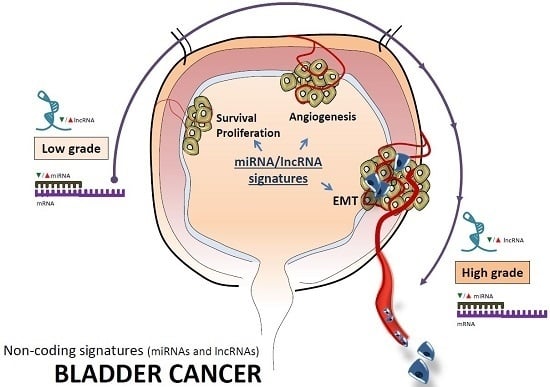
Understanding the Role of Non-Coding RNAs in Bladder Cancer: From Dark Matter to Valuable Therapeutic Targets
Abstract
:1. Introduction
2. Non-Coding RNAs
2.1. Micro-RNAs
2.2. Long Non-Coding RNAs
2.3. Cell Free microRNAs
2.4. Mitochondrial miRNAs (mitoMiRs)
2.5. The Role of Non-Coding RNAs in Warburg Effect
3. Challenges and Future Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BC | Bladder cancer |
| MIBC | Muscle invasive bladder cancer |
| NMIBC | Non-muscle invasive bladder cancer |
| mitoMir | Mitochondrial microRNA |
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, B.W.; Burger, M.; Lotan, Y.; Solsona, E.; Stief, C.G.; Sylvester, R.J.; Witjes, J.A.; Zlotta, A.R. Recurrence and progression of disease in non-muscle-invasive bladder cancer: From epidemiology to treatment strategy. Eur. Urol. 2009, 56, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Drayton, R.M.; Catto, J.W. Molecular mechanisms of cisplatin resistance in bladder cancer. Expert Rev. Anticancer Ther. 2012, 12, 271–281. [Google Scholar] [CrossRef]
- Carballido, E.M.; Rosenberg, J.E. Optimal treatment for metastatic bladder cancer. Curr. Oncol. Rep. 2014, 16, 404. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Lee, C.T.; Montie, J.E. Bladder cancer in 2010: How far have we come? CA Cancer J. Clin. 2010, 60, 244–272. [Google Scholar] [CrossRef] [PubMed]
- Roupret, M.; Hupertan, V.; Yates, D.R.; Comperat, E.; Catto, J.W.; Meuth, M.; Lackmichi, A.; Ricci, S.; Lacave, R.; Gattegno, B.; et al. A comparison of the performance of microsatellite and methylation urine analysis for predicting the recurrence of urothelial cell carcinoma, and definition of a set of markers by Bayesian network analysis. BJU Int. 2008, 101, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Wang, L.; Castillo-Martin, M.; McBride, R.; Galsky, M.D.; Zhu, J.; Boffetta, P.; Zhang, D.Y.; Cordon-Cardo, C. Biomarkers for bladder cancer management: Present and future. Am. J. Clin. Exp. Urol. 2014, 2, 1–14. [Google Scholar] [PubMed]
- Massari, F.; Ciccarese, C.; Santoni, M.; Iacovelli, R.; Mazzucchelli, R.; Piva, F.; Scarpelli, M.; Berardi, R.; Tortora, G.; Lopez-Beltran, A.; et al. Metabolic phenotype of bladder cancer. Cancer Treat. Rev. 2016, 45, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef]
- Gibb, E.A.; Brown, C.J.; Lam, W.L. The functional role of long non-coding RNA in human carcinomas. Mol. Cancer 2011, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Taft, R.J.; Pang, K.C.; Mercer, T.R.; Dinger, M.; Mattick, J.S. Non-coding RNAs: Regulators of disease. J. Pathol. 2010, 220, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Shih, I.H.; Jones-Rhoades, M.W.; Bartel, D.P.; Burge, C.B. Prediction of mammalian microRNA targets. Cell 2003, 115, 787–798. [Google Scholar] [CrossRef]
- Martens-Uzunova, E.S.; Bottcher, R.; Croce, C.M.; Jenster, G.; Visakorpi, T.; Calin, G.A. Long noncoding RNA in prostate, bladder, and kidney cancer. Eur. Urol. 2014, 65, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Farazi, T.A.; Hoell, J.I.; Morozov, P.; Tuschl, T. MicroRNAs in human cancer. Adv. Exp. Med. Biol. 2013, 774, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Berindan-Neagoe, I.; Monroig Pdel, C.; Pasculli, B.; Calin, G.A. Micrornaome genome: A treasure for cancer diagnosis and therapy. CA Cancer J. Clin. 2014, 64, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.W.; Ferland-McCollough, D.; Jackson, T.J.; Bushell, M. microRNAs in cancer management. Lancet Oncol. 2012, 13, e249–e258. [Google Scholar] [CrossRef]
- Fevrier, B.; Raposo, G. Exosomes: Endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 2004, 16, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Tomuleasa, C.; Monroig, P.; Cucuianu, A.; Berindan-Neagoe, I.; Calin, G.A. Exosomes as divine messengers: Are they the hermes of modern molecular oncology? Cell Death Differentiation 2015, 22, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Berindan-Neagoe, I.; Calin, G.A. Molecular pathways: microRNAs, cancer cells, and microenvironment. Clin. Cancer Res. 2014, 20, 6247–6253. [Google Scholar] [CrossRef] [PubMed]
- Itesako, T.; Seki, N.; Yoshino, H.; Chiyomaru, T.; Yamasaki, T.; Hidaka, H.; Yonezawa, T.; Nohata, N.; Kinoshita, T.; Nakagawa, M.; et al. The microRNA expression signature of bladder cancer by deep sequencing: The functional significance of the miR-195/497 cluster. PLoS ONE 2014, 9, e84311. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chen, J.; Zhao, X.; Liang, C.; Wang, Y.; Sun, L.; Jiang, Z.; Zhang, Z.; Yang, R.; Chen, J.; et al. MicroRNA expression signatures of bladder cancer revealed by deep sequencing. PLoS ONE 2011, 6, e18286. [Google Scholar] [CrossRef] [PubMed]
- Canturk, K.M.; Ozdemir, M.; Can, C.; Oner, S.; Emre, R.; Aslan, H.; Cilingir, O.; Ciftci, E.; Celayir, F.M.; Aldemir, O.; et al. Investigation of key miRNAs and target genes in bladder cancer using miRNA profiling and bioinformatic tools. Mol. Biol. Rep. 2014, 41, 8127–8135. [Google Scholar] [CrossRef] [PubMed]
- Ratert, N.; Meyer, H.A.; Jung, M.; Lioudmer, P.; Mollenkopf, H.J.; Wagner, I.; Miller, K.; Kilic, E.; Erbersdobler, A.; Weikert, S.; et al. miRNA profiling identifies candidate mirnas for bladder cancer diagnosis and clinical outcome. J. Mol. Diagn. 2013, 15, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Dyrskjot, L.; Ostenfeld, M.S.; Bramsen, J.B.; Silahtaroglu, A.N.; Lamy, P.; Ramanathan, R.; Fristrup, N.; Jensen, J.L.; Andersen, C.L.; Zieger, K.; et al. Genomic profiling of microRNAs in bladder cancer: miR-129 is associated with poor outcome and promotes cell death in vitro. Cancer Res. 2009, 69, 4851–4860. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M.; Liang, G.; Liu, C.C.; Wolff, E.M.; Tsai, Y.C.; Ye, W.; Zhou, X.; Jones, P.A. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009, 69, 2623–2629. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Dong, W.; Huang, J.; Pan, Q.; Fan, X.; Zhang, C.; Huang, L. MicroRNA-143 as a tumor suppressor for bladder cancer. J. Urol. 2009, 181, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Kamalapuram, S.K.; Kanwar, R.K. Survivin signaling in clinical oncology: A multifaceted dragon. Med. Res. Rev. 2013, 33, 765–789. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, E.D.; Bramsen, J.B.; Hulf, T.; Dyrskjot, L.; Ramanathan, R.; Hansen, T.B.; Villadsen, S.B.; Gao, S.; Ostenfeld, M.S.; Borre, M.; et al. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int. J. Cancer 2011, 128, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Gaur, A.B.; Lengyel, E.; Peter, M.E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008, 22, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, R.; Seki, N.; Chiyomaru, T.; Inoguchi, S.; Ishihara, T.; Goto, Y.; Nishikawa, R.; Mataki, H.; Tatarano, S.; Itesako, T.; et al. Tumour-suppressive microRNA-144–5p directly targets CCNE1/2 as potential prognostic markers in bladder cancer. Br. J. Cancer 2015, 113, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, B.; Hui, K.; Zeng, J.; Fan, J.; Wang, X.; Hsieh, J.T.; He, D.; Wu, K. miR-92b targets DAB2IP to promote EMT in bladder cancer migration and invasion. Oncol. Rep. 2016, 36, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Majid, S.; Dar, A.A.; Saini, S.; Deng, G.; Chang, I.; Greene, K.; Tanaka, Y.; Dahiya, R.; Yamamura, S. MicroRNA-23b functions as a tumor suppressor by regulating Zeb1 in bladder cancer. PLoS ONE 2013, 8, e67686. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, S.; Lin, Y.; Chen, H.; Hu, Z.; Mao, Y.; Xu, X.; Wu, J.; Zhu, Y.; Zheng, X.; et al. MicroRNA-124–3p inhibits cell migration and invasion in bladder cancer cells by targeting ROCK1. J. Trans. Med. 2013, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, S.; Hu, L.; Liu, F.; Zhang, Q.; Zhang, D. miR-199a-5p suppresses human bladder cancer cell metastasis by targeting CCR7. BMC Urol. 2016, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Lu, Q.; Wu, D.; Li, P.; Xu, B.; Qing, W.; Wang, M.; Zhang, Z.; Zhang, W. MicroRNA-21 modulates cell proliferation and sensitivity to doxorubicin in bladder cancer cells. Oncol. Rep. 2011, 25, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.N.; Wang, K.F.; Xu, Z.Q.; Li, S.J.; Liu, Q.; Fu, D.H.; Wang, X.; Wu, B. MiR-133b regulates bladder cancer cell proliferation and apoptosis by targeting Bcl-w and Akt1. Cancer Cell Int. 2014, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, G.; Cao, G.; Chen, X.; Huang, J.; Jiang, X.; Hou, J. MicroRNA335 inhibits bladder cancer cell growth and migration by targeting mitogenactivated protein kinase 1. Mol. Med. Rep. 2016, 14, 1765–1770. