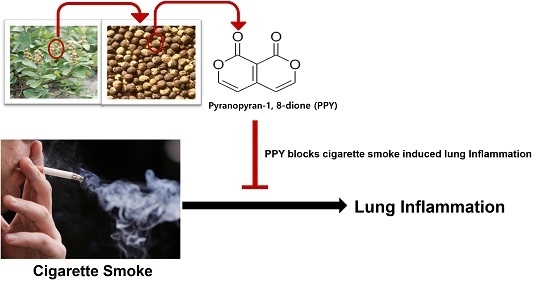
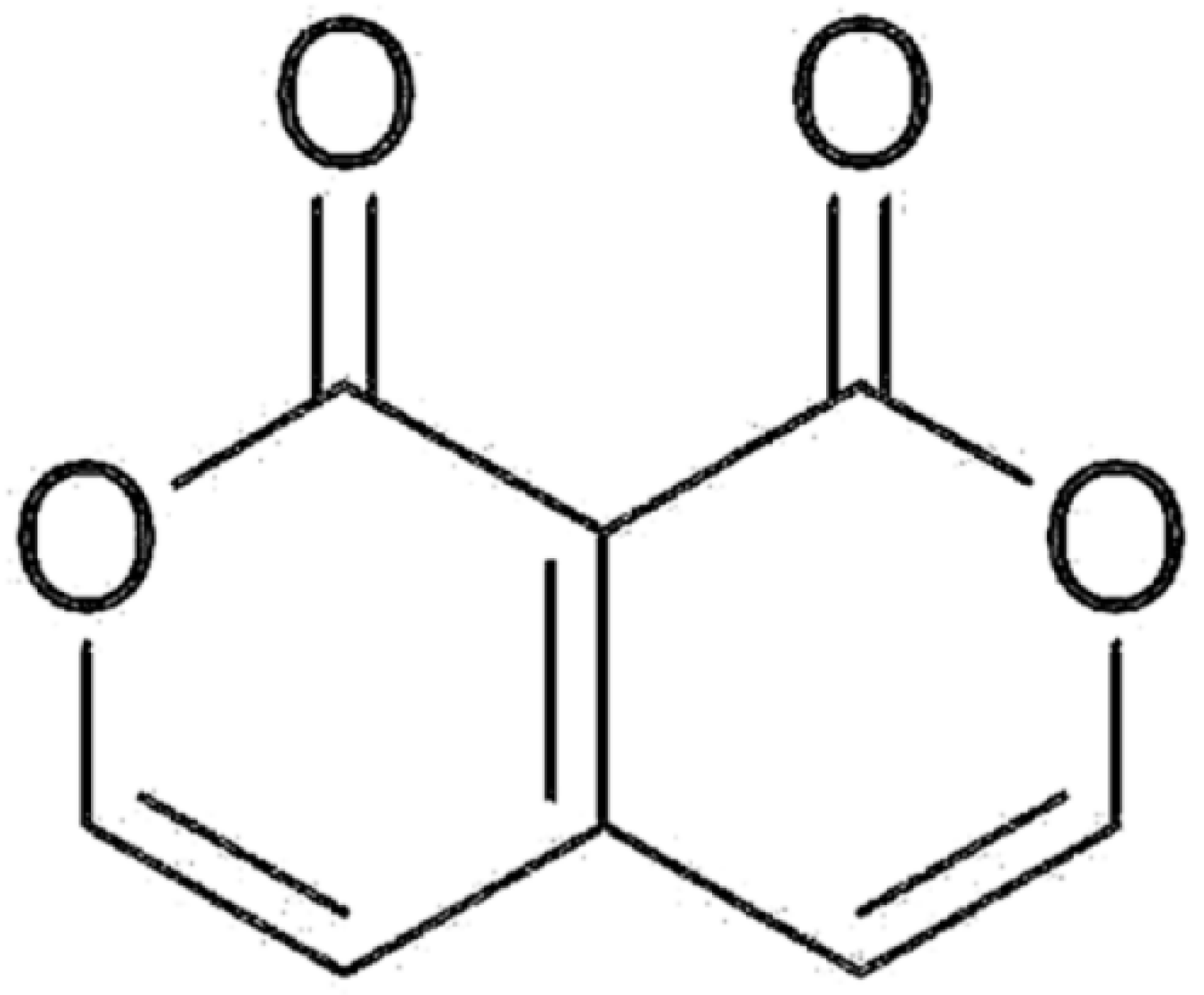
Pyranopyran-1,8-dione, an Active Compound from Vitices Fructus, Attenuates Cigarette-Smoke Induced Lung Inflammation in Mice
Abstract
:1. Introduction
2. Results
2.1. PPY Reduced Levels of Proinflammatory Cytokines and Chemokine KC in Bronchoalveolar Lavage (BAL) Fluid
2.2. PPY Inhibited the Recruitment of Inflammatory Cells into BAL Fluid
2.3. PPY Decreased the Morphological Change in Lung Tissue
2.4. Effect of PPY on Goblet Cell Hyperplasia
3. Discussion
4. Materials and Methods
4.1. Reagent
4.2. Animal
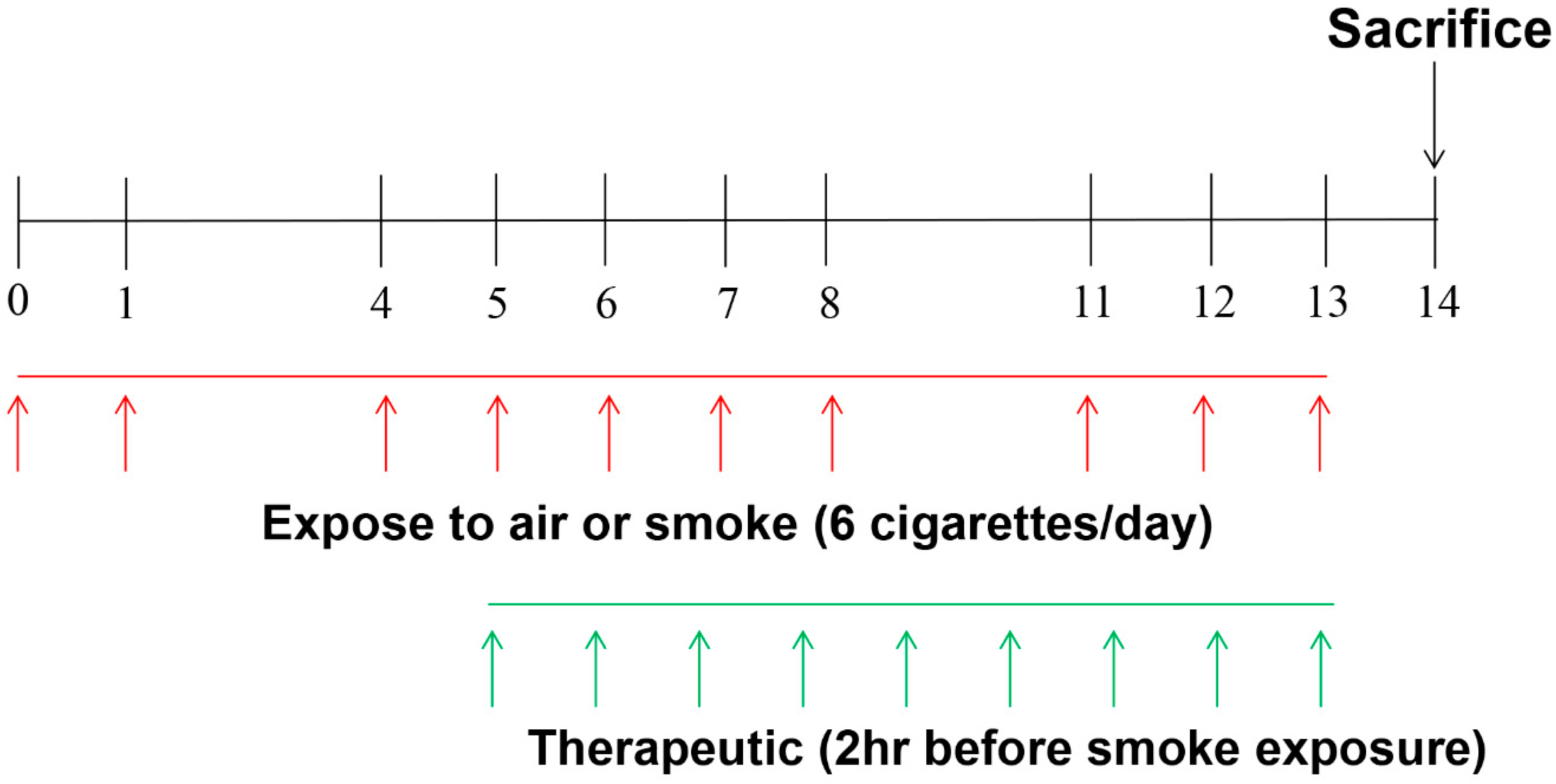
4.3. Exposure to CS and Animal Treatment
4.4. Analysis of Inflammatory Cell Profiles in BAL Fluid
4.5. ELISA Analysis of IL-6, TNF-α, and CXCL1/KC
4.6. Immunohistochemistry of Lungs
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rainsford, K.D. Anti-inflammatory drugs in the 21st century. Subcell. Biochem. 2007, 42, 3–27. [Google Scholar] [PubMed]
- D’Acquisto, F.; Maione, F.; Pederzoli-Ribeil, M. From il-15 to il-33: The never-ending list of new players in inflammation. Is it time to forget the humble aspirin and move ahead? Biochem. Pharmacol. 2010, 79, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Kang, M.; Cho, C.; Parvez, S.; Park, C.H.; Kim, D.; Oh, J.; Kim, H.; Shin, M.; Hong, M.; et al. Inhibition of nitric oxide and tumor necrosis factor-α by moutan cortex in activated mouse peritoneal macrophages. Biol. Pharm. Bull. 2007, 30, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.H.; Ko, E.; Oh, B.G.; Kim, S.H.; Kim, Y.; Shin, M.; Hong, M.; Bae, H. Inhibition effects of vitex rotundifolia on inflammatory gene expression in a549 human epithelial cells. Ann. Allergy Asthma Immunol. 2009, 103, 152–159. [Google Scholar] [CrossRef]
- Sohn, S.H.; Ko, E.; Oh, B.G.; Kim, J.; Choi, E.; Kim, S.H.; Kim, Y.; Shin, M.; Hong, M.; Bae, H. Global gene analysis of erigeron canadensis-treated TNF-α-, IL-4- and IL-1β-stimulated A549 human epithelial cells. Ann. Nutr. Metab. 2009, 54, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.H.; Ko, E.; Kim, Y.; Shin, M.; Hong, M.; Bae, H. Genomewide expression profile of forsythia suspensa on lipopolysaccaride-induced activation in microglial cells. Mol. Cell Toxicol. 2008, 4, 113–123. [Google Scholar]
