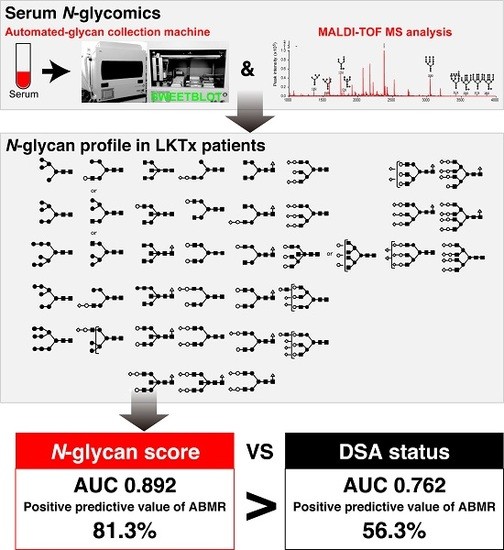
Serum Aberrant N-Glycan Profile as a Marker Associated with Early Antibody-Mediated Rejection in Patients Receiving a Living Donor Kidney Transplant
Abstract
:1. Introduction
2. Results
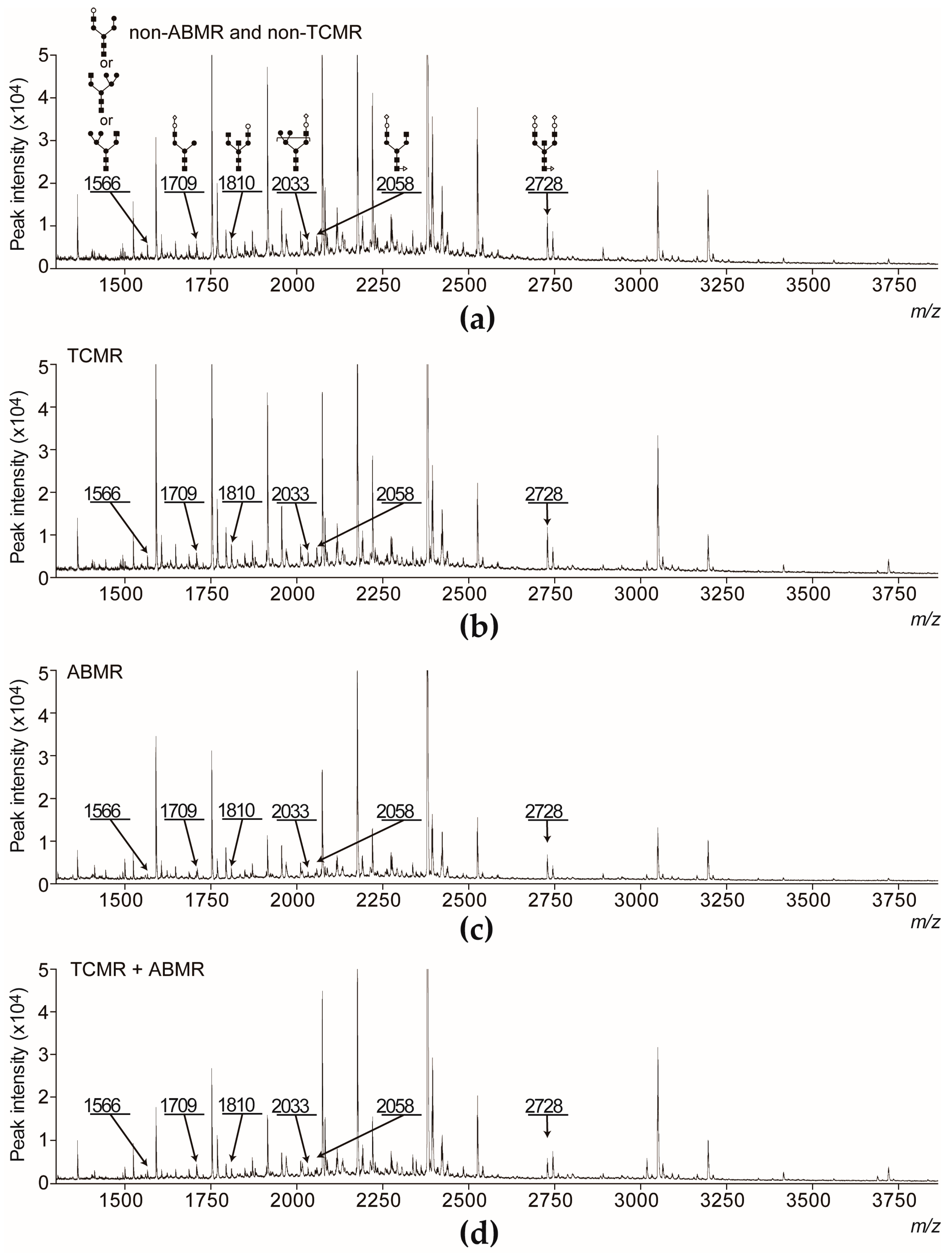
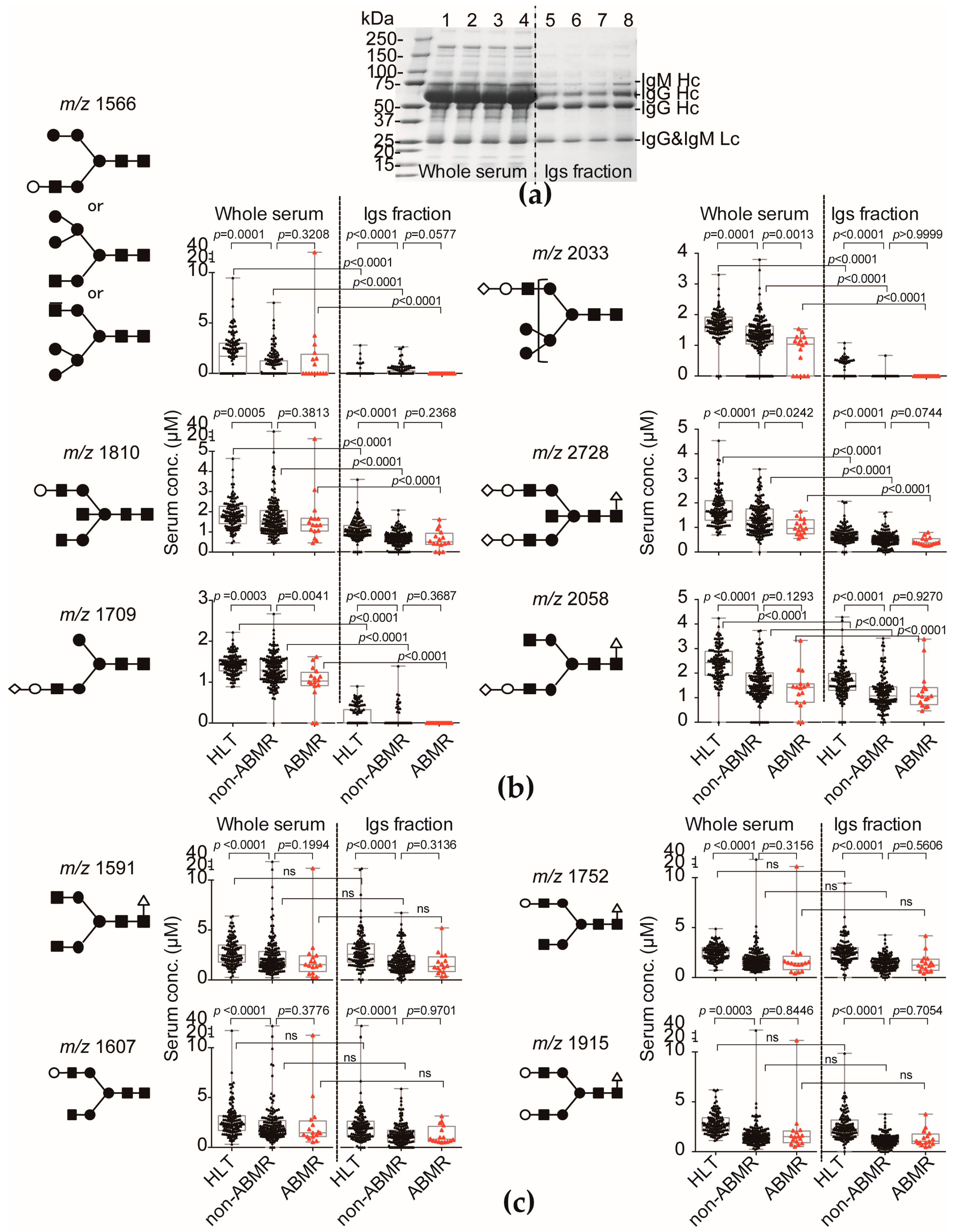
2.1. The Level of Serum Sialyl Hybrid Type and Sialyl Bisecting Type N-Glycans Were Associated with Recipients Who Developed ABMR and Much Lower than That of Recipients Who Did Not Develop ABMR
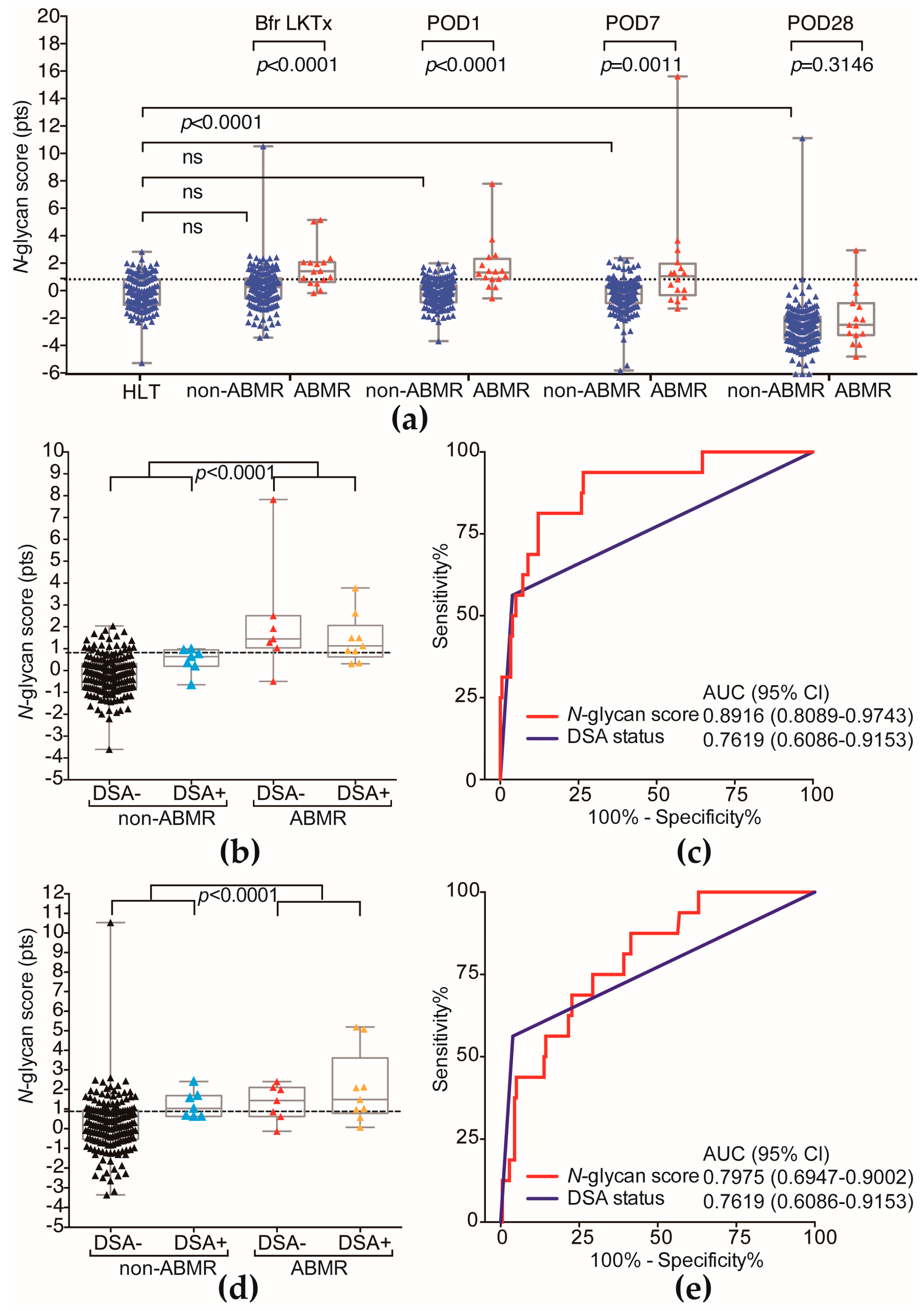
2.2. The ABMR Predictive Performance of N-Glycan Score Based on the Aberrant Serum N-Glycan Profile Was Far Superior to That of Preformed Donor-Specific Antibody Status
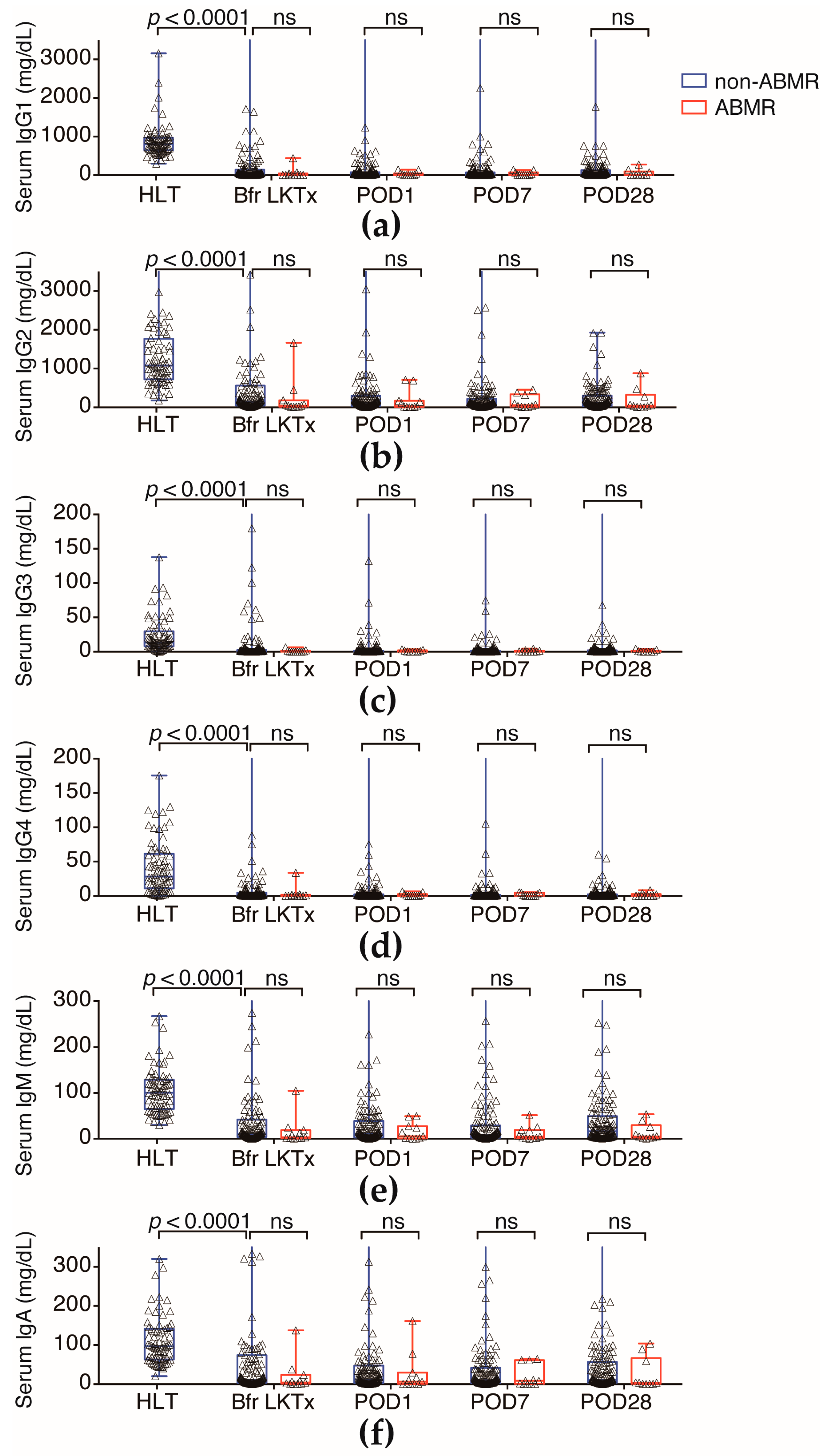
2.3. N-Glycan-Carrying Serum Immunoglobulin (Igs) Levels Were Not Significantly Different between the ABMR Group and Non-ABMR Group
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Serum Samples and Diagnosis of ABMR
4.3. Glycoblotting Method and Mass Spectrometry
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABMR | Antibody-mediated rejection |
| LKTx | Living donor kidney transplant |
| TCMR | T-cell-mediated rejection |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
| DSA | Donor-specific anti-HLA antibodies |
| IgG | Immunoglobulin G |
| Gal | Galactose |
| Man | Mannose |
| GlcNAc | N-acetylglucosamine |
| Fuc | Fucose |
| Sia | Sialic acid |
| Hex | Hexose |
| HexNAc | N-acetylhexosamine |
| dHex | Deoxyhexose |
| MALDI-TOF MS | Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry |
| BOA | Benzyloxyamine |
| ABOi | ABO incompatible |
| KTx | Kidney transplant |
| ODF | One degree of freedom |
| TDF | Two degrees of freedom |
| Bfr | Before |
| POD | Postoperative day |
| PRA | Panel reactive antibody |
| Igs | Immunoglobulins |
References
