Correlation of Leukocyte Telomere Length Measurement Methods in Patients with Dyskeratosis Congenita and in Their Unaffected Relatives
Abstract
:1. Introduction
2. Results
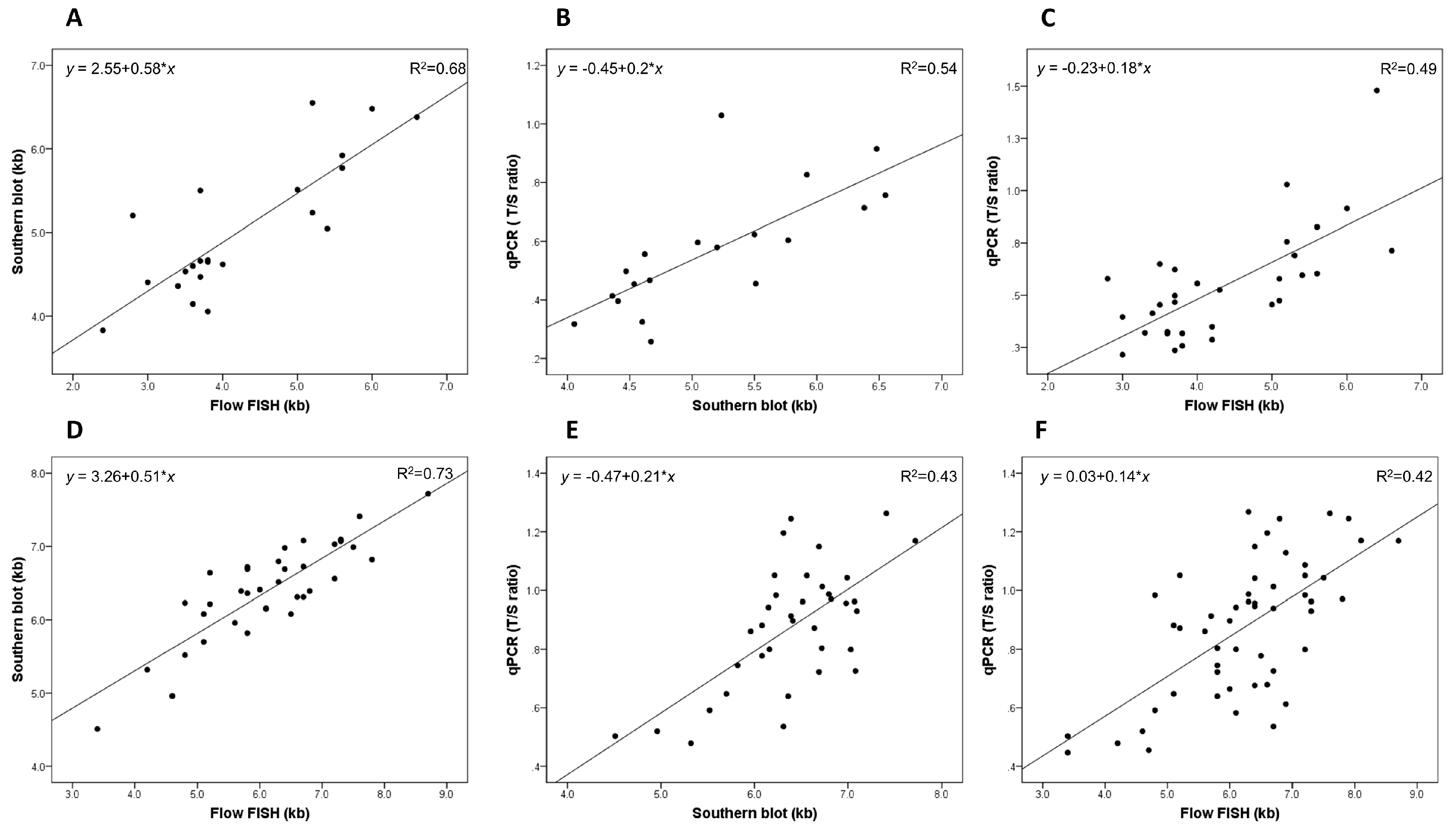
2.1. Telomere Length Measurement (TLM) Correlations in Patients with Dyskeratosis Congenita (DC)
2.2. TLM Correlations in Unaffected Relatives
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. DNA Extraction
4.3. Quantitative Polymerase Chain Reaction (qPCR) Assay
4.4. Southern Blot Assay
4.5. Flow cytometry with fluorescence in situ hybridization (Flow FISH)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ballew, B.J.; Savage, S.A. Updates on the biology and management of dyskeratosis congenita and related telomere biology disorders. Expert. Rev. Hematol. 2013, 6, 327–337. [Google Scholar] [CrossRef] [PubMed]
- De Lange, T.; Shiue, L.; Myers, R.M.; Cox, D.R.; Naylor, S.L.; Killery, A.M.; Varmus, H.E. Structure and variability of human chromosome ends. Mol. Cell. Biol. 1990, 10, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.L.; Newman, A.B. Telomere length in epidemiology: A biomarker of aging, age-related disease, both, or neither? Epidemiol. Rev. 2013, 35, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Lansdorp, P.M. Telomeres and aging. Physiol. Rev. 2008, 88, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, S.M.; Cawthon, R.; Giri, N.; Alter, B.P.; Savage, S.A. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging 2010, 2, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Aston, K.I.; Hunt, S.C.; Susser, E.; Kimura, M.; Factor-Litvak, P.; Carrell, D.; Aviv, A. Divergence of sperm and leukocyte age-dependent telomere dynamics: Implications for male-driven evolution of telomere length in humans. Mol. Hum. Reprod. 2012, 18, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; De, V.I.; Liu, Y.; Han, J.; Prescott, J.; Hunter, D.J.; Rimm, E.B. Associations between diet, lifestyle factors, and telomere length in women. Am. J. Clin. Nutr. 2010, 91, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.C.; Horvath, K.; Kark, J.D.; Susser, E.; Tishkoff, S.A.; Aviv, A. Telomere length and the cancer-atherosclerosis trade-off. PLoS Genet. 2016, 12, e1006144. [Google Scholar] [CrossRef] [PubMed]
- Dokal, I. Dyskeratosis congenita. Hematology Am. Soc. Hematol. Educ. Program 2011, 2011, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Alter, B.P.; Baerlocher, G.M.; Savage, S.A.; Chanock, S.J.; Weksler, B.B.; Willner, J.P.; Peters, J.A.; Giri, N.; Lansdorp, P.M. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood 2007, 110, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Alter, B.P.; Rosenberg, P.S.; Giri, N.; Baerlocher, G.M.; Lansdorp, P.M.; Savage, S.A. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica 2012, 97, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Hills, M.; Lansdorp, P.M. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutat. Res. 2012, 730, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, G.M.; Mak, J.; Tien, T.; Lansdorp, P.M. Telomere length measurement by fluorescence in situ hybridization and flow cytometry: Tips and pitfalls. Cytometry 2002, 47, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Stone, R.C.; Hunt, S.C.; Skurnick, J.; Lu, X.; Cao, X.; Harley, C.B.; Aviv, A. Measurement of telomere length by the southern blot analysis of terminal restriction fragment lengths. Nat. Protoc. 2010, 5, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, S.M.; Khincha, P.P.; Katki, H.A.; Giri, N.; Wong, J.Y.; Spellman, S.; Yanovski, J.A.; Han, J.C.; de Vivo, I.; Alter, B.P.; et al. The limitations of qPCR telomere length measurement in diagnosing dyskeratosis congenita. Mol. Genet. Genomic Med. 2016, 4, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Aviv, A.; Hunt, S.C.; Lin, J.; Cao, X.; Kimura, M.; Blackburn, E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by southern blots and qPCR. Nucleic Acids Res. 2011, 39, e134. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, G.M.; Vulto, I.; De, J.G.; Lansdorp, P.M. Flow cytometry and fish to measure the average length of telomeres (flow FISH). Nat. Protoc. 2006, 1, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Baerlocher, G.M.; Vulto, I.; Poon, S.S.; Lansdorp, P.M. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLoS Genet. 2012, 8, e1002696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, J.M.; Johnson, R.A.; Litzelman, K.; Skinner, H.G.; Seo, S.; Engelman, C.D.; Vanderboom, R.J.; Kimmel, G.W.; Gangnon, R.E.; Riegert-Johnson, D.L.; et al. Telomere length varies by DNA extraction method: Implications for epidemiologic research. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, S.; Susser, E.; Factor-Litvak, P.R.; Simons, M.J.; Benetos, A.; Steenstrup, T.; Kark, J.D.; Aviv, A. Commentary: The reliability of telomere length measurements. Int. J. Epidemiol. 2015, 44, 1683–1686. [Google Scholar] [CrossRef] [PubMed]
- Raschenberger, J.; Lamina, C.; Haun, M.; Kollerits, B.; Coassin, S.; Boes, E.; Kedenko, L.; Kottgen, A.; Kronenberg, F. Influence of DNA extraction methods on relative telomere length measurements and its impact on epidemiological studies. Sci. Rep. 2016, 6, 25398. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.T.; Kuzawa, C.W.; Hayes, M.G. Improving qPCR telomere length assays: Controlling for well position effects increases statistical power. Am. J. Hum. Biol. 2015, 27, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Elbers, C.C.; Garcia, M.E.; Kimura, M.; Cummings, S.R.; Nalls, M.A.; Newman, A.B.; Park, V.; Sanders, J.L.; Tranah, G.J.; Tishkoff, S.A.; et al. Comparison between southern blots and qPCR analysis of leukocyte telomere length in the health abc study. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 69, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ruiz, C.M.; Baird, D.; Roger, L.; Boukamp, P.; Krunic, D.; Cawthon, R.; Dokter, M.M.; van der Harst, P.; Bekaert, S.; de Meyer, T.; et al. Reproducibility of telomere length assessment: Authors’ response to damjan krstajic and ljubomir buturovic. Int. J. Epidemiol. 2015, 44, 1739–1741. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Rodrigues, F.; Santana-Lemos, B.A.; Scheucher, P.S.; Alves-Paiva, R.M.; Calado, R.T. Direct comparison of flow-fish and qPCR as diagnostic tests for telomere length measurement in humans. PLoS ONE 2014, 9, e113747. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere measurement by quantitative pcr. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Alter, B.P.; Giri, N.; Savage, S.A.; Peters, J.A.; Loud, J.T.; Leathwood, L.; Carr, A.G.; Greene, M.H.; Rosenberg, P.S. Malignancies and survival patterns in the national cancer institute inherited bone marrow failure syndromes cohort study. Br. J. Haematol. 2010, 150, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Vulliamy, T.J.; Marrone, A.; Knight, S.W.; Walne, A.; Mason, P.J.; Dokal, I. Mutations in dyskeratosis congenita: Their impact on telomere length and the diversity of clinical presentation. Blood 2006, 107, 2680–2685. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, S.M.; Wang, T.; Dagnall, C.; Haagenson, M.; Spellman, S.R.; Hicks, B.; Jones, K.; Katki, H.A.; Lee, S.J.; Savage, S.A. Effect of recipient age and stem cell source on the association between donor telomere length and survival after allogeneic unrelated hematopoietic cell transplantation for severe aplastic anemia. Biol. Blood Marrow Transplant. 2016, 22, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
| Header | DC (n = 35) | Unaffected Relatives (n = 53) |
|---|---|---|
| Age in years, median (range) | 27 (5–70) | 41 (3–69) |
| Gender, male:female | 2:1 | 1:1 |
| Genetic Mutation Number (%) | ||
| TERC | 9 (26%) | Pathogenic variant(s) causative of DC in the family were not present |
| TERT | 6 (17%) | |
| DKC1 | 5 (14%) | |
| TINF2 | 5 (14%) | |
| WRAP53 | 1 (3%) | |
| RTEL1 | 1 (3%) | |
| Unknown gene | 8 (23%) | |
| TL Median (Range) | ||
| qPCR (T/S ratio) * | 0.50 (0.21–1.48) | 0.91 (0.45–1.27) |
| Flow FISH lymphocytes (kb) * | 3.8 (2.4–6.6) | 6.4 (3.4–8.7) |
| Southern blot (kb) * | 4.6 (3.8–6.6) | 6.4 (4.5–7.7) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khincha, P.P.; Dagnall, C.L.; Hicks, B.; Jones, K.; Aviv, A.; Kimura, M.; Katki, H.; Aubert, G.; Giri, N.; Alter, B.P.; et al. Correlation of Leukocyte Telomere Length Measurement Methods in Patients with Dyskeratosis Congenita and in Their Unaffected Relatives. Int. J. Mol. Sci. 2017, 18, 1765. https://doi.org/10.3390/ijms18081765
Khincha PP, Dagnall CL, Hicks B, Jones K, Aviv A, Kimura M, Katki H, Aubert G, Giri N, Alter BP, et al. Correlation of Leukocyte Telomere Length Measurement Methods in Patients with Dyskeratosis Congenita and in Their Unaffected Relatives. International Journal of Molecular Sciences. 2017; 18(8):1765. https://doi.org/10.3390/ijms18081765
Chicago/Turabian StyleKhincha, Payal P., Casey L. Dagnall, Belynda Hicks, Kristine Jones, Abraham Aviv, Masayuki Kimura, Hormuzd Katki, Geraldine Aubert, Neelam Giri, Blanche P. Alter, and et al. 2017. "Correlation of Leukocyte Telomere Length Measurement Methods in Patients with Dyskeratosis Congenita and in Their Unaffected Relatives" International Journal of Molecular Sciences 18, no. 8: 1765. https://doi.org/10.3390/ijms18081765