- Hu, Y.; Hou, T.T.; Xin, H.L.; Zhang, Q.Y.; Zheng, H.C.; Rahman, K.; Qin, L.P. Estrogen-like activity of volatile components from Vitex rotundifolia L. Indian J. Med. Res. 2007, 126, 68–72. [Google Scholar] [PubMed]
- Okuyama, E.; Fujimori, S.; Yamazaki, M.; Deyama, T. Pharmacologically active components of viticis fructus (Vitex rotundifolia). Ii. The components having analgesic effects. Chem. Pharm. Bull. (Tokyo) 1998, 46, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.Y.; Kim, S.H.; Lim, J.P.; Suh, E.S.; Jeong, H.J.; Kim, B.D.; Park, E.J.; Hwang, W.J.; Rye, D.G.; Baek, S.H.; et al. Effect of Vitex rotundifolia on immediate-type allergic reaction. J. Ethnopharmacol. 2000, 72, 443–450. [Google Scholar] [CrossRef]
- Bao, M.J.; Shen, J.; Jia, Y.L.; Li, F.F.; Ma, W.J.; Shen, H.J.; Shen, L.L.; Lin, X.X.; Zhang, L.H.; Dong, X.W.; et al. Apple polyphenol protects against cigarette smoke-induced acute lung injury. Nutrition 2012, 29, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Han, A.R.; Kim, Y.; Choi, S.H.; Ko, E.; Lee, N.Y.; Jeong, J.H.; Kim, S.H.; Bae, H. A new compound, 1H,8H-Pyrano[3,4-C]Pyran-1,8-Dione, suppresses airway epithelial cell inflammatory responses in a murine model of asthma. Int. J. Immunopathol. Pharmacol. 2009, 22, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Li, Y.; Li, M.; Liu, X.; McSharry, C.; Xu, D. Anti-interleukin-33 inhibits cigarette smoke-induced lung inflammation in mice. Immunology 2013, 138, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Siafakas, N.M.; Tzortzaki, E.G. Few smokers develop copd. Why? Respir. Med. 2002, 96, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Cavarra, E.; Bartalesi, B.; Lucattelli, M.; Fineschi, S.; Lunghi, B.; Gambelli, F.; Ortiz, L.A.; Martorana, P.A.; Lungarella, G. Effects of cigarette smoke in mice with different levels of α(1)-proteinase inhibitor and sensitivity to oxidants. Am. J. Respir. Crit. Care Med. 2001, 164, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.R.; Davies, R.J.; Devalia, J.L. Airway epithelial cells, cytokines, and pollutants. Am. J. Respir. Crit. Care Med. 1999, 160, S38–S43. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.; Capelli, A.; Lusuardi, M.; Balbo, P.; Vecchio, C.; Maestrelli, P.; Mapp, C.E.; Fabbri, L.M.; Donner, C.F.; Saetta, M. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am. J. Respir. Crit. Care Med. 1998, 158, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Mio, T.; Romberger, D.J.; Thompson, A.B.; Robbins, R.A.; Heires, A.; Rennard, S.I. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am. J. Respir. Crit. Care Med. 1997, 155, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Witherden, I.R.; Vanden Bon, E.J.; Goldstraw, P.; Ratcliffe, C.; Pastorino, U.; Tetley, T.D. Primary human alveolar type II epithelial cell chemokine release: Effects of cigarette smoke and neutrophil elastase. Am. J. Respir. Cell Mol. Biol. 2004, 30, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Murugan, V.; Peak, M.J. Signal transduction pathways linking the actication of alveolar macrophages with the recruitment of neutrophils to lungs in chronic obstructive pulmonary disease. Exp. Lung Res. 2009, 35, 439–485. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.B.; Foster, D.C.; Mathewes, S.L.; Osborn, S.G.; Kuijper, J.L.; Forstrom, J.W.; Martin, T.R. Molecular cloning of porcine alveolar macrophage-derived neutrophil chemotactic factors I and II; identification of porcine IL-8 and another intercrine-α protein. Biochemistry 1992, 31, 10483–10490. [Google Scholar] [CrossRef] [PubMed]
- Huvenne, W.; Perez-Novo, C.A.; Derycke, L.; De Ruyck, N.; Krysko, O.; Maes, T.; Pauwels, N.; Robays, L.; Bracke, K.R.; Joos, G.; et al. Different regulation of cigarette smoke induced inflammation in upper versus lower airways. Respir. Res. 2010, 11, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichinose, M. Differences of inflammatory mechanisms in asthma and copd. Allergol. Int. 2009, 58, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Churg, A.; Wright, J.L. Animal models of cigarette smoke-induced chronic obstructive lung disease. Contrib. Microbiol. 2007, 14, 113–125. [Google Scholar] [PubMed]
- Drost, E.M.; MacNee, W. Potential role of il-8, platelet-activating factor and TNF-α in the sequestration of neutrophils in the lung: Effects on neutrophil deformability, adhesion receptor expression, and chemotaxis. Eur. J. Immunol. 2002, 32, 393–403. [Google Scholar] [CrossRef]