- Nankivell, B.J.; Fenton-Lee, C.A.; Kuypers, D.R.; Cheung, E.; Allen, R.D.; O’Connell, P.J.; Chapman, J.R. Effect of histological damage on long-term kidney transplant outcome. Transplantation 2001, 71, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, C.; Loupy, A.; Hill, G.S.; Andrade, J.; Nochy, D.; Antoine, C.; Gautreau, C.; Charron, D.; Glotz, D.; Suberbielle-Boissel, C. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J. Am. Soc. Nephrol. 2010, 21, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Sequeira, F.; Tyan, D.B. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Hum. Immunol. 2011, 72, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Yabu, J.M.; Higgins, J.P.; Chen, G.; Sequeira, F.; Busque, S.; Tyan, D.B. C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation 2011, 91, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Lefaucheur, C.; Vernerey, D.; Prugger, C.; Duong van Huyen, J.P.; Mooney, N.; Suberbielle, C.; Fremeaux-Bacchi, V.; Mejean, A.; Desgrandchamps, F.; et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N. Engl. J. Med. 2013, 369, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, C.; Viglietti, D.; Bentlejewski, C.; Duong van Huyen, J.P.; Vernerey, D.; Aubert, O.; Verine, J.; Jouven, X.; Legendre, C.; Glotz, D.; et al. IgG donor-specific anti-human HLA antibody subclasses and kidney allograft antibody-mediated injury. J. Am. Soc. Nephrol. 2016, 27, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Malard-Castagnet, S.; Dugast, E.; Degauque, N.; Pallier, A.; Soulillou, J.P.; Cesbron, A.; Giral, M.; Harb, J.; Brouard, S. Sialylation of antibodies in kidney recipients with de novo donor specific antibody, with or without antibody mediated rejection. Hum. Immunol. 