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Cojocneanu-Petric, R.; Chira, S.; Truta, A.; Floares, A.; Petrut, B.; Achimas-Cadariu, P.; Berindan-Neagoe, I. Clinical and pathological implications of mirna in bladder cancer. Int. J. Nanomed. 2015, 10, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Shang, C.; Zhang, H.; Guo, Y.; Tong, X. Up-regulation of miR-9 target CBX7 to regulate invasion ability of bladder transitional cell carcinoma. Med. Sci. Monit. 2015, 21, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, W.; Zuo, Y.; Ding, M.; Ke, C.; Yan, R.; Zhan, H.; Liu, J.; Wang, J. miR-9 promotes cell proliferation and inhibits apoptosis by targeting LASS2 in bladder cancer. Tumour Biol. 2015, 36, 9631–9640. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, J.; Kang, Y.; He, Y.; Liang, B.; Yang, P.; Yu, Z. miR-19a acts as an oncogenic microRNA and is up-regulated in bladder cancer. J. Exp. Clin. Cancer Res. 2014, 33, 67. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Jia, Z.; Dou, Z. MiR-24–3p regulates bladder cancer cell proliferation, migration, invasion and autophagy by targeting DEDD. Oncol. Rep. 2017, 37, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ke, C.; Ma, X.; Zhao, Q.; Yang, M.; Zhang, W.; Wang, J. MicroRNA-92 promotes invasion and chemoresistance by targeting GSK3β and activating Wnt signaling in bladder cancer cells. Tumour Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, K.; Wang, Y.; Xu, Z.; Meng, J.; Gu, S. Upregulation of microRNA-96 and its oncogenic functions by targeting CDKN1A in bladder cancer. Cancer Cell Int. 2015, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Egawa, H.; Jingushi, K.; Hirono, T.; Ueda, Y.; Kitae, K.; Nakata, W.; Fujita, K.; Uemura, M.; Nonomura, N.; Tsujikawa, K. The miR-130 family promotes cell migration and invasion in bladder cancer through FAK and Akt phosphorylation by regulating PTEN. Sci. Rep. 2016, 6, 20574. [Google Scholar] [CrossRef]
- Mao, X.P.; Zhang, L.S.; Huang, B.; Zhou, S.Y.; Liao, J.; Chen, L.W.; Qiu, S.P.; Chen, J.X. Mir-135a enhances cellular proliferation through post-transcriptionally regulating PHLPP2 and FOXO1 in human bladder cancer. J. Trans. Med. 2015, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Y.; Liu, Z.; Xia, S.; Jin, C.; Yin, H.; Zhao, W.; Wu, Q. MicroRNA-137 upregulation increases bladder cancer cell proliferation and invasion by targeting PAQR3. PLoS ONE 2014, 9, e109734. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, M.; Liang, H.; Guo, S.; Guo, X.; Yuan, M.; Lian, H.; Yan, X.; Zhang, S.; Chen, X.; et al. miR-138–5p contributes to cell proliferation and invasion by targeting Survivin in bladder cancer cells. Mol. Cancer 2016, 15, 82. [Google Scholar] [CrossRef]
- Lei, Y.; Hu, X.; Li, B.; Peng, M.; Tong, S.; Zu, X.; Wang, Z.; Qi, L.; Chen, M. miR-150 modulates cisplatin chemosensitivity and invasiveness of muscle-invasive bladder cancer cells via targeting PDCD4 in vitro. Med. Sci. Monit. 2014, 20, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Dong, W.; Lin, T.X.; Zhong, G.Z.; Liao, B.; Wang, B.; Gu, P.; Huang, L.; Xie, Y.; Lu, F.D.; et al. MicroRNA-155 promotes bladder cancer growth by repressing the tumor suppressor DMTF1. Oncotarget 2015, 6, 16043–16058. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Ueno, K.; Shahryari, V.; Tanaka, Y.; Tabatabai, Z.L.; Hinoda, Y.; Dahiya, R. Oncogenic miRNA-182–5p targets Smad4 and RECK in human bladder cancer. PLoS ONE 2012, 7, e51056. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Lv, L.; Li, Y.; Zhang, C.; Meng, F.; Pu, Y.; Xiao, J.; Qian, L.; Zhao, W.; Liu, Q.; et al. miR-193a-3p regulates the multi-drug resistance of bladder cancer by targeting the LOXL4 gene and the oxidative stress pathway. Mol. Cancer 2014, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Adam, L.; Zhong, M.; Choi, W.; Qi, W.; Nicoloso, M.; Arora, A.; Calin, G.; Wang, H.; Siefker-Radtke, A.; McConkey, D.; et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin. Cancer Res. 2009, 15, 5060–5072. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.P.; Hu, Z.M.; Li, K.; Xia, K. miR-222 attenuates cisplatin-induced cell death by targeting the PPP2R2A/Akt/mTOR Axis in bladder cancer cells. J. Cell. Mol. Med. 2016, 20, 559–567. [Google Scholar] [CrossRef]
- Tan, M.; Mu, X.; Liu, Z.; Tao, L.; Wang, J.; Ge, J.; Qiu, J. microRNA-495 promotes bladder cancer cell growth and invasion by targeting phosphatase and tensin homolog. Biochem. Biophys. Res. Commun. 2017, 483, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Chiyomaru, T.; Enokida, H.; Kawakami, K.; Tatarano, S.; Uchida, Y.; Kawahara, K.; Nishiyama, K.; Seki, N.; Nakagawa, M. Functional role of LASP1 in cell viability and its regulation by microRNAs in bladder cancer. Urol. Oncol. 2012, 30, 434–443. [Google Scholar] [CrossRef]
- Yoshino, H.; Chiyomaru, T.; Enokida, H.; Kawakami, K.; Tatarano, S.; Nishiyama, K.; Nohata, N.; Seki, N.; Nakagawa, M. The tumour-suppressive function of miR-1 and miR-133a targeting TAGLN2 in bladder cancer. Br. J. Cancer 2011, 104, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Enokida, H.; Chiyomaru, T.; Tatarano, S.; Hidaka, H.; Yamasaki, T.; Gotannda, T.; Tachiwada, T.; Nohata, N.; Yamane, T.; et al. Tumor suppressive microRNA-1 mediated novel apoptosis pathways through direct inhibition of splicing factor serine/arginine-rich 9 (SRSF9/SRp30c) in bladder cancer. Biochem. Biophys. Res. Commun. 2012, 417, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Yoshino, H.; Enokida, H.; Hidaka, H.; Chiyomaru, T.; Nohata, N.; Kinoshita, T.; Fuse, M.; Seki, N.; Nakagawa, M. Novel molecular targets regulated by tumor suppressors microRNA-1 and microRNA-133a in bladder cancer. Int. J. Oncol. 2012, 40, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yuan, J.; Feng, N.; Li, Y.; Lin, Z.; Jiang, Z.; Gui, Y. Hsa-miR-1 downregulates long non-coding RNA urothelial cancer associated 1 in bladder cancer. Tumour Biol. 2014, 35, 10075–10084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, C.; Liu, W.; Zheng, W.; Zhang, Y.; Wang, S.; Huang, D.; Liu, X.; Bai, Z. MicroRNA-24 upregulation inhibits proliferation, metastasis and induces apoptosis in bladder cancer cells by targeting CARMA3. Int. J. Oncol. 2015, 47, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, S.; Seki, N.; Chiyomaru, T.; Ishihara, T.; Matsushita, R.; Mataki, H.; Itesako, T.; Tatarano, S.; Yoshino, H.; Goto, Y.; et al. Tumour-suppressive microRNA-24–1 inhibits cancer cell proliferation through targeting FOXM1 in bladder cancer. FEBS Lett. 2014, 588, 3170–3179. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, H.; Hu, Z.; Mao, Y.; Xu, X.; Zhu, Y.; Xu, X.; Wu, J.; Li, S.; Mao, Q.; et al. MiR-26a inhibits proliferation and motility in bladder cancer by targeting HMGA1. FEBS Lett. 2013, 587, 2467–2473. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Seki, N.; Matsushita, R.; Yonemori, M.; Yoshino, H.; Nakagawa, M.; Enokida, H. Tumour-suppressive miRNA-26a-5p and miR-26b-5p inhibit cell aggressiveness by regulating PLOD2 in bladder cancer. Br. J. Cancer 2016, 115, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Drayton, R.M.; Dudziec, E.; Peter, S.; Bertz, S.; Hartmann, A.; Bryant, H.E.; Catto, J.W. Reduced expression of miRNA-27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin. Cancer Res. 2014, 20, 1990–2000. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Bai, H.; Hu, H. rs11671784 G/A Variation in miR-27a decreases chemo-sensitivity of bladder cancer by decreasing miR-27a and increasing the target RUNX-1 expression. Biochem. Biophys. Res. Commun. 2015, 458, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.D.; Wu, X.H.; Fan, Y.R.; Tan, B.; Quan, Z.; Luo, C.L. Exosome-derived microRNA-29c induces apoptosis of BIU-87 cells by down regulating BCL-2 and MCL-1. Asian Pac. J. Cancer Prev. 2014, 15, 3471–3476. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Huang, S.; Wan, X.; Luo, H.; Wu, D. MiRNA-29c regulates cell growth and invasion by targeting CDK6 in bladder cancer. Am. J. Trans. Res. 2015, 7, 1382–1389. [Google Scholar]