- Rozema, E.; Atanasov, A.G.; Fakhrudin, N.; Singhuber, J.; Namduang, U.; Heiss, E.H.; Reznicek, G.; Huck, C.W.; Bonn, G.K.; Dirsch, V.M.; et al. Selected extracts of chinese herbal medicines: Their effect on NF-κB, PPARα and PPARγ and the respective bioactive compounds. Evid. Based Complement. Altern. Med. 2012, 2012, 983023. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of copd. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef] [PubMed]
- Calverley, P.M.; Rabe, K.F.; Goehring, U.M.; Kristiansen, S.; Fabbri, L.M.; Martinez, F.J. M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: Two randomised clinical trials. Lancet 2009, 374, 685–694. [Google Scholar] [CrossRef]
- Rabe, K.F.; Bateman, E.D.; O’Donnell, D.; Witte, S.; Bredenbroker, D.; Bethke, T.D. Roflumilast—An oral anti-inflammatory treatment for chronic obstructive pulmonary disease: A randomised controlled trial. Lancet 2005, 366, 563–571. [Google Scholar] [CrossRef]
- Page, C.P.; Spina, D. Phosphodiesterase inhibitors in the treatment of inflammatory diseases. Handb. Exp. Pharmacol. 2011, 391–414. [Google Scholar]
- Blanc, P.D.; Trupin, L.; Earnest, G.; Katz, P.P.; Yelin, E.H.; Eisner, M.D. Alternative therapies among adults with a reported diagnosis of asthma or rhinosinusitis: Data from a population-based survey. Chest 2001, 120, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.E.; Arnold, E.; Lasserson, T.J.; Wu, T. Herbal interventions for chronic asthma in adults and children: A systematic review and meta-analysis. Prim. Care Respir. J. 2010, 19, 307–314. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Ioannides-Demos, L.L.; Santamaria, N.M.; Kong, D.C.; Stewart, K. Use of complementary and alternative medicines by patients with chronic obstructive pulmonary disease. Med. J. Aust. 2004, 181, 248–251. [Google Scholar] [PubMed]
- Maione, F.; Russo, R.; Khan, H.; Mascolo, N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2016, 30, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Khan, R.; Raza, M.; Khan, H.; Pervez, S.; de Feo, V.; Maione, F.; Mascolo, N. Suppression of inflammatory response by chrysin, a flavone isolated from potentilla evestita th. Wolf. In silico predictive study on its mechanistic effect. Fitoterapia 2015, 103, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Hayden, M.S. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Schiekofer, S.; Schwaninger, M.; Andrassy, M.; Humpert, P.M.; Chen, J.; Hong, M.; Luther, T.; Henle, T.; Kloting, I.; et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-κb. Diabetes 2001, 50, 2792–2808. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.L.; Cosio, M.; Churg, A. Animal models of chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L1–L15. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Hallman, A.H.; Roberts, W.J.; Wagner, P.D.; Hogan, M.C. Superoxide release from contracting skeletal muscle in pulmonary TNF-α overexpression mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R75–R81. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Lucas, K.; Fortuna, C.A.; Chuang, C.C.; Best, T.M. Molecular regulation of toll-like receptors in asthma and copd. Front. Physiol. 2015, 6, 312. [Google Scholar] [CrossRef] [PubMed]
- Lore, N.I.; Bragonzi, A.; Cigana, C. The IL-17A/IL-17RA axis in pulmonary defence and immunopathology. Cytokine Growth Factor Rev. 2016, 30, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.R.; Siegel, L.; Leith, A.; Mohn, D.; Escobar, S.; Wannberg, S.; Misura, K.; Rickel, E.; Rottman, J.B.; Comeau, M.R.; et al. IL-17RA signaling in airway inflammation and bronchial hyperreactivity in allergic asthma. Am. J. Respir. Cell Mol. Biol. 2015, 53, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Haam, K.K.; Park, S.; Kim, Y.; Lee, S.R.; Lee, G.; Kim, M.; Hong, M.; Shin, M.; Jung, S.; et al. The standardized herbal formula, PM014, ameliorated cigarette smoke-induced lung inflammation in a murine model of chronic obstructive pulmonary disease. BMC Complement. Altern. Med. 2013, 13, 219. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.; Jung, K.-H.; Ji, E.S.; Bae, H. Pyranopyran-1,8-dione, an Active Compound from Vitices Fructus, Attenuates Cigarette-Smoke Induced Lung Inflammation in Mice. Int. J. Mol. Sci. 2017, 18, 1602. https://doi.org/10.3390/ijms18071602
Lee G, Jung K-H, Ji ES, Bae H. Pyranopyran-1,8-dione, an Active Compound from Vitices Fructus, Attenuates Cigarette-Smoke Induced Lung Inflammation in Mice. International Journal of Molecular Sciences. 2017; 18(7):1602. https://doi.org/10.3390/ijms18071602
Chicago/Turabian StyleLee, Gihyun, Kyung-Hwa Jung, Eun Seok Ji, and Hyunsu Bae. 2017. "Pyranopyran-1,8-dione, an Active Compound from Vitices Fructus, Attenuates Cigarette-Smoke Induced Lung Inflammation in Mice" International Journal of Molecular Sciences 18, no. 7: 1602. https://doi.org/10.3390/ijms18071602