2016, 77, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S.; Amano, M.; Tobisawa, Y.; Yoneyama, T.; Tsuchiya, N.; Habuchi, T.; Nishimura, S.I.; Ohyama, C. Serum N-Glycan alteration associated with renal cell carcinoma detected by high throughput glycan analysis. J. Urol. 2014, 191, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Tobisawa, Y.; Hatakeyama, S.; Ohashi, T.; Tanaka, M.; Narita, S.; Koie, T.; Habuchi, T.; Nishimura, S.I.; Ohyama, C.; et al. Serum tri- and tetra-antennary N-glycan is a potential predictive biomarker for castration-resistant prostate cancer. Prostate 2014, 74, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, K.; Nouso, K.; Miyake, Y.; Nakamura, S.; Obi, S.; Amano, M.; Hirose, K.; Nishimura, S.I.; Yamamoto, K. Serum glycan as a prognostic marker in patients with advanced hepatocellular carcinoma treated with sorafenib. Hepatology 2014, 59, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S.; Amano, M.; Tobisawa, Y.; Yoneyama, T.; Tsushima, M.; Hirose, K.; Yoneyama, T.; Hashimoto, Y.; Koie, T.; Saitoh, H.; et al. Serum N-Glycan profiling predicts prognosis in patients undergoing hemodialysis. Sci. World J. 2013, 2013, 268407. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Yamaguchi, M.; Takegawa, Y.; Yamashita, T.; Terashima, M.; Furukawa, J.I.; Miura, Y.; Shinohara, Y.; Iwasaki, N.; Minami, A.; et al. Threshold in stage-specific embryonic glycotypes uncovered by a full portrait of dynamic N-glycan expression during cell differentiation. Mol. Cell Proteom. 2010, 9, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, K.; Nouso, K.; Saito, S.; Hiraoka, S.; Harada, K.; Takahashi, S.; Morimoto, Y.; Kobayashi, S.; Ikeda, F.; Miyake, Y.; et al. Serum glycan markers for evaluation of disease activity and prediction of clinical course in patients with ulcerative colitis. PLoS ONE 2013, 8, e74861. [Google Scholar] [CrossRef] [PubMed]