- Xu, T.; Qin, L.; Zhu, Z.; Wang, X.; Liu, Y.; Fan, Y.; Zhong, S.; Wang, X.; Zhang, X.; Xia, L.; et al. MicroRNA-31 functions as a tumor suppressor and increases sensitivity to mitomycin-C in urothelial bladder cancer by targeting integrin α5. Oncotarget 2016, 7, 27445–27457. [Google Scholar] [CrossRef] [PubMed]
- Vinall, R.L.; Ripoll, A.Z.; Wang, S.; Pan, C.X.; deVere White, R.W. MiR-34a chemosensitizes bladder cancer cells to cisplatin treatment regardless of p53-Rb pathway status. Int. J. Cancer 2012, 130, 2526–2538. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yao, W.; Xiao, W.; Li, H.; Xu, H.; Lang, B. MicroRNA-34a functions as an anti-metastatic microRNA and suppresses angiogenesis in bladder cancer by directly targeting CD44. J. Exp. Clin. Cancer Res. 2014, 33, 779. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tian, J.; Xian, W.; Xie, T.; Yang, X. miR-34a inhibits proliferation and invasion of bladder cancer cells by targeting orphan nuclear receptor HNF4G. Dis. Markers 2015, 2015, 879254. [Google Scholar] [CrossRef] [PubMed]
- Catto, J.W.; Miah, S.; Owen, H.C.; Bryant, H.; Myers, K.; Dudziec, E.; Larre, S.; Milo, M.; Rehman, I.; Rosario, D.J.; et al. Distinct microRNA alterations characterize high- and low-grade bladder cancer. Cancer Res. 2009, 69, 8472–8481. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhou, Y.; Pan, H.; Zhou, J.; Fan, Y.; Qu, P. MicroRNA-99a inhibiting cell proliferation, migration and invasion by targeting fibroblast growth factor receptor 3 in bladder cancer. Oncol. Lett. 2014, 7, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zeng, Q.; Xu, W.; Jiao, L.; Chen, Y.; Zhang, Z.; Wu, C.; Jin, T.; Pan, A.; Wei, R.; et al. MiRNA-100 inhibits human bladder urothelial carcinogenesis by directly targeting mTOR. Mol. Cancer Ther. 2013, 12, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Fang, Y.; Cao, Y.; Chen, Q.; Liu, Y. Enforced expression of miR-101 enhances cisplatin sensitivity in human bladder cancer cells by modulating the cyclooxygenase-2 pathway. Mol. Med. Rep. 2014, 10, 2203–2209. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Li, B.; Tong, S.; Qi, L.; Hu, X.; Cui, Y.; Li, Z.; He, W.; Zu, X.; Wang, Z.; et al. MiR-101 suppresses vascular endothelial growth factor C that inhibits migration and invasion and enhances cisplatin chemosensitivity of bladder cancer cells. PLoS ONE 2015, 10, e0117809. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Wu, Z.; Yang, X.; Chen, L.; Han, Z.; Zhang, Y.; Liu, J.; Liu, W.; Liu, X. MicroRNA-101 inhibits the proliferation and invasion of bladder cancer cells via targeting c-FOS. Mol. Med. Rep. 2016, 14, 2651–2656. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Park, S.S.; Hwang, B.; Kim, W.T.; Choi, Y.H.; Kim, W.J.; Moon, S.K. MicroRNA-106a suppresses proliferation, migration, and invasion of bladder cancer cells by modulating MAPK signaling, cell cycle regulators, and Ets-1-mediated MMP-2 expression. Oncol. Rep. 2016, 36, 2421–2429. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, Q.F.; Liu, X.Q.; Guo, Z.J.; Li, C.Y.; Sun, G. MiR-122 targets VEGFC in bladder cancer to inhibit tumor growth and angiogenesis. Am. J. Trans. Res. 2016, 8, 3056–3066. [Google Scholar]
- Zhang, T.; Wang, J.; Zhai, X.; Li, H.; Li, C.; Chang, J. MiR-124 retards bladder cancer growth by directly targeting CDK4. Acta Biochim. Biophys. Sinica 2014, 46, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Q.; Xu, B.; Wang, P.; Fan, W.; Cai, Y.; Gu, X.; Meng, F. MiR-124 exerts tumor suppressive functions on the cell proliferation, motility and angiogenesis of bladder cancer by fine-tuning UHRF1. FEBS J. 2015, 282, 4376–4388. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Luo, J.; Cai, Q.; Pan, Q.; Zeng, H.; Guo, Z.; Dong, W.; Huang, J.; Lin, T. MicroRNA-125b suppresses the development of bladder cancer by targeting E2F3. Int. J. Cancer 2011, 128, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ding, J.; Wang, L.; Pan, H.; Zhou, Z.; Zhou, J.; Qu, P. MicroRNA-125b inhibits cell migration and invasion by targeting matrix metallopeptidase 13 in bladder cancer. Oncol. Lett. 2013, 5, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, W.; Li, J.; Huang, S.; Wan, X.; Luo, H.; Wu, D. MiRNA-125b inhibits proliferation and migration by targeting SphK1 in bladder cancer. Am. J. Trans. Res. 2015, 7, 2346–2354. [Google Scholar]
- Jia, A.Y.; Castillo-Martin, M.; Bonal, D.M.; Sanchez-Carbayo, M.; Silva, J.M.; Cordon-Cardo, C. MicroRNA-126 inhibits invasion in bladder cancer via regulation of ADAM9. Br. J. Cancer 2014, 110, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Lin, H.Y.; Zhu, Y.Y.; Zhu, Y.P.; Chen, L.W. MiR-126 regulates proliferation and invasion in the bladder cancer BLS cell line by targeting the PIK3R2-mediated PI3K/Akt signaling pathway. OncoTargets Ther. 2016, 9, 5181–5193. [Google Scholar] [CrossRef]
- Zhou, X.U.; Qi, L.; Tong, S.; Cui, Y.U.; Chen, J.; Huang, T.; Chen, Z.; Zu, X.B. MiR-128 downregulation promotes growth and metastasis of bladder cancer cells and involves VEGF-C upregulation. Oncol. Lett. 2015, 10, 3183–3190. [Google Scholar] [CrossRef] [PubMed]
- Majid, S.; Dar, A.A.; Saini, S.; Shahryari, V.; Arora, S.; Zaman, M.S.; Chang, I.; Yamamura, S.; Chiyomaru, T.; Fukuhara, S.; et al. MicroRNA-1280 inhibits invasion and metastasis by targeting ROCK1 in bladder cancer. PLoS ONE 2012, 7, e46743. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhong, Z.; Chi, H.; Huang, M.; Jiang, R.; Chen, J. Genome-wide screen of miRNAs and targeting mRNAs reveals the negatively regulatory effect of miR-130b-3p on PTEN by PI3K and integrin β1 signaling pathways in bladder carcinoma. Int. J. Mol. Sci. 2016, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, D.; Tao, J.; Qu, P.; Zhou, Z.; Hou, J. MicroRNA-133 inhibits cell proliferation, migration and invasion by targeting epidermal growth factor receptor and its downstream effector proteins in bladder cancer. Scand. J. Urol. 2013, 47, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.K.; Wang, J.M.; Zhang, P.; Wang, Y.Q. MicroRNA-138 regulates metastatic potential of bladder cancer through ZEB2. Cell. Physiol. Biochem. 2015, 37, 2366–2374. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, M.; Seki, N.; Yoshino, H.; Matsushita, R.; Miyamoto, K.; Nakagawa, M.; Enokida, H. Dual tumor-suppressors miR-139–5p and miR-139–3p targeting matrix metalloprotease 11 in bladder cancer. Cancer Sci. 2016, 107, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Q.; Niu, X.; Wang, G.; Zheng, S.; Fu, G.; Wang, Z. MiR-143 inhibits bladder cancer cell proliferation and enhances their sensitivity to gemcitabine by repressing IGF-1R signaling. Oncol. Lett. 2017, 13, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Villadsen, S.B.; Bramsen, J.B.; Ostenfeld, M.S.; Wiklund, E.D.; Fristrup, N.; Gao, S.; Hansen, T.B.; Jensen, T.I.; Borre, M.; Orntoft, T.F.; et al. The miR-143/-145 cluster regulates plasminogen activator inhibitor-1 in bladder cancer. Br. J. Cancer 2012, 106, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ying, L.; Tian, Y.; Yang, P.; Zhu, Y.; Wang, Z.; Qiu, F.; Lin, J. MiR-144 downregulation increases bladder cancer cell proliferation by targeting EZH2 and regulating Wnt signaling. FEBS J. 2013, 280, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Chiyomaru, T.; Enokida, H.; Tatarano, S.; Kawahara, K.; Uchida, Y.; Nishiyama, K.; Fujimura, L.; Kikkawa, N.; Seki, N.; Nakagawa, M. MiR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br. J. Cancer 2010, 102, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Yamada, N.; Kumazaki, M.; Yasui, Y.; Iwasaki, J.; Naito, S.; Akao, Y. socs7, a target gene of microRNA-145, regulates interferon-β induction through STAT3 nuclear translocation in bladder cancer cells. Cell Death Dis. 2013, 4, e482. [Google Scholar] [CrossRef] [PubMed]
