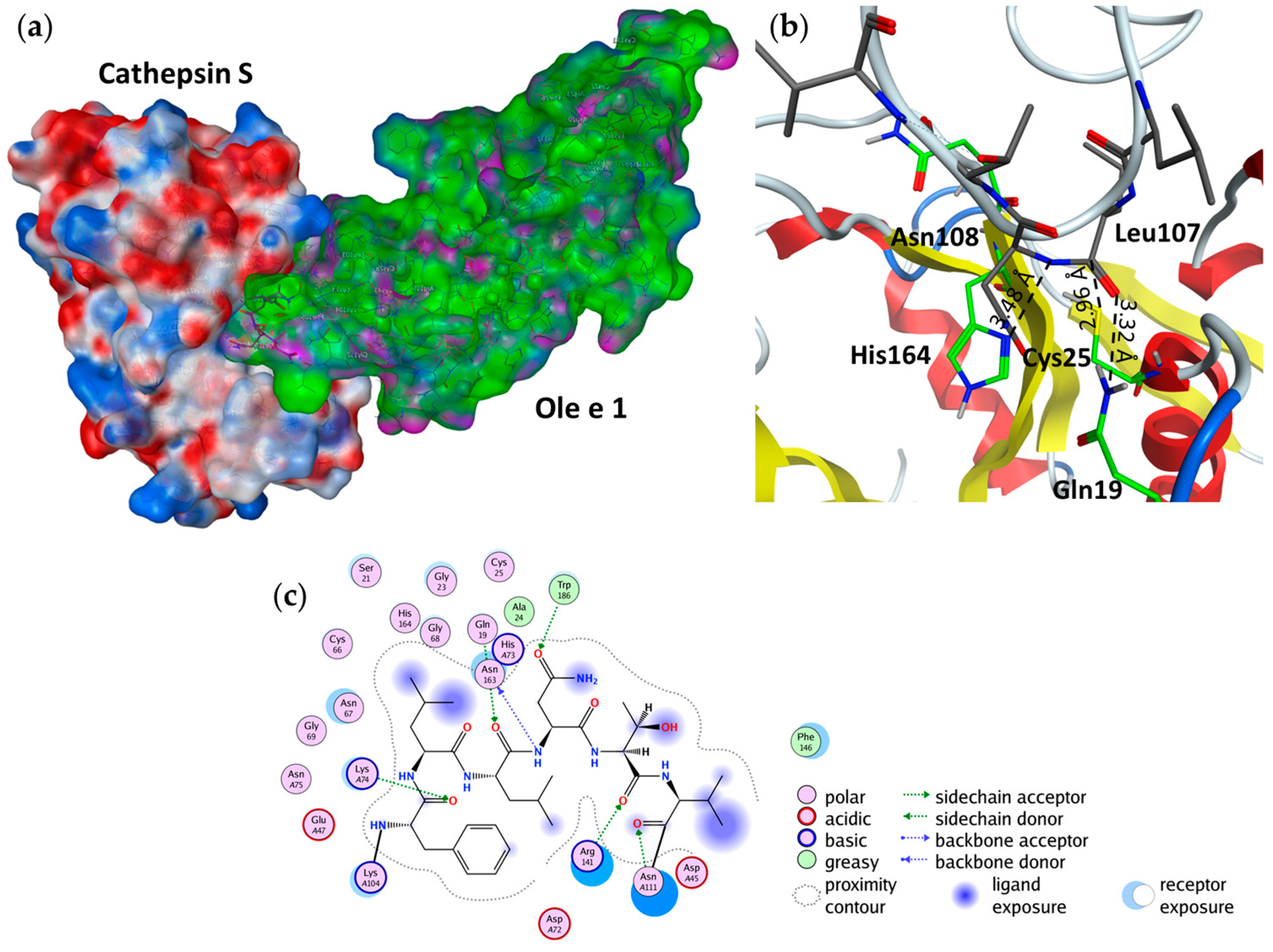
2.4. Substrate Binding Studies
2.5. Cathepsin S Inhibition
3. Discussion
4. Materials and Methods
4.1. Recombinant Production of Ole e 1-Like Allergens
4.2. Amino Acid Analysis
4.3. Endolysosomal Degradation Assay
4.4. Cathepsin S Inhibition Assays
4.5. Gel Electrophoresis
4.6. T Cell Epitope Prediction
4.7. Mass Spectrometry
4.8. Homology Modeling and Molecular Dynamics
4.9. Structure Preparation and Docking
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACE | Acetyl |
| DC | Dendritic cell |
| HCl | Hydrochloric acid |
| MHC | Major histocompatibility complex |
| NME | N-methyl |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
Appendix A
| Amino Acid | P4 | P3 | P2 | P1 | P1′ | P2′ | P3′ | P4′ |
|---|---|---|---|---|---|---|---|---|
| Ala | 63 | 70 | 22 | 87 | 107 | 98 | 82 | 53 |
| Arg | 6 | 7 | 1 | 13 | 6 | 12 | 8 | 8 |
| Asn | 30 | 34 | 4 | 41 | 28 | 30 | 39 | 30 |
| Asp | 37 | 19 | 0 | 28 | 33 | 27 | 71 | 77 |
| Cys | 9 | 20 | 14 | 14 | 15 | 19 | 6 | 14 |
| Gln | 38 | 33 | 14 | 82 | 67 | 51 | 37 | 47 |
| Glu | 59 | 53 | 2 | 81 | 68 | 38 | 42 | 80 |
| Gly | 48 | 63 | 5 | 128 | 118 | 86 | 80 | 91 |
| His | 20 | 17 | 3 | 17 | 26 | 29 | 55 | 40 |
| Ile | 25 | 58 | 76 | 9 | 19 | 34 | 21 | 27 |
| Leu | 40 | 71 | 244 | 44 | 24 | 31 | 23 | 22 |
| Lys | 7 | 9 | 1 | 8 | 6 | 26 | 15 | 23 |
| Met | 20 | 20 | 55 | 15 | 10 | 8 | 2 | 6 |
| Phe | 30 | 42 | 69 | 10 | 14 | 16 | 22 | 12 |
| Pro | 42 | 51 | 5 | 1 | 0 | 63 | 106 | 79 |
| Ser | 51 | 57 | 11 | 85 | 128 | 72 | 51 | 51 |
| Thr | 22 | 41 | 17 | 76 | 40 | 59 | 39 | 46 |
| Trp | 10 | 7 | 11 | 6 | 4 | 4 | 8 | 4 |
| Tyr | 14 | 14 | 28 | 5 | 13 | 6 | 16 | 10 |
| Val | 48 | 47 | 172 | 6 | 24 | 41 | 27 | 30 |

References
- Radauer, C.; Bublin, M.; Wagner, S.; Mari, A.; Breiteneder, H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J. Allergy Clin. Immunol. 2008, 121, 847.e7–852.e7. [Google Scholar] [CrossRef] [PubMed]
- Stemeseder, T.; Freier, R.; Wildner, S.; Fuchs, J.E.; Briza, P.; Lang, R.; Batanero, E.; Lidholm, J.; Liedl, K.R.; Campo, P.; et al. Crystal structure of Pla l 1 reveals both structural similarity and allergenic divergence within the Ole e 1-like protein family. J. Allergy Clin. Immunol. 2017, 140, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Batanero, E.; Villalba, M.; Rodriguez, R. Glycosylation site of the major allergen from olive tree pollen. Allergenic implications of the carbohydrate moiety. Mol. Immunol. 1994, 31, 31–37. [Google Scholar] [CrossRef]
- Van Ree, R.; Cabanes-Macheteau, M.; Akkerdaas, J.; Milazzo, J.P.; Loutelier-Bourhis, C.; Rayon, C.; Villalba, M.; Koppelman, S.; Aalberse, R.; Rodriguez, R.; et al. β(1,2)-Xylose and α(1,3)-fucose residues have a strong contribution in IgE binding to plant glycoallergens. J. Biol. Chem. 2000, 275, 11451–11458. [Google Scholar] [CrossRef] [PubMed]