- Nouso, K.; Amano, M.; Ito, Y.M.; Miyahara, K.; Morimoto, Y.; Kato, H.; Tsutsumi, K.; Tomoda, T.; Yamamoto, N.; Nakamura, S.; et al. Clinical utility of high-throughput glycome analysis in patients with pancreatic cancer. J. Gastroenterol. 2013, 48, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.; Winkler, A.; Lorenz, A.K.; Holecska, V.; Blanchard, V.; Eiglmeier, S.; Schoen, A.L.; Bitterling, J.; Stoehr, A.D.; Petzold, D.; et al. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. J. Clin. Investig. 2013, 123, 3788–3796. [Google Scholar] [CrossRef] [PubMed]
- Quast, I.; Keller, C.W.; Maurer, M.A.; Giddens, J.P.; Tackenberg, B.; Wang, L.X.; Munz, C.; Nimmerjahn, F.; Dalakas, M.C.; Lunemann, J.D. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J. Clin. Investig. 2015, 125, 4160–4170. [Google Scholar] [CrossRef] [PubMed]
- Seino, J.; Fujihira, H.; Nakakita, S.I.; Masahara-Negishi, Y.; Miyoshi, E.; Hirabayashi, J.; Suzuki, T. Occurrence of free sialyl oligosaccharides related to N-glycans (sialyl free N-glycans) in animal sera. Glycobiology 2016, 26, 1072–1085. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, W.; Havenaar, E.C.; Brinkman-van der Linden, E.C. α1-acid glycoprotein (orosomucoid): Pathophysiological changes in glycosylation in relation to its function. Glycoconj. J. 1995, 12, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.E.; Larsen, V.A.; Bog-Hansen, T.C. The microheterogeneity of α1-acid glycoprotein in inflammatory lung disease, cancer of the lung and normal health. Clin. Chim. Acta 1984, 138, 41–47. [Google Scholar] [CrossRef]
- Haas, M. The revised (2013) banff classification for antibody-mediated rejection of renal allografts: Update, difficulties, and future considerations. Am. J. Transplant. 2016, 16, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Hato, M.; Shinohara, Y.; Kuramoto, H.; Furukawa, J.I.; Kurogochi, M.; Shimaoka, H.; Tada, M.; Nakanishi, K.; Ozaki, M.; et al. BlotGlycoABCTM, an integrated glycoblotting technique for rapid and large scale clinical glycomics. Mol. Cell Proteom. 