- Kou, B.; Gao, Y.; Du, C.; Shi, Q.; Xu, S.; Wang, C.Q.; Wang, X.; He, D.; Guo, P. MiR-145 inhibits invasion of bladder cancer cells by targeting PAK1. Urol. Oncol. 2014, 32, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xu, T.; Wang, L.; Wang, X.; Zhong, S.; Xu, C.; Shen, Z. MicroRNA-145 directly targets the insulin-like growth factor receptor I in human bladder cancer cells. FEBS Lett. 2014, 588, 3180–3185. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, R.; Yoshino, H.; Enokida, H.; Goto, Y.; Miyamoto, K.; Yonemori, M.; Inoguchi, S.; Nakagawa, M.; Seki, N. Regulation of UHRF1 by dual-strand tumor-suppressor microRNA-145 (miR-145-5p and miR-145-3p): Inhibition of bladder cancer cell aggressiveness. Oncotarget 2016, 7, 28460–28487. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Wu, X.; Huang, C.; Wang, M.; Zhao, X.; Luo, G.; Li, Y.; Jiang, G.; Xiao, X.; Zeng, F. PTTG1 regulated by miR-146–3p promotes bladder cancer migration, invasion, metastasis and growth. Oncotarget 2017, 8, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhai, W.; Hu, G.; Huang, C.; Xie, T.; Zhang, J.; Xu, Y. MicroRNA-206 acts as a tumor suppressor in bladder cancer via targeting YRDC. Am. J. Trans. Res. 2016, 8, 4705–4715. [Google Scholar]
- Hirata, H.; Hinoda, Y.; Ueno, K.; Shahryari, V.; Tabatabai, Z.L.; Dahiya, R. MicroRNA-1826 targets VEGFC, β-catenin (CTNNB1) and MEK1 (MAP2K1) in human bladder cancer. Carcinogenesis 2012, 33, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; He, L.; Gan, Y.; Zeng, Q.; Dai, Y.; Tan, J. MiR-186 suppresses the growth and metastasis of bladder cancer by targeting NSBP1. Diagn. Pathol. 2015, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Li, Y.; Deng, H.; Zhang, C.; Pu, Y.; Qian, L.; Xiao, J.; Zhao, W.; Liu, Q.; Zhang, D.; et al. MiR-193a-3p promotes the multi-chemoresistance of bladder cancer by targeting the HOXC9 gene. Cancer Lett. 2015, 357, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhuang, Q.; Cui, L. MiR-194 inhibits cell proliferation and invasion via repression of RAP2B in bladder cancer. Biomed. Pharmacother. 2016, 80, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, J.; Chen, H.; Mao, Y.; Liu, Y.; Mao, Q.; Yang, K.; Zheng, X.; Xie, L. Cyclin-dependent kinase 4 is a novel target in micoRNA-195-mediated cell cycle arrest in bladder cancer cells. FEBS Lett. 2012, 586, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Qi, L.; Chen, M.; Liu, L.; Yan, W.; Tong, S.; Zu, X. MicroRNA-195 inhibits cell proliferation in bladder cancer via inhibition of cell division control protein 42 homolog/signal transducer and activator of transcription-3 signaling. Exp. Ther. Med. 2015, 10, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Yoshino, H.; Yonemori, M.; Miyamoto, K.; Sugita, S.; Matsushita, R.; Itesako, T.; Tatarano, S.; Nakagawa, M.; Enokida, H. Regulation of ITGA3 by the dual-stranded microRNA-199 family as a potential prognostic marker in bladder cancer. Br. J. Cancer 2017. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhang, X.; Yang, G.; Song, Y.; Cai, W. Decrement of miR-199a-5p contributes to the tumorigenesis of bladder urothelial carcinoma by regulating MLK3/NF-κB pathway. Am. J. Trans. Res. 2015, 7, 2786–2794. [Google Scholar]
- Liu, L.; Qiu, M.; Tan, G.; Liang, Z.; Qin, Y.; Chen, L.; Chen, H.; Liu, J. MiR-200c inhibits invasion, migration and proliferation of bladder cancer cells through down-regulation of BMI-1 and E2F3. J. Trans. Med. 2014, 12, 305. [Google Scholar] [CrossRef] [PubMed]
- Bo, J.; Yang, G.; Huo, K.; Jiang, H.; Zhang, L.; Liu, D.; Huang, Y. MicroRNA-203 suppresses bladder cancer development by repressing bcl-w expression. FEBS J. 2011, 278, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Arora, S.; Majid, S.; Shahryari, V.; Chen, Y.; Deng, G.; Yamamura, S.; Ueno, K.; Dahiya, R. Curcumin modulates microRNA-203-mediated regulation of the Src-Akt axis in bladder cancer. Cancer Prev. Res. 2011, 4, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Du, P.; Yuan, W.; Du, Z.; Yu, M.; Yu, X.; Hu, T. Long non-coding RNA HOTAIR regulates cyclin J via inhibition of microRNA-205 expression in bladder cancer. Cell Death Dis. 2015, 6, e1907. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Wang, L.; Yang, Y.; Dong, Z.; Wang, H.; Du, L.; Wang, C. MicroRNA-214 suppresses oncogenesis and exerts impact on prognosis by targeting PDRG1 in bladder cancer. PLoS ONE 2015, 10, e0118086. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yang, X.; Deng, X.; Zhang, X.; Li, P.; Tao, J.; Lu, Q. MicroRNA-218 inhibits bladder cancer cell proliferation, migration, and invasion by targeting BMI-1. Tumour Biol. 2015, 36, 8015–8023. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yang, X.; Cheng, Y.; Zhang, X.; Yang, C.; Deng, X.; Li, P.; Tao, J.; Yang, H.; Wei, J.; et al. MicroRNA-218 increases the sensitivity of bladder cancer to cisplatin by targeting Glut1. Cell. Physiol. Biochem. 2017, 41, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, J.; Lin, Y.; Zhu, Y.; Xu, X.; Xu, X.; Liang, Z.; Li, S.; Hu, Z.; Zheng, X.; et al. MicroRNA-320c inhibits tumorous behaviors of bladder cancer by targeting Cyclin-dependent kinase 6. J. Exp. Clin. Cancer Res. 2014, 33, 69. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Zhang, H.; Guo, Y.; Hong, Y.; Liu, Y.; Xue, Y. MiR-320a down-regulation mediates bladder carcinoma invasion by targeting ITGB3. Mol. Biol. Rep. 2014, 41, 2521–2527. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Niu, X.; Pan, H.; Zhou, Y.; Qu, P.; Zhou, J. MicroRNA-335 is downregulated in bladder cancer and inhibits cell growth, migration and invasion via targeting ROCK1. Mol. Med. Rep. 2016, 13, 4379–4385. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, H.; Lin, Y.; Hu, Z.; Mao, Y.; Wu, J.; Xu, X.; Zhu, Y.; Li, S.; Zheng, X.; et al. MicroRNA-409–3p inhibits migration and invasion of bladder cancer cells via targeting c-Met. Mol. Cells 2013, 36, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.L.; Ho, J.Y.; Chou, S.C.; Yu, D.S. MiR-429 reverses epithelial-mesenchymal transition by restoring E-cadherin expression in bladder cancer. Oncotarget 2016, 7, 26593–26603. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, X.; Zhu, X.; Zhong, Z.; Xu, R.; Wang, Z.; Cao, J.; Hou, Y. Decreased expression of miR-430 promotes the development of bladder cancer via the upregulation of CXCR7. Mol. Med. Rep. 2013, 8, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, Y.; Liang, Z.; Li, S.; Xu, X.; Wang, X.; Wu, J.; Hu, Z.; Meng, S.; Liu, B.; et al. c-Met and CREB1 are involved in miR-433-mediated inhibition of the epithelial-mesenchymal transition in bladder cancer by regulating Akt/GSK-3β/Snail signaling. Cell Death Dis. 2016, 7, e2088. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Shi, J.; Pan, Y.; Chen, Q.; Leng, P.; Wang, Y. miR-451 suppresses bladder cancer cell migration and invasion via directly targeting c-Myc. Oncol. Rep. 2016, 36, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Q.; Wang, S.; Zhang, J. miR4855p inhibits bladder cancer metastasis by targeting HMGA2. Int. J. Mol. Med. 2015, 36, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, X.; Xu, X.; Hu, Z.; Wu, J.; Zhu, Y.; Chen, H.; Mao, Y.; Lin, Y.; Luo, J.; et al. MicroRNA-490–5p inhibits proliferation of bladder cancer by targeting c-Fos. Biochem. Biophys. Res. Commun. 2013, 441, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Yang, L.; Xie, X.; Peng, L.; Wang, Y. MicroRNA-490–5p is a novel tumor suppressor targeting c-FOS in human bladder cancer. Arch. Med. Sci. 2015, 11, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Guil, S.; Esteller, M. Cis-acting noncoding RNAs: Friends and foes. Nat. Struct. Mol. Biol. 2012, 19, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T. Epigenetic regulation by long noncoding RNAs. Science 2012, 338, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Bussemakers, M.J.; van Bokhoven, A.; Verhaegh, G.W.; Smit, F.P.; Karthaus, H.F.; Schalken, J.A.; Debruyne, F.M.; Ru, N.; Isaacs, W.B. DD3: A new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999, 59, 5975–5979. [Google Scholar] [PubMed]