- Barderas, R.; Purohit, A.; Papanikolaou, I.; Rodriguez, R.; Pauli, G.; Villalba, M. Cloning, expression, and clinical significance of the major allergen from ash pollen, Fra e 1. J. Allergy Clin. Immunol. 2005, 115, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Calabozo, B.; Barber, D.; Polo, F. Studies on the carbohydrate moiety of Pla l 1 allergen. Identification of a major N-glycan and significance for the immunoglobulin E-binding activity. Clin. Exp. Allergy 2002, 32, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.; Rodriguez, R.; Batanero, E. The spectrum of olive pollen allergens. From structures to diagnosis and treatment. Methods 2014, 66, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Marknell DeWitt, A.; Niederberger, V.; Lehtonen, P.; Spitzauer, S.; Sperr, W.R.; Valent, P.; Valenta, R.; Lidholm, J. Molecular and immunological characterization of a novel timothy grass (phleum pratense) pollen allergen, Phl p 11. Clin. Exp. Allergy 2002, 32, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.J.; Alche, J.D.; Calabozo, B.; Rodriguez-Garcia, M.I.; Polo, F. Pla 1 1 and Ole e 1 pollen allergens share common epitopes and similar ultrastructural localization. J. Investig. Allergol. Clin. Immunol. 2007, 17, 41–47. [Google Scholar] [PubMed]
- Villalba, M.; Batanero, E.; Lopez-Otin, C.; Sanchez, L.M.; Monsalve, R.I.; Gonzalez de la Pena, M.A.; Lahoz, C.; Rodriguez, R. The amino acid sequence of Ole e I, the major allergen from olive tree (Olea europaea) pollen. Eur. J. Biochem. 1993, 216, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Batanero, E.; Crespo, J.F.; Monsalve, R.I.; Martin-Esteban, M.; Villalba, M.; Rodriguez, R. IgE-binding and histamine-release capabilities of the main carbohydrate component isolated from the major allergen of olive tree pollen, Ole e 1. J. Allergy Clin. Immunol. 1999, 103, 147–153. [Google Scholar] [CrossRef]
- Palomares, O.; Swoboda, I.; Villalba, M.; Balic, N.; Spitzauer, S.; Rodriguez, R.; Valenta, R. The major allergen of olive pollen Ole e 1 is a diagnostic marker for sensitization to Oleaceae. Int. Arch. Allergy Immunol. 2006, 141, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.; Barderas, R.; Mas, S.; Colas, C.; Batanero, E.; Rodriguez, R. Amaranthaceae pollens: Review of an emerging allergy in the mediterranean area. J. Investig. Allergol. Clin. Immunol. 2014, 24, 371–381. [Google Scholar] [PubMed]
- Castro, L.; Mas, S.; Barderas, R.; Colas, C.; Garcia-Selles, J.; Barber, D.; Rodriguez, R.; Villalba, M. Sal k 5, a member of the widespread Ole e 1-like protein family, is a new allergen of russian thistle (Salsola kali) pollen. Int. Arch. Allergy Immunol. 2014, 163, 142–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barderas, R.; Villalba, M.; Lombardero, M.; Rodriguez, R. Identification and characterization of Che a 1 allergen from chenopodium album pollen. Int. Arch. Allergy Immunol. 2002, 127, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Gadermaier, G.; Eichhorn, S.; Vejvar, E.; Weilnbock, L.; Lang, R.; Briza, P.; Huber, C.G.; Ferreira, F.; Hawranek, T. Plantago lanceolata: An important trigger of summer pollinosis with limited IgE cross-reactivity. J. Allergy Clin. Immunol. 2014, 134, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Cardaba, B.; del Pozo, V.; Jurado, A.; Gallardo, S.; Cortegano, I.; Arrieta, I.; del Amo, A.; Tramon, P.; Florido, F.; Sastre, J.; et al. Olive pollen allergy: Searching for immunodominant T-cell epitopes on the Ole e 1 molecule. Clin. Exp. Allergy 1998, 28, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Jurets, A.; Wallner, M.; Briza, P.; Ruzek, S.; Hainzl, S.; Pichler, U.; Kitzmuller, C.; Bohle, B.; Huber, C.G.; et al. Assessing protein immunogenicity with a dendritic cell line-derived endolysosomal degradome. PLoS ONE 2011, 6, e17278. [Google Scholar] [CrossRef] [PubMed]