2008, 7, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Motoi, T.; Maho, A.; Hiroshi, K.; Taiji, T.; Naoya, M.; Kazuko, H.; Tetsu, O.; Shin-Ichiro, N. N- and O-glycome analysis of serum and urine from bladder cancer patients using a high-throughput glycoblotting method. J. Glycom. Lipidom. 2013, 3, 1000108. [Google Scholar]
- Harrell, F.E.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]




| No. | m/z | Composition |
|---|---|---|
| 1 | 1362.5 | (Hex)2 + (Man)3(GlcNAc)2 |
| 2 | 1524.5 | (Hex)3 + (Man)3(GlcNAc)2 |
| 3 | 1565.5 | (Hex)5 + (HexNAc)3 |
| 4 | 1590.6 | (HexNAc)2(dHex)1 + (Man)3(GlcNAc)2 |
| 5 | 1606.6 | (Hex)1(HexNAc)2 + (Man)3(GlcNAc)2 |
| 6 | 1647.6 | (HexNAc)3 + (Man)3(GlcNAc)2 |
| 7 | 1686.6 | (Hex)4 + (Man)3(GlcNAc)2 |
| 8 | 1708.6 | (Hex)1(HexNAc)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 9 | 1752.6 | (Hex)1(HexNAc)2(dHex)1 + (Man)3(GlcNAc)2 |
| 10 | 1768.6 | (Hex)2(HexNAc)2 + (Man)3(GlcNAc)2 |
| 11 | 1793.7 | (HexNAc)3(dHex)1 + (Man)3(GlcNAc)2 |
| 12 | 1809.7 | (Hex)1(HexNAc)3 + (Man)3(GlcNAc)2 |
| 13 | 1848.6 | (Hex)5 + (Man)3(GlcNAc)2 |
| 14 | 1870.7 | (Hex)2(HexNAc)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 15 | 1914.7 | (Hex)2(HexNAc)2(dHex)1 + (Man)3(GlcNAc)2 |
| 16 | 1955.7 | (Hex)1(HexNAc)3(dHex)1 + (Man)3(GlcNAc)2 |
| 17 | 2010.7 | (Hex)6 + (Man)3(GlcNAc)2 |
| 18 | 2032.7 | (Hex)3(HexNac)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 19 | 2057.8 | (Hex)1(HexNAc)2(dHex)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 20 | 2073.8 | (Hex)2(HexNAc)2(NeuAc)1 + (Man)3(GlcNAc)2 |
| 21 | 2219.8 | (Hex)2(HexNAc)2(dHex)1(NeuAc)1 + (Man)3(GlcNAc)2 |
| 22 | 2336.9 | (Hex)3(HexNAc)4 + (Man)3(GlcNAc)2 |
| 23 | 2348.9 1 | Internal standard (BOA-labeled A2 amide) |
| 24 | 2378.9 | (Hex)2(HexNAc)2(NeuAc)2 + (Man)3(GlcNAc)2 |