- Fang, Y.; Fullwood, M.J. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genom. Proteom. Bioinf. 2016, 14, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, M.; Ferro, M.; Terreri, S.; Durso, M.; Romanelli, A.; Avitabile, C.; De Cobelli, O.; Messere, A.; Bruzzese, D.; Vannini, I.; et al. Long non-coding RNA containing ultraconserved genomic region 8 promotes bladder cancer tumorigenesis. Oncotarget 2016, 7, 20636–20654. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Lin, T.; He, W.; Han, J.; Zhu, D.; Hu, K.; Li, W.; Zheng, Z.; Huang, J.; Xie, W. Knockdown of a novel lincRNA AATBC suppresses proliferation and induces apoptosis in bladder cancer. Oncotarget 2015, 6, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, X.; Song, Y.; Zhang, P.; Xiao, Y.; Xing, Y. Long non-coding RNA ANRIL is up-regulated in bladder cancer and regulates bladder cancer cell proliferation and apoptosis through the intrinsic pathway. Biochem. Biophys. Res. Commun. 2015, 467, 223–228. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Liu, Y.; Chen, Z.; Li, J.; Chen, M.; Liu, L.; Liao, X.; Lv, Z.; Zhan, Y.; Zhuang, C.; et al. Over-expression of long noncoding RNA BANCR inhibits malignant phenotypes of human bladder cancer. J. Exp. Clin. Cancer Res. 2016, 35, 125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, W.; Jiang, J.; Bao, E.; Xu, D.; Zeng, Y.; Tao, L.; Qiu, J. Downregulation of GAS5 promotes bladder cancer cell proliferation, partly by regulating CDK6. PLoS ONE 2013, 8, e73991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, Y.; Song, Y.; Shang, C. Long noncoding RNA GAS5 inhibits malignant proliferation and chemotherapy resistance to doxorubicin in bladder transitional cell carcinoma. Cancer Chemother. Pharmacol. 2017, 79, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Zhu, J.L.; Bao, W.S.; Chen, D.K.; Huang, W.W.; Weng, Z.L. Long noncoding RNA GHET1 promotes the development of bladder cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 7196–7205. [Google Scholar] [PubMed]
- Amit, D.; Hochberg, A. Development of targeted therapy for bladder cancer mediated by a double promoter plasmid expressing diphtheria toxin under the control of H19 and IGF2-P4 regulatory sequences. J. Trans. Med. 2010, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Li, Z.; Wang, W.; Zeng, Y.; Liu, Z.; Qiu, J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013, 333, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Li, Z.; Wang, W.; Zeng, Y.; Liu, Z.; Qiu, J. Upregulated H19 contributes to bladder cancer cell proliferation by regulating ID2 expression. FEBS J. 2013, 280, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, Z.; Chen, S.S.; Li, F.; Lei, C.Y.; Chen, X.X.; Bao, J.M.; Luo, Y.; Lin, G.Z.; Pang, S.Y.; et al. The YAP1 oncogene contributes to bladder cancer cell proliferation and migration by regulating the H19 long noncoding RNA. Urol. Oncol. 2015, 33, 427.e1–427.e10. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhuang, C.; Liu, Y.; Li, J.; Dai, F.; Xia, M.; Zhan, Y.; Lin, J.; Chen, Z.; He, A.; et al. Tetracycline-inducible shRNA targeting antisense long non-coding RNA HIF1A-AS2 represses the malignant phenotypes of bladder cancer. Cancer Lett. 2016, 376, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.H.; Lu, S.W.; Huang, Y.Q.; Que, G.B.; Chen, J.H.; Chen, Y.P.; Zhang, H.B.; Liang, X.L.; Jiang, J.H. Upregulation of the long noncoding RNA HOTAIR predicts recurrence in stage Ta/T1 bladder cancer. Tumour Biol. 2014, 35, 10249–10257. [Google Scholar] [CrossRef] [PubMed]
- Heubach, J.; Monsior, J.; Deenen, R.; Niegisch, G.; Szarvas, T.; Niedworok, C.; Schulz, W.A.; Hoffmann, M.J. The long noncoding RNA HOTAIR has tissue and cell type-dependent effects on HOX gene expression and phenotype of urothelial cancer cells. Mol. Cancer 2015, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Guo, Y.; Zhang, H.; Xue, Y.X. Long noncoding RNA HOTAIR is a prognostic biomarker and inhibits chemosensitivity to doxorubicin in bladder transitional cell carcinoma. Cancer Chemother. Pharmacol. 2016, 77, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Berrondo, C.; Flax, J.; Kucherov, V.; Siebert, A.; Osinski, T.; Rosenberg, A.; Fucile, C.; Richheimer, S.; Beckham, C.J. Expression of the long non-coding RNA HOTAIR Correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes. PLoS ONE 2016, 11, e0147236. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fernandez, M.; Feber, A.; Duenas, M.; Segovia, C.; Rubio, C.; Fernandez, M.; Villacampa, F.; Duarte, J.; Lopez-Calderon, F.F.; Gomez-Rodriguez, M.J.; et al. Analysis of the Polycomb-related lncRNAs HOTAIR and ANRIL in bladder cancer. Clin. Epigenetics 2015, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Wu, Z.Y.; Wang, G.C.; Liu, K.; Niu, X.B.; Gu, S.; Meng, J.S. LINC00312 inhibits the migration and invasion of bladder cancer cells by targeting miR-197–3p. Tumour Biol. 2016, 37, 14553–14563. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Cai, Q.; Sun, F.; Zhong, G.; Wang, P.; Liu, H.; Luo, J.; Yu, H.; Huang, J.; Lin, T. Linc-UBC1 physically associates with polycomb repressive complex 2 (PRC2) and acts as a negative prognostic factor for lymph node metastasis and survival in bladder cancer. Biochim. Biophys. 2013, 1832, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Dai, B.; Zhang, H.; Shi, G.; Shen, Y.; Ye, D. Long non-coding RNA LOC572558 inhibits bladder cancer cell proliferation and tumor growth by regulating the AKT-MDM2-p53 signaling axis. Cancer Lett. 2016, 380, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Chen, Q.; Wang, Y.; Zhou, Z.; Huang, Y.; Qiu, F. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol. Biosys. 2012, 8, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, Y.; Nie, L.; Gui, Y.; Cai, Z. Inducing cell proliferation inhibition, apoptosis, and motility reduction by silencing long noncoding ribonucleic acid metastasis-associated lung adenocarcinoma transcript 1 in urothelial carcinoma of the bladder. Urology 2013, 81, 209.e1–209.e7. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, Y.; Zhang, H.; Wang, T.; Diao, R.; Jiang, Z.; Gui, Y.; Cai, Z. Hsa-miR-125b suppresses bladder cancer development by down-regulating oncogene SIRT7 and oncogenic long non-coding RNA MALAT1. FEBS Lett. 2013, 587, 3875–3882. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shen, B.; Tan, M.; Mu, X.; Qin, Y.; Zhang, F.; Liu, Y. TGF-β-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin. Cancer Res. 2014, 20, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Ma, G.; Zhang, Z.; Hua, Q.; Chu, H.; Tong, N.; Yuan, L.; Qin, C.; Yin, C.; Zhang, Z.; et al. A novel antisense long noncoding RNA regulates the expression of MDC1 in bladder cancer. Oncotarget 2015, 6, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Huang, Y.; Chen, H.; Wang, Y.; Xia, L.; Chen, Y.; Liu, Y.; Qiu, F. Downregulated MEG3 activates autophagy and increases cell proliferation in bladder cancer. Mol. BioSyst. 2013, 9, 407–411. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Chen, Z.; Mei, H.; Liu, Y. Decreased expression of LncRNA MIR31HG in human bladder cancer. Cancer Biomark. 2016, 17, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xie, W.; Xie, L.; Sun, Y.; Zhang, Y.; Shen, Z.; Sha, N.; Xu, H.; Wu, Z.; Hu, H.; et al. Expression of long noncoding RNA lncRNA-n336928 is correlated with tumor stage and grade and overall survival in bladder cancer. Biochem. Biophys. Res. Commun. 2015, 468, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, M.; Li, Z.; Kong, C.; Bi, J.; Li, J.; Gao, Z.; Li, Z. ncRAN, A newly identified long noncoding RNA, enhances human bladder tumor growth, invasion, and survival. Urology 2011, 77, 510.e1–510.e5. [Google Scholar] [CrossRef] [PubMed]