- Kitzmuller, C.; Zulehner, N.; Roulias, A.; Briza, P.; Ferreira, F.; Fae, I.; Fischer, G.F.; Bohle, B. Correlation of sensitizing capacity and T-cell recognition within the Bet v 1 family. J. Allergy Clin. Immunol. 2015, 136, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Delamarre, L.; Couture, R.; Mellman, I.; Trombetta, E.S. Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J. Exp. Med. 2006, 203, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Delamarre, L.; Pack, M.; Chang, H.; Mellman, I.; Trombetta, E.S. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 2005, 307, 1630–1634. [Google Scholar] [CrossRef] [PubMed]
- Machado, Y.; Freier, R.; Scheiblhofer, S.; Thalhamer, T.; Mayr, M.; Briza, P.; Grutsch, S.; Ahammer, L.; Fuchs, J.E.; Wallnoefer, H.G.; et al. Fold stability during endolysosomal acidification is a key factor for allergenicity and immunogenicity of the major birch pollen allergen. J. Allergy Clin. Immunol. 2016, 137, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Pluger, E.B.; Boes, M.; Alfonso, C.; Schroter, C.J.; Kalbacher, H.; Ploegh, H.L.; Driessen, C. Specific role for cathepsin S in the generation of antigenic peptides in vivo. Eur. J. Immunol. 2002, 32, 467–476. [Google Scholar] [CrossRef]
- Rudensky, A.; Beers, C. Lysosomal cysteine proteases and antigen presentation. Ernst Schering Res. Found. Workshop 2006, 56, 81–95. [Google Scholar]
- Cai, J.; Robinson, J.; Belshaw, S.; Everett, K.; Fradera, X.; van Zeeland, M.; van Berkom, L.; van Rijnsbergen, P.; Popplestone, L.; Baugh, M.; et al. Trifluoromethylphenyl as P2 for ketoamide-based cathepsin S inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 6890–6894. [Google Scholar] [CrossRef] [PubMed]
- Ward, Y.D.; Thomson, D.S.; Frye, L.L.; Cywin, C.L.; Morwick, T.; Emmanuel, M.J.; Zindell, R.; McNeil, D.; Bekkali, Y.; Hrapchak, M.; et al. Design and synthesis of dipeptide nitriles as reversible and potent cathepsin S inhibitors. J. Med. Chem. 2002, 45, 5471–5482. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.K.; Schiffler, M.A.; Gavardinas, K.; Kim, E.J.; Matthews, D.P.; Staszak, M.A.; Coffey, D.S.; Shaw, B.W.; Cassidy, K.C.; Brier, R.A.; et al. Discovery of cathepsin S inhibitor ly3000328 for the treatment of abdominal aortic aneurysm. ACS Med. Chem. Lett. 2014, 5, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Reese, G.; Gadermaier, G.; Schulten, V.; Lauer, I.; Egger, M.; Briza, P.; Randow, S.; Wolfheimer, S.; Kigongo, V.; et al. Protein unfolding strongly modulates the allergenicity and immunogenicity of Pru p 3, the major peach allergen. J. Allergy Clin. Immunol. 2011, 128, 1022.e7–1030.e7. [Google Scholar] [CrossRef] [PubMed]