| 25 | 2524.9 | (Hex)2(HexNAc)2(dHex)1(NeuAc)2 + (Man)3(GlcNAc)2 |
| 26 | 2727.9 | (Hex)2(HexNAc)3(dHex)1(NeuAc)2 + (Man)3(GlcNAc)2 |
| 27 | 2743.9 | (Hex)3(HexNAc)3(NeuAc)2 + (Man)3(GlcNAc)2 |
| 28 | 2890.1 | (Hex)3(HexNAc)3(dHex)1(NeuAc)2 + (Man)3(GlcNAc)2 |
| 29 | 3049.1 | (Hex)3(HexNAc)3(NeuAc)3 + (Man)3(GlcNAc)2 |
| 30 | 3109.1 | (Hex)4(HexNAc)4(NeuAc)2 + (Man)3(GlcNAc)2 |
| 31 | 3195.2 | (Hex)3(HexNAc)3(dHex)1(NeuAc)3 + (Man)3(GlcNAc)2 |
| 32 | 3341.2 | (Hex)3 (HexNAc)3 (Deoxyhexose)2 (NeuAc)3 + (Man)3(GlcNAc)2 |
| 33 | 3414.2 | (Hex)4(HexNAc)4(NeuAc)3 + (Man)3(GlcNAc)2 |
| 34 | 3560.3 | (Hex)4(HexNAc)4(dHex)1(NeuAc)3 + (Man)3(GlcNAc)2 |
| 35 | 3719.3 | (Hex)4(HexNAc)4(NeuAc)4 + (Man)3(GlcNAc)2 |
| 36 | 3865.4 | (Hex)4(HexNAc)4(dHex)1(NeuAc)4 + (Man)3(GlcNAc)2 |
| HLT 5 | All LKTx 2 Cases | Non-ABMR a | ABMR b | p Value (a vs. b) | ||
|---|---|---|---|---|---|---|
| No Event | TCMR | ABMR ± TCMR | ||||
| Number of recipients | 135 | 197 | 141 | 40 | 16 | |
| Recipient’s sex (male/female) | 93/42 | 108/89 | 76/65 | 26/14 | 6/10 | ns 1 |
| Recipient’s age at LKTx 2 (median, IQR 3) | 38 (24–67) | 52 (40–59) | 52 (40–59) | 51 (39–58) | 51 (43–58) | ns 1 |
| ABOi KTx 4 | 97 (49%) | 63 (44%) | 24 (60%) | 10 (62.5%) | ns 1 | |
| Rituximab | 79 (40%) | 50 (36%) | 17 (43%) | 12 (75%) | 0.0003 | |
| Preformed DSA-positive status | 16 (8.1%) | 6 (4%) | 1 (3%) | 9 (56%) | 0.0003 | |
| Non-ABMR survival, month (range) | 48.5 (0.001–117.9) | 40.8 (7.7–117.9) | 75.1 (16.2–114.7) | 1 (0.001–4) | <0.0001 | |
| Non-TCMR survival, month (range) | 24.1 (0.25–117.9) | 40.4 (7.7–117.9) | 1 (1–12) | 8.8 (0.25–96.3) | 0.0039 | |
| Variables | Wilk’s Lambda | F Value | ODF 1 | TDF 2 | p Value | Function Value |
|---|---|---|---|---|---|---|
| Recipient sex | 0.9735 | 4.3294 | 1 | 159 | 0.0391 | −0.9142 |
| Recipient Age at LKTx 3 | 0.9999 | 0.0201 | 1 | 159 | 0.8873 | −0.0024 |