- XianGuo, C.; ZongYao, H.; Jun, Z.; Song, F.; GuangYue, L.; LiGang, Z.; KaiPing, Z.; YangYang, Z.; ChaoZhao, L. Promoting progression and clinicopathological significance of NEAT1 over-expression in bladder cancer. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Zhuang, C.; Xu, W.; Fu, X.; Lv, Z.; Wu, H.; Mou, L.; Zhao, G.; Cai, Z.; et al. Inducing cell growth arrest and apoptosis by silencing long non-coding RNA PCAT-1 in human bladder cancer. Tumour Biol. 2015, 36, 7685–7689. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Li, J.; Liu, Y.; Chen, M.; Yuan, J.; Fu, X.; Zhan, Y.; Liu, L.; Lin, J.; Zhou, Q.; et al. Tetracycline-inducible shRNA targeting long non-coding RNA PVT1 inhibits cell growth and induces apoptosis in bladder cancer cells. Oncotarget 2015, 6, 41194–41203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, Z.; Nan, Y.; Li, M. Inhibiting malignant phenotypes of the bladder cancer cells by silencing long noncoding RNA SChLAP1. Int. Urol. Nephrol. 2016, 48, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Zhao, Z.H.; Xu, W.C.; Hou, J.Q.; Du, X.Y. Increased expression of SPRY4-IT1 predicts poor prognosis and promotes tumor growth and metastasis in bladder cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 1954–1960. [Google Scholar] [PubMed]
- Liu, D.; Li, Y.; Luo, G.; Xiao, X.; Tao, D.; Wu, X.; Wang, M.; Huang, C.; Wang, L.; Zeng, F.; et al. LncRNA SPRY4-IT1 sponges miR-101–3p to promote proliferation and metastasis of bladder cancer cells through up-regulating EZH2. Cancer Lett. 2017, 388, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, Y.; Gui, Y.; Cai, Z. Long intergenic non-coding RNA TUG1 is overexpressed in urothelial carcinoma of the bladder. J. Surg. Oncol. 2013, 107, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, H.; Cheng, H.; Li, Y.; Li, X.; Zhu, C. Downregulation of long noncoding RNA TUG1 inhibits proliferation and induces apoptosis through the TUG1/miR-142/ZEB2 axis in bladder cancer cells. OncoTargets Ther. 2017, 10, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Qiu, K.; Li, M.; Liang, Y. Double-negative feedback loop between long non-coding RNA TUG1 and miR-145 promotes epithelial to mesenchymal transition and radioresistance in human bladder cancer cells. FEBS Lett. 2015, 589, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Iliev, R.; Kleinova, R.; Juracek, J.; Dolezel, J.; Ozanova, Z.; Fedorko, M.; Pacik, D.; Svoboda, M.; Stanik, M.; Slaby, O. Overexpression of long non-coding RNA TUG1 predicts poor prognosis and promotes cancer cell proliferation and migration in high-grade muscle-invasive bladder cancer. Tumour Biol. 2016, 37, 13385–13390. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shen, B.; Tan, M.; Mu, X.; Qin, Y.; Zhang, F.; Liu, Y. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. FEBS J. 2014, 281, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, Y.; Jin, B.; Wu, C.; Yang, J.; Wang, L.; Zhang, Z.; Mao, Z. Long non-coding RNA urothelial carcinoma associated 1 induces cell replication by inhibiting BRG1 in 5637 cells. Oncol. Rep. 2014, 32, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Singh, P.K.; Rath, S.K.; Dalela, D.; Goel, M.M.; Bhatt, M.L. Appraisal of diagnostic ability of UCA1 as a biomarker of carcinoma of the urinary bladder. Tumour Biol. 2014, 35, 11435–11442. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Matboli, M.; Essawy, N.O.; Kotb, Y.M. Integrative functional genetic-epigenetic approach for selecting genes as urine biomarkers for bladder cancer diagnosis. Tumour Biol. 2015, 36, 9545–9552. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Matboli, M.; Essawy, N.O.; Shehta, M.; Kotb, Y.M. Rapid detection of urinary long non-coding RNA urothelial carcinoma associated one using a PCR-free nanoparticle-based assay. Biomarkers 2015, 20, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Li, X.; Pang, H.; Pan, J.J.; Xie, X.J.; Chen, W. Long non-coding RNA UCA1 promotes glutamine metabolism by targeting miR-16 in human bladder cancer. Jpn. J. Clin. Oncol. 2015, 45, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, W.; Yang, C.; Wu, W.; Wu, S.; Qin, X.; Li, X. Long non-coding RNA UCA1a(CUDR) promotes proliferation and tumorigenesis of bladder cancer. Int. J. Oncol. 2012, 41, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhong, G.; He, W.; Yu, H.; Huang, J.; Lin, T. lncRNA up-regulated in nonmuscle invasive bladder cancer facilitates tumor growth and acts as a negative prognostic factor of recurrence. J. Urol. 2016, 196, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Lu, Q.; Shen, B.; Huang, X.; Shen, L.; Zheng, X.; Huang, R.; Yan, J.; Guo, H. TGFβ1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci. Rep. 2015, 5, 11924. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Pang, H.; Li, X.; Li, H.; Pan, J.; Chen, W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016, 107, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, X.; Wu, S.; Xue, M.; Chen, W. Long non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci. 2014, 105, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Borkowska, E.; Drayton, R.M.; Rakhit, C.P.; Noon, A.; Chen, W.; Catto, J.W. Identification of differentially expressed long noncoding RNAs in bladder cancer. Clin. Cancer Res. 2014, 20, 5311–5321. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Wang, N.; Qi, J.; Gu, Z.; Shen, H. Long noncoding RNAGAS5 acts as a tumor suppressor in bladder transitional cell carcinoma via regulation of chemokine (CC motif) ligand 1 expression. Mol. Med. Rep. 2016, 13, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, X.; Wang, Y.; Zhao, L.; Chen, W. Long non-coding RNA UCA1 regulated cell cycle distribution via CREB through PI3-K dependent pathway in bladder carcinoma cells. Gene 2012, 496, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Calin, G.A.; Meng, Q.H. Circulating microRNAs as Promising Tumor Biomarkers. Adv. Clin. Chem. 2014, 67, 189–214. [Google Scholar] [CrossRef] [PubMed]
- Fabris, L.; Ceder, Y.; Chinnaiyan, A.M.; Jenster, G.W.; Sorensen, K.D.; Tomlins, S.; Visakorpi, T.; Calin, G.A. The potential of MicroRNAs as prostate cancer biomarkers. Eur. Urol. 2016, 70, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, H.; Carney, G.E. Evidence and potential in vivo functions for biofluid miRNAs: From expression profiling to functional testing: Potential roles of extracellular miRNAs as indicators of physiological change and as agents of intercellular information exchange. BioEssays 2016, 38, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Ostenfeld, M.S.; Jeppesen, D.K.; Laurberg, J.R.; Boysen, A.T.; Bramsen, J.B.; Primdal-Bengtson, B.; Hendrix, A.; Lamy, P.; Dagnaes-Hansen, F.; Rasmussen, M.H.; et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014, 74, 5758–5771. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Du, L.; Duan, W.; Wang, R.; Yan, K.; Wang, L.; Li, J.; Zheng, G.; Zhang, X.; Yang, Y.; et al. Serum microRNA expression signatures as novel noninvasive biomarkers for prediction and prognosis of muscle-invasive bladder cancer. Oncotarget 2016, 7, 36733–36742. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Du, L.; Wang, L.; Li, J.; Liu, Y.; Zheng, G.; Qu, A.; Zhang, X.; Pan, H.; Yang, Y.; et al. Serum microRNA expression signatures identified from genome-wide microRNA profiling serve as novel noninvasive biomarkers for diagnosis and recurrence of bladder cancer. Int. J. Cancer 2015, 136, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Dai, W.; Wang, X.; Chen, W.; Shen, C.; Ye, G.; Li, L. Circulating miR-205: A promising biomarker for the detection and prognosis evaluation of bladder cancer. Tumour Biol. 2016, 37, 8075–8082. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Shi, D.; Yuan, L.; Li, P.; Chu, H.; Qin, C.; Yin, C.; Zhang, Z.; Wang, M. Circulating miR-497 and miR-663b in plasma are potential novel biomarkers for bladder cancer. Sci. Rep. 2015, 5, 10437. [Google Scholar] [CrossRef] [PubMed]