- Mutschlechner, S.; Egger, M.; Briza, P.; Wallner, M.; Lackner, P.; Karle, A.; Vogt, A.B.; Fischer, G.F.; Bohle, B.; Ferreira, F. Naturally processed T cell-activating peptides of the major birch pollen allergen. J. Allergy Clin. Immunol. 2010, 125, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Gadermaier, G.; Hauser, M.; Egger, M.; Ferrara, R.; Briza, P.; Santos, K.S.; Zennaro, D.; Girbl, T.; Zuidmeer-Jongejan, L.; Mari, A.; et al. Sensitization prevalence, antibody cross-reactivity and immunogenic peptide profile of Api g 2, the non-specific lipid transfer protein 1 of celery. PLoS ONE 2011, 6, e24150. [Google Scholar] [CrossRef] [PubMed]
- Barderas, R.; Villalba, M.; Rodriguez, R. Che a 1: Recombinant expression, purification and correspondence to the natural form. Int. Arch. Allergy Immunol. 2004, 135, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Huecas, S.; Villalba, M.; Gonzalez, E.; Martinez-Ruiz, A.; Rodriguez, R. Production and detailed characterization of biologically active olive pollen allergen Ole e 1 secreted by the yeast pichia pastoris. Eur. J. Biochem. 1999, 261, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Calabozo, B.; Diaz-Perales, A.; Salcedo, G.; Barber, D.; Polo, F. Cloning and expression of biologically active plantago lanceolata pollen allergen Pla l 1 in the yeast pichia pastoris. Biochem. J. 2003, 372, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Rammensee, H.G.; Friede, T.; Stevanoviic, S. MHC ligands and peptide motifs: First listing. Immunogenetics 1995, 41, 178–228. [Google Scholar] [CrossRef] [PubMed]
- Soria-Guerra, R.E.; Nieto-Gomez, R.; Govea-Alonso, D.O.; Rosales-Mendoza, S. An overview of bioinformatics tools for epitope prediction: Implications on vaccine development. J. Biomed. Inform. 2015, 53, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Finn, R. Twenty years of the merops database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2016, 44, D343–D350. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.E.; Palmer, J.T.; Bromme, D.; Somoza, J.R. Crystal structure of human cathepsin S. Protein Sci. 1998, 7, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Bromme, D.; Bonneau, P.R.; Lachance, P.; Wiederanders, B.; Kirschke, H.; Peters, C.; Thomas, D.Y.; Storer, A.C.; Vernet, T. Functional expression of human cathepsin S in saccharomyces cerevisiae. Purification and characterization of the recombinant enzyme. J. Biol. Chem. 1993, 268, 4832–4838. [Google Scholar] [PubMed]
- Lennon-Dumenil, A.M.; Bakker, A.H.; Maehr, R.; Fiebiger, E.; Overkleeft, H.S.; Rosemblatt, M.; Ploegh, H.L.; Lagaudriere-Gesbert, C. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J. Exp. Med. 2002, 196, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using clustal omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Raghava, G.P. Propred: Prediction of HLA-DR binding sites. Bioinformatics 2001, 17, 1236–1237. [Google Scholar] [CrossRef] [PubMed]
- Southwood, S.; Sidney, J.; Kondo, A.; del Guercio, M.F.; Appella, E.; Hoffman, S.; Kubo, R.T.; Chesnut, R.W.; Grey, H.M.; Sette, A. Several common HLA-DR types share largely overlapping peptide binding repertoires. J. Immunol. 1998, 160, 3363–3373. [Google Scholar] [PubMed]