| m/z 1362 | 0.9992 | 0.1254 | 1 | 159 | 0.7238 | 0.3670 |
| m/z 1525 | 0.9997 | 0.0457 | 1 | 159 | 0.8311 | 0.2994 |
| m/z 1566 | 0.9821 | 2.8998 | 1 | 159 | 0.0905 | 0.2181 |
| m/z 1591 | 0.9969 | 0.4931 | 1 | 159 | 0.4836 | 0.3501 |
| m/z 1607 | 0.9999 | 0.0224 | 1 | 159 | 0.8811 | −0.0316 |
| m/z 1648 | 0.9938 | 0.9880 | 1 | 159 | 0.3217 | −0.4001 |
| m/z 1687 | 0.9996 | 0.0581 | 1 | 159 | 0.8098 | 0.2314 |
| m/z 1709 | 0.9857 | 2.3116 | 1 | 159 | 0.1304 | −1.9646 |
| m/z 1753 | 0.9882 | 1.8956 | 1 | 159 | 0.1705 | −1.3974 |
| m/z 1769 | 0.9966 | 0.5473 | 1 | 159 | 0.4605 | −0.1732 |
| m/z 1794 | 0.9969 | 0.5001 | 1 | 159 | 0.4805 | −0.4953 |
| m/z 1810 | 0.9817 | 2.9575 | 1 | 159 | 0.0874 | 1.6191 |
| m/z 1849 | 0.9928 | 1.1510 | 1 | 159 | 0.2850 | −1.0837 |
| m/z 1871 | 0.9995 | 0.0823 | 1 | 159 | 0.7746 | 0.1763 |
| m/z 1915 | 1.0000 | 0.0005 | 1 | 159 | 0.9818 | −0.0203 |
| m/z 1956 | 0.9967 | 0.5323 | 1 | 159 | 0.4667 | 0.7082 |
| m/z 2011 | 0.9962 | 0.6075 | 1 | 159 | 0.4369 | −0.6413 |
| m/z 2033 | 0.9860 | 2.2501 | 1 | 159 | 0.1356 | −0.6367 |
| m/z 2058 | 0.9870 | 2.1008 | 1 | 159 | 0.1492 | 0.9117 |
| m/z 2074 | 0.9999 | 0.0216 | 1 | 159 | 0.8834 | 0.2810 |
| m/z 2220 | 0.9975 | 0.3938 | 1 | 159 | 0.5312 | 0.9062 |
| m/z 2337 | 0.9992 | 0.1319 | 1 | 159 | 0.7169 | 0.9508 |
| m/z 2379 | 0.9991 | 0.1483 | 1 | 159 | 0.7006 | 1.0798 |
| m/z 2525 | 0.9998 | 0.0373 | 1 | 159 | 0.8472 | 0.2243 |
| m/z 2728 | 0.9846 | 2.4851 | 1 | 159 | 0.1169 | −1.4954 |
| m/z 2744 | 0.9989 | 0.1678 | 1 | 159 | 0.6826 | 1.0543 |
| m/z 2890 | 0.9963 | 0.5937 | 1 | 159 | 0.4421 | −1.1626 |
| m/z 3049 | 0.9998 | 0.0383 | 1 | 159 | 0.8451 | 0.4017 |
| m/z 3109 | 0.9938 | 0.9901 | 1 | 159 | 0.3212 | −0.5170 |
| m/z 3195 | 0.9996 | 0.0689 | 1 | 159 | 0.7933 | −0.3882 |
| m/z 3341 | 0.9956 | 0.7013 | 1 | 159 | 0.4036 | 0.1788 |
| m/z 3414 | 0.9986 | 0.2210 | 1 | 159 | 0.6389 | 0.6209 |