- Pospisilova, S.; Pazourkova, E.; Horinek, A.; Brisuda, A.; Svobodova, I.; Soukup, V.; Hrbacek, J.; Capoun, O.; Hanus, T.; Mares, J.; et al. MicroRNAs in urine supernatant as potential non-invasive markers for bladder cancer detection. Neoplasma 2016, 63, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Fendler, A.; Stephan, C.; Yousef, G.M.; Kristiansen, G.; Jung, K. The translational potential of microRNAs as biofluid markers of urological tumours. Nat. Rev. Urol. 2016, 13, 734–752. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Ryu, S.Y.; Sheu, S.S. Distinctive characteristics and functions of multiple mitochondrial Ca2+ influx mechanisms. Sci. China Life Sci. 2011, 54, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.A.; Reichert, A.S. Impaired quality control of mitochondria: Aging from a new perspective. Exp. Gerontol. 2010, 45, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Poyton, R.O.; McEwen, J.E. Crosstalk between nuclear and mitochondrial genomes. Annu. Rev. Biochem. 1996, 65, 563–607. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Neph, S.; Dinger, M.E.; Crawford, J.; Smith, M.A.; Shearwood, A.M.; Haugen, E.; Bracken, C.P.; Rackham, O.; Stamatoyannopoulos, J.A.; et al. The human mitochondrial transcriptome. Cell 2011, 146, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Sripada, L.; Tomar, D.; Singh, R. Mitochondria: One of the destinations of miRNAs. Mitochondrion 2012, 12, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Kren, B.T.; Wong, P.Y.; Sarver, A.; Zhang, X.; Zeng, Y.; Steer, C.J. MicroRNAs identified in highly purified liver-derived mitochondria may play a role in apoptosis. RNA Biol. 2009, 6, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Latronico, M.V.; Condorelli, G. The might of microRNA in mitochondria. Circ. Res. 2012, 110, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Barrey, E.; Saint-Auret, G.; Bonnamy, B.; Damas, D.; Boyer, O.; Gidrol, X. Pre-microRNA and mature microRNA in human mitochondria. PLoS ONE 2011, 6, e20220. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [PubMed]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation. Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Fogg, V.C.; Lanning, N.J.; Mackeigan, J.P. Mitochondria in cancer: At the crossroads of life and death. Chin. J. Cancer 2011, 30, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Zhang, L.F.; Zhang, H.W.; Hu, S.; Lu, M.H.; Liang, S.; Li, B.; Li, Y.; Li, D.; Wang, E.D.; et al. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J. 2012, 31, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Xiao, T.; Fang, Z.; Sun, Y.; Li, F.; Gao, Y.; Feng, Y.; Li, L.; Wang, Y.; Liu, X.; et al. MicroRNA-143 (miR-143) regulates cancer glycolysis via targeting hexokinase 2 gene. J. Biol. Chem. 2012, 287, 23227–23235. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, M.; Nocchi, L.; Staffolani, S.; Manzella, N.; Amati, M.; Goodwin, J.; Kluckova, K.; Nguyen, M.; Strafella, E.; Bajzikova, M.; et al. MicroRNA-126 suppresses mesothelioma malignancy by targeting IRS1 and interfering with the mitochondrial function. Antioxid. Redox Signaling 2014, 21, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, X.P.; Jiang, L.; Lu, J.; Liu, X.; Chen, S.J. Silencing of COX-2 in nasopharyngeal carcinoma cells with a shRNAmir lentivirus vector. J. South. Med. Univ. 2009, 29, 1111–1114. [Google Scholar]
- Ritterson Lew, C.; Guin, S.; Theodorescu, D. Targeting glycogen metabolism in bladder cancer. Nat. Rev. Urol. 2015, 12, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Qi, M.; Wu, B.; Song, Y.; Wang, Y.; Li, T. MicroRNA-195–5p suppresses glucose uptake and proliferation of human bladder cancer T24 cells by regulating GLUT3 expression. FEBS Lett. 2012, 586, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Takai, T.; Yoshikawa, Y.; Inamoto, T.; Minami, K.; Taniguchi, K.; Sugito, N.; Kuranaga, Y.; Shinohara, H.; Kumazaki, M.; Tsujino, T.; et al. A Novel Combination RNAi toward warburg effect by replacement with miR-145 and Silencing of PTBP1 Induces apoptotic cell death in bladder cancer cells. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, G.; Hasler, R.; El Mokhtari, N.E.; Konig, I.R.; Loos, B.G.; Jepsen, S.; Rosenstiel, P.; Schreiber, S.; Schaefer, A.S. The large non-coding RNA ANRIL, which is associated with atherosclerosis, periodontitis and several forms of cancer, regulates ADIPOR1, VAMP3 and C11ORF10. Hum. Mol. Genet. 2013, 22, 4516–4527. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, H.; Mei, Y.; Wu, M. Reciprocal regulation of HIF-1α and lincRNA-p21 modulates the Warburg effect. Mol. Cell 2014, 53, 88–100. [Google Scholar] [CrossRef] [PubMed]
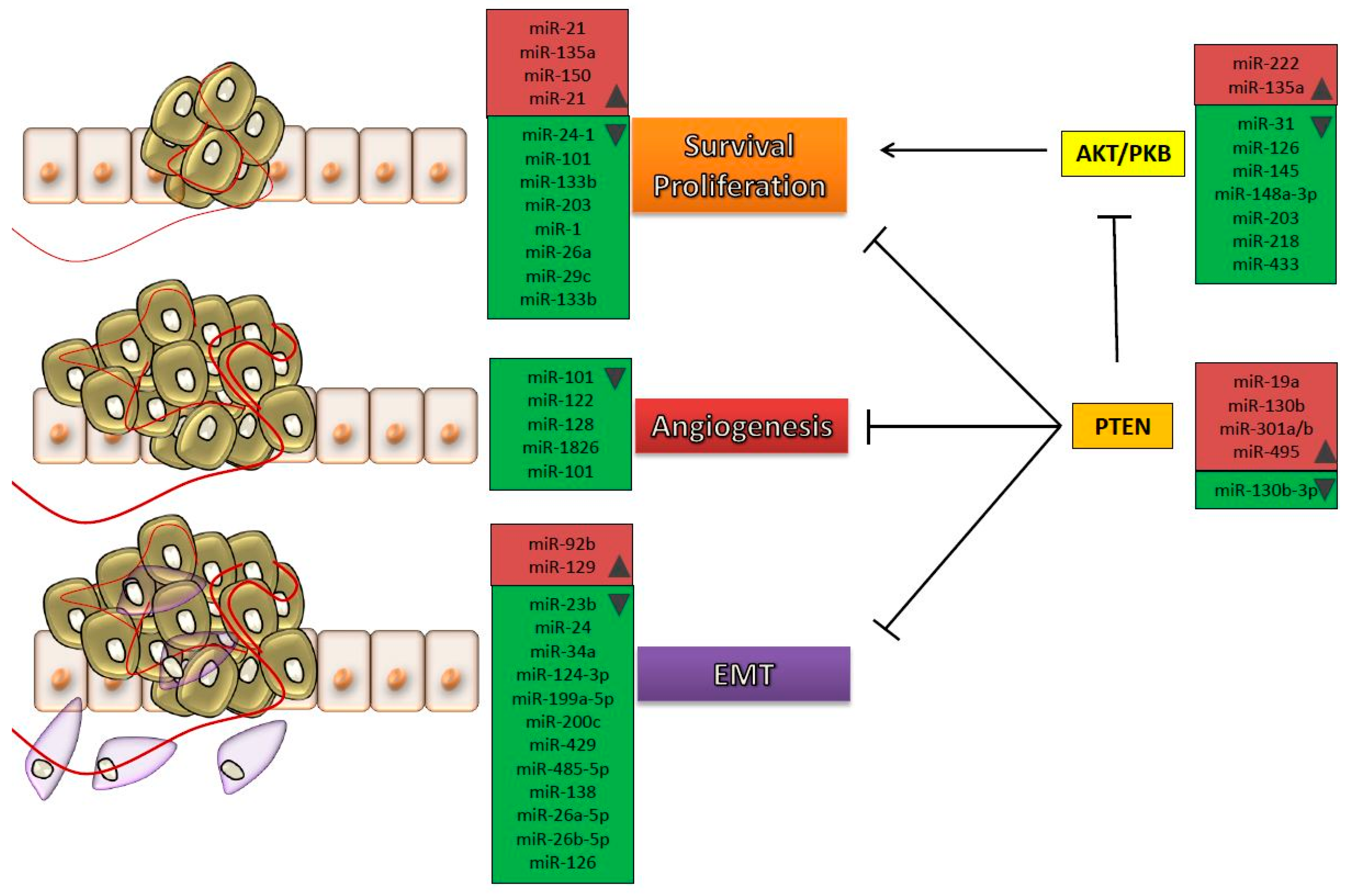

| miRNA | Expression | Target Genes | Pathway | Reference |
|---|---|---|---|---|
| miR-9 | ↑ | CBX7 | [40] | |
| miR-9 | ↑ | LASS2 | [41] | |
| miR-19a | ↑ | PTEN | AKT/PKB signaling | [42] |
| miR-21 | ↑ | BCL-2 | PI3K-AKT | [36] |
| miR-24-3p | ↑ | DEDD | [43] | |
| miR-92 | ↑ | GSK3β | Wnt/c-myc/MMP7 signaling | [44] |
| miR-92b | ↑ | DAB2IP | EMT | [32] |
| miR-96 | ↑ | CDKN1A | [45] | |
| miR-129 | ↑ | SOX4 | [25] | |
| miR-130b | ↑ | PTEN | PI3K/AKT signaling | [46] |
| miR-135a | ↑ | PHLPP2, FOXO1 | AKT signaling | [47] |
| miR-137 | ↑ | PAQR3 | [48] | |
| miR-138-5p | ↑ | BIRC5 | [49] | |
| miR-150 | ↑ | PDCD4 | [50] | |
| miR-155 | ↑ | DMTF1 | [51] | |
| miR-182-5p | ↑ | RECK, Smad4 | [52] | |
| miR-193a-3p | ↑ | LOXL4, SRSF2 | Oxidative Stress | [53] |
| miR-200 | ↑ | ERRFI-1 | EGFR inhibitor resistance | [54] |
| miR-222 | ↑ | PPP2R2A | PPP2R2A/AKT/mTOR signaling | [55] |
| miR-301a/b | ↑ | PTEN | PI3K/AKT signaling | [46] |
| miR-495 | ↑ | PTEN | [56] | |
| miR-1 | ↓ | LASP1 | [57] | |
| miR-1 | ↓ | TAGLN2 | [58] | |
| miR-1 | ↓ | SRSF9 | [59] | |
| miR-1 | ↓ | PTMA, PNP | [60] | |
| miR-1 | ↓ | UCA1 | [61] | |
| miR-23b | ↓ | Zeb-1 | EMT | [33] |
| miR-24 | ↓ | CARMA3 | EMT | [62] |
| miR-24-1 | ↓ | FOXM1 | [63] | |
| miR-26a | ↓ | HMGA1 | [64] | |
| miR-26a-5p | ↓ | PLOD2 | [65] | |
| miR-26b-5p | ↓ | PLOD2 | [65] | |
| miR-27a | ↓ | SLC7A11 | Glutathione biosynthesis | [66] |
| miR-27a | ↓ | RUNX-1 | [67] | |
| miR-29c | ↓ | BCL-2, MCL1 | [68] | |
| miR-29c | ↓ | CDK6 | G1 phase arrest | [69] |
| miR-31 | ↓ | ITGA5 | AKT and ERK | [70] |
| miR-34a | ↓ | Cdk-6, SIRT-1 | [71] | |
| miR-34a | ↓ | CD44 | EMT | [72] |
| miR-34a | ↓ | HNF4G | [73] | |
| miR-99a | ↓ | FGFR3 | [74] | |
| miR-99a | ↓ | FGFR3 | [75] | |
| miR-100 | ↓ | FGFR3 | [74] | |
| miR-100 | ↓ | mTOR | [76] | |
| miR-101 | ↓ | COX-2 | [77] | |
| miR-101 | ↓ | VEGF-C | [78] | |
| miR-101 | ↓ | c-FOS | [79] | |
| miR-106a | ↓ | cyclin D1/CDK6 | [80] | |
| miR-122 | ↓ | VEGFC | mTOR and AKT | [81] |
| miR-124 | ↓ | Cdk4 | [82] | |
| miR-124 | ↓ | UHRF1 | [83] | |
| miR-124-3p | ↓ | ROCK1 | EMT | [34] |
| miR-125b | ↓ | E2F3 | E2F3–Cyclin A2 signaling | [84] |
| miR-125b | ↓ | MMP13 | [85] | |
| miR-125b | ↓ | SphK1 | G1 phase arrest | [86] |
| miR-126 | ↓ | ADAM9 | [87] | |
| miR-126 | ↓ | PIK3R2 | PI3K/AKT signaling | [88] |
| miR-128 | ↓ | VEGF-C | [89] | |
| miR-1280 | ↓ | ROCK1 | [90] | |
| miR-130b-3p | ↓ | PTEN | PI3K and integrin β1/FAK signaling | [91] |
| miR-133a | ↓ | LASP1 | [57] | |
| miR-133a | ↓ | TAGLN2 | [58] | |
| miR-133a | ↓ | PTMA, PNP | [60] | |
| miR-133a/b | ↓ | EGFR, MMP-2 | [92] | |
| miR-133b | ↓ | Bcl-w, AKT1 | [37] | |
| miR-138 | ↓ | ZEB2 | [93] | |
| miR-139-3p/5p | ↓ | MMP11 | [94] | |
| miR-143 | ↓ | IGF-1R | [95] | |
| miR-143/145 cluster | ↓ | PAI-1 | [96] | |
| miR-144 | ↓ | EZH2 | Wnt signaling/EZH2/Nkd1 | [97] |
| miR-144-5p | ↓ | CCNE1/2, CDC25A | [31] | |
| miR-145 | ↓ | FSCN1 | [98] | |
| miR-145 | ↓ | SOCS7 | PI3K/AKT signaling | [99] |
| miR-145 | ↓ | PAK1 | [100] | |
| miR-145 | ↓ | IGF-IR | [101] | |
| miR-145-3p/5p | ↓ | UHRF1 | [102] | |
| miR-146-3p | ↓ | PTTG1 | [103] | |
| miR-148a-3p | ↓ | ERBB3, DNMT1, AKT2 | [104] | |
| miR-1826 | ↓ | VEGFC, CTNNB1, MAP2K1 | MAPK-ERK signal transduction | [105] |
| miR-186 | ↓ | NSBP1 | [106] | |
| miR-193-3p | ↓ | HOXC9 | DNA damage response and oxidative stress | [107] |
| miR-194 | ↓ | RAP2B | [108] | |
| miR-195 | ↓ | Cdk4 | G1-phase arrest | [109] |
| miR-195 | ↓ | Cdc42 | Cdc42/STAT3 signaling | [110] |
| miR-195/497 cluster | ↓ | BIRC5, WNT7A | [21] | |
| miR-199-3p/5p | ↓ | ITGA3 | [111] | |
| miR-199a-3p/5p | ↓ | ITGA3 | [111] | |