- Kavan, D.; Man, P. Mstools-web based application for visualization and presentation of HXMS data. Int. J. Mass Spectrom. 2011, 302, 53–58. [Google Scholar] [CrossRef]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The swiss-model workspace: A web-based environment for protein structure homology modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE), version 2016.08; H3A 2R7; Chemical Computing Group Inc.: Montreal, QC, Canada, 2017.
- Bond, C.S.; Schuttelkopf, A.W. Aline: A wysiwyg protein-sequence alignment editor for publication-quality alignments. Acta Crystallogr. Sect. D 2009, 65, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Valiev, M.; Bylaska, E.J.; Govind, N.; Kowalski, K.; Straatsma, T.P.; van Dam, H.J.J.; Wang, D.; Nieplocha, J.; Apra, E.; Windus, T.L.; et al. NWchem: A comprehensive and scalable open-source solution for large scale molecular simulations. Comp. Phys. Commun. 2010, 181, 1477–1489. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]

| Allergen | Plant | Accession Number | Host Organism | Protein Modification |
|---|---|---|---|---|
| Ole e 1.0101 | Olea europaea | P19963.2 | P. pastoris | A99V, K106L, N111Q, deamidation, oxidation |
| Olive | ||||
| Fra e 1.0101 | Fraxinus excelsior | AAQ08947.1 | P. pastoris | minor deamidations, yeast-specific glycosylation at N111 |
| Ash | ||||
| Sal k 5.0101 | Salsola kali | ADK22842.1 | P. pastoris | K3N, G84D, I91V, deamidation, yeast-specific glycosylation at N43 |
| Russian thistle | ||||
| Che a 1.0101 | Chenopodium album | Q8LGR0.1 | P. pastoris | deamidation, methionine oxidation, yeast-specific glycosylation at N39 |
| Lamb’s quarters | ||||
| Phl p 11.0101 | Phleum pratense | Q8H6L7.1 | P. pastoris | N24Q |
| Timothy grass | ||||
| Pla l 1.0101 | Plantago lanceolata | P82242.2 | E. coli | None |
| English plantain |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wildner, S.; Elsässer, B.; Stemeseder, T.; Briza, P.; Soh, W.T.; Villalba, M.; Lidholm, J.; Brandstetter, H.; Gadermaier, G. Endolysosomal Degradation of Allergenic Ole e 1-Like Proteins: Analysis of Proteolytic Cleavage Sites Revealing T Cell Epitope-Containing Peptides. Int. J. Mol. Sci. 2017, 18, 1780. https://doi.org/10.3390/ijms18081780
Wildner S, Elsässer B, Stemeseder T, Briza P, Soh WT, Villalba M, Lidholm J, Brandstetter H, Gadermaier G. Endolysosomal Degradation of Allergenic Ole e 1-Like Proteins: Analysis of Proteolytic Cleavage Sites Revealing T Cell Epitope-Containing Peptides. International Journal of Molecular Sciences. 2017; 18(8):1780. https://doi.org/10.3390/ijms18081780
Chicago/Turabian StyleWildner, Sabrina, Brigitta Elsässer, Teresa Stemeseder, Peter Briza, Wai Tuck Soh, Mayte Villalba, Jonas Lidholm, Hans Brandstetter, and Gabriele Gadermaier. 2017. "Endolysosomal Degradation of Allergenic Ole e 1-Like Proteins: Analysis of Proteolytic Cleavage Sites Revealing T Cell Epitope-Containing Peptides" International Journal of Molecular Sciences 18, no. 8: 1780. https://doi.org/10.3390/ijms18081780