| m/z 3560 | 0.9991 | 0.1355 | 1 | 159 | 0.7133 | 0.1269 |
| m/z 3719 | 0.9904 | 1.5426 | 1 | 159 | 0.2161 | −1.1201 |
| m/z 3865 | 0.9962 | 0.6102 | 1 | 159 | 0.4359 | 0.4113 |
| constant term | 1.5761 | |||||
| Cutoff Value of N-glycan Score | ||||
| Diagnostic result | <0.8770 | ≥0.8770 | Predictive value (%) | |
| Non-ABMR | 157 | 24 | 86.74 | NPV 1 |
| ABMR | 3 | 13 | 81.25 | PPV 2 |
| Total | 160 | 37 | 86.29 | Accuracy |
| Preformed DSA status | ||||
| Diagnostic result | Negative | Positive | Predictive value (%) | |
| Non-ABMR | 174 | 7 | 96.13 | NPV 1 |
| ABMR | 7 | 9 | 56.25 | PPV 2 |
| Total | 160 | 16 | 92.89 | Accuracy |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noro, D.; Yoneyama, T.; Hatakeyama, S.; Tobisawa, Y.; Mori, K.; Hashimoto, Y.; Koie, T.; Tanaka, M.; Nishimura, S.-I.; Sasaki, H.; et al. Serum Aberrant N-Glycan Profile as a Marker Associated with Early Antibody-Mediated Rejection in Patients Receiving a Living Donor Kidney Transplant. Int. J. Mol. Sci. 2017, 18, 1731. https://doi.org/10.3390/ijms18081731
Noro D, Yoneyama T, Hatakeyama S, Tobisawa Y, Mori K, Hashimoto Y, Koie T, Tanaka M, Nishimura S-I, Sasaki H, et al. Serum Aberrant N-Glycan Profile as a Marker Associated with Early Antibody-Mediated Rejection in Patients Receiving a Living Donor Kidney Transplant. International Journal of Molecular Sciences. 2017; 18(8):1731. https://doi.org/10.3390/ijms18081731
Chicago/Turabian StyleNoro, Daisuke, Tohru Yoneyama, Shingo Hatakeyama, Yuki Tobisawa, Kazuyuki Mori, Yasuhiro Hashimoto, Takuya Koie, Masakazu Tanaka, Shin-Ichiro Nishimura, Hideo Sasaki, and et al. 2017. "Serum Aberrant N-Glycan Profile as a Marker Associated with Early Antibody-Mediated Rejection in Patients Receiving a Living Donor Kidney Transplant" International Journal of Molecular Sciences 18, no. 8: 1731. https://doi.org/10.3390/ijms18081731