| miR-199a-5p | ↓ | MLK3 | MLK3/IκB/NF-κB | [112] |
| miR-199a-5p | ↓ | CCR7 | EMT | [35] |
| miR-200c | ↓ | BMI-1, E2F3 | EMT | [113] |
| miR-203 | ↓ | bcl-w | [114] | |
| miR-203 | ↓ | AKT2, Src | PI3K/AKT signaling | [115] |
| miR-205 | ↓ | CCNJ | [116] | |
| miR-206 | ↓ | YRDC | [104] | |
| miR-214 | ↓ | PDRG1 | [117] | |
| miR-218 | ↓ | LASP1 | [57] | |
| miR-218 | ↓ | BMI-1 | PI3K/AKT signaling | [118] |
| miR-218 | ↓ | Glut1 | [119] | |
| miR-320c | ↓ | Cdk6 | G1-phase arrest | [120] |
| miR-320s | ↓ | ITGB3 | [121] | |
| miR-335 | ↓ | ROCK1 | [122] | |
| miR-335 | ↓ | MAPK1 | [38] | |
| miR-409-3p | ↓ | c-Met | [123] | |
| miR-429 | ↓ | E-cad, ZEB1/2, β-catenin | EMT | [124] |
| miR-430 | ↓ | CXCR3 | [125] | |
| miR-433 | ↓ | CREB, c-Met | c-Met/AKT/GSK-3β/Snail signaling, EMT | [126] |
| miR-451 | ↓ | c-Myc | [127] | |
| miR-485-5p | ↓ | HMGA2 | EMT | [128] |
| miR-490-5p | ↓ | c-FOS | G1-phase arrest | [129] |
| miR-490-5p | ↓ | c-FOS | [130] |
| lncRNA | Tumor Type | Study | Expression | Reference |
|---|---|---|---|---|
| AATBC | Bladder cancer | 90 patients 90 adjacent normal tissue | ↑ 54 cases ↓ 36 cases | [137] |
| ANRIL | Bladder cancer | 51 patients 51 adjacent non-tumor tissues | ↑ | [138] |
| BANCR | Bladder cancer | 54 patients 54matched adjacent normal tissues | ↓ | [139] |
| GAS5 | Urothelial carcinoma | 28 patients 28 adjacent normal mucosa | ↓ | [140] |
| GAS5 | Transitional cell carcinoma | 82 patients 37 normal bladder tissues | ↓ | [141] |
| GHET-1 | Bladder cancer | 80 patients 80 adjacent normal tissues | ↑ | [142] |
| H19 | Transitional cell carcinoma | 39 patients | ↑ | [143] |
| H19 | Urothelial carcinoma | 41 patients 41 adjacent normal mucosa | ↑ | [144] |
| H19 | Urothelial carcinoma | 24 patients 24 adjacent normal control tissues | ↑ | [145] |
| H19 | Bladder cancer | 40 patients 19 adjacent normal tissues | ↑ | [146] |
| HIF1A-AS2 | Bladder cancer | 44 patients 44 matched normal peritumoral tissues | ↑ | [147] |
| HOTAIR | Urothelial carcinoma | 110 patients 110 matched normal tissues | ↑ 90 cases ↓ 20 cases | [148] |
| HOTAIR | Urothelial carcinoma | Set 1: 19 patients, 10 normal bladder tissues Set 2: 108 patients, 7 normal bladder tissues | Set 1: ↑ 9 cases Set 2: No significant differences | [149] |
| HOTAIR | Transitional cell carcinoma | 35 patients 16 normal bladder transitional cell | ↑ | [150] |
| HOTAIR MALAT-1 | Bladder cancer | 10 patients 10 distal normal tissue | ↑ ↑ | [151] |
| HOTAIR | NMIBC | 64 patients 64 distant normal mucosa | ↑ | [152] |
| LINC00312 | Bladder cancer | 110 patients | ↓ | [153] |
| Linc-UBC1 | Bladder cancer | 102 patients 102 adjacent normal mucosa | ↑ 60 cases ↓ 42 cases | [154] |
| LOC572558 | Bladder cancer | 24 patients 24 matched normal bladder tissue | ↓ | [155] |
| MALAT-1 | Bladder cancer | 22 patients 22 matched normal tissues | ↑ | [156] |
| MALAT-1 | Urothelial carcinoma | 36 patients 36 matched histologically normal urothelium | ↑ | [157] |
| MALAT-1 | Urothelial carcinoma | 27 patients 27 adjacent histologically normal tissues | ↑ | [158] |
| MALAT-1 | Urothelial carcinoma | 95 patients 95 matched normal tissues | ↑ | [159] |
| MDC1-AS | Bladder cancer | 32 patients 32 adjacent normal tissue | ↓ | [160] |
| MEG3 | Urothelial carcinoma | 31 patients 31 adjacent benign tissues | ↓ | [161] |
| MIR31HG | Bladder cancer | 55 patients 55 matched non-tumor bladder tissues | ↓ | [162] |
| MIR31HG | Bladder cancer | 55 patients 55 adjacent normal tissue | ↓ | [162] |
| n336928 | Bladder cancer | 95 patients 95 adjacent non-tumor tissues | ↑ | [163] |
| ncRAN | Transitional cell carcinoma | 40 patients 40 paired adjacent noncancerous tissues | ↑ | [164] |
| NEAT1 | Bladder cancer | 65 patients 65 adjacent normal tissue | ↑ 48 cases | [165] |
| PCAT-1 | Urothelial carcinoma | 36 patients 36 matched normal tissues | ↑ | [166] |
| PVT1 | Bladder cancer | 32 patients 32 matched para-cancer tissues | ↑ 20 cases | [167] |
| SChLAP1 | Urothelial carcinoma | 36 patients 36 paired normal bladder tissue | ↑ | [168] |
| SPRY4-IT1 | Urothelial carcinoma | 68 patients 68 adjacent non-tumor tissues | ↑ | [169] |
| SPRY4-IT1 | Bladder cancer | 60 patients 60 matched normal adjacent tissue | ↑ | [170] |
| TUG1 | Urothelial carcinoma | 44 patients 44 matched histologically normal urothelium | ↑ | [171] |
| TUG1 | Bladder cancer | 36 patients 36 adjacent normal tissue | ↑ | [172] |
| TUG1 | Bladder cancer | 54 patients | ↑ | [173] |
| TUG1 | Bladder cancer | 47 patients 47 adjacent non-tumor bladder tissues | ↑ | [174] |
| UCA1 | Urothelial carcinoma | 34 patients (Cisplatin-based chemotherapy) | ↑ | [175] |
| UCA1 | Urothelial carcinoma | 20 patients 20 matched normal tissues | ↑ 17 cases ↓ 3 cases | [176] |
| UCA1 | Urothelial carcinoma | 25 patients 25 matched normal tissues | ↑ | [61] |
| UCA1 | Transitional cell carcinoma | 117 patients 74 non bladder cancer patients | ↑ | [177] |
| UCA1 | Bladder cancer | 94 patients urine samples from patients with benign disease (56) or healthy volunteers (60) | ↑ | [178] |
| UCA1 | Bladder cancer | 184 patients (139 malignant disease+45 benign disease) 36 healthy volunteers | ↑ | [179] |
| UCA1 | Bladder cancer | 35 patients 6 normal bladder tissues 10 adjacent normal tissues | ↑ | [180] |
| UCA1a (CUDR) | Transitional cell carcinoma | 8 patients 8 adjacent normal mucosa | ↑ | [181] |
| uc.8+ (ultraconserved RNA 8+) | Bladder cancer | 24 patients 17 normal bladder epithelium | ↑ | [136] |
| UNMIBC | NMIBC | 75 patients 75 adjacent normal mucosa | ↑ 45 cases | [182] |
| ZEB2NAT | Urothelial carcinoma | 30 patients 30 adjacent normal tissue | ↑ | [183] |
| lncRNA | Association with Genes | Pathway | Reference |
|---|---|---|---|
| AATBC | ↑ NRF2 | JNK signaling | [137] |
| AB074278 | ↓ EMP-1 | [186] | |
| ANRIL | ↓ cleaved Caspase 9/3, cleaved PARP, Bax, Smac, cyt C ↑ Bcl-2 | Intrinsic pathway | [138] |
| GAS5 | ↓ CDK6 | [140] | |
| GAS5 | ↓ CCL1 | [187] | |
| GAS5 | ↓ Bcl-2 | [141] | |
| GHET-1 | ↓ Snail, Slug, Twist, ZEB1 | EMT | [142] |
| H19 | EHZ2 ↓ E-cadherin | Wnt/β-catenin | [144] |
| H19 | ↑ ID2 | [145] | |
| HIF1A-AS2 | ↑ caspase 3 | [147] | |
| HOTAIR | ↓ WIF-1 | Wnt/β-catenin signaling | [148] |
| HOTAIR | ↓ miR-205 | [152] | |
| HOTAIR | ↑ SNAI1, ZEB1, TWIST1, MMP1 | EMT | [151] |
| LINC00312 | ↓ miR-197-3p | [153] | |
| Linc-UBC1 | PRC complex | [154] | |
| LOC572558 | ↑ AKT, MDM2, p53 | AKT-MDM2-p53 signaling axis | [155] |
| MALAT-1 | ↓ ZEB1, ZEB2, SLU, E-cadherin | Wnt/β-catenin | [156] |
| MALAT-1 | ↓ E-cadherin SUZ12 | EMT | [159] |
| SPRY4-IT1 | ↓ miR-101-3p ↑ EZH2 | EMT EZH2 pathway | [170] |
| SPRY4-IT1 | ↑ miR-101-3p ↓ EZH2 | [170] | |
| TUG1 | ↑ ZEB2 ↓ miR-142 | Wnt/β-catenin pathway | [172] |
| TUG1 | ↓ miR-145 | EMT | [173] |
| UCA-1 | ↑ HK2 ↓ miR-143 | Glycolysis mTOR–STAT3 pathway | [185] |
| UCA-1 | ↑ CREB | PI3-K/AKT | [188] |
| UCA-1 | ↑ Wnt6 | Wnt signaling | [175] |
| UCA-1 | ↓ BRG1 | [176] | |
| UCA-1 | ↑ GLS2 | Cell redox state Glutaminolysis | [180] |
| UCA-1 | ↑ ZEB1/2 ↓ miR-145 | EMT miR-145–ZEB1/2–FSCN1 | [184] |
| UNMIBC | EZH2, SUZ12 | [182] | |
| ZEB2NAT | ↑ TGF-β1, ZEB2 | EMT | [183] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop-Bica, C.; Gulei, D.; Cojocneanu-Petric, R.; Braicu, C.; Petrut, B.; Berindan-Neagoe, I. Understanding the Role of Non-Coding RNAs in Bladder Cancer: From Dark Matter to Valuable Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 1514. https://doi.org/10.3390/ijms18071514
Pop-Bica C, Gulei D, Cojocneanu-Petric R, Braicu C, Petrut B, Berindan-Neagoe I. Understanding the Role of Non-Coding RNAs in Bladder Cancer: From Dark Matter to Valuable Therapeutic Targets. International Journal of Molecular Sciences. 2017; 18(7):1514. https://doi.org/10.3390/ijms18071514
Chicago/Turabian StylePop-Bica, Cecilia, Diana Gulei, Roxana Cojocneanu-Petric, Cornelia Braicu, Bogdan Petrut, and Ioana Berindan-Neagoe. 2017. "Understanding the Role of Non-Coding RNAs in Bladder Cancer: From Dark Matter to Valuable Therapeutic Targets" International Journal of Molecular Sciences 18, no. 7: 1514. https://doi.org/10.3390/ijms18071514