Autophagy Induced FHL2 Upregulation Promotes IL-6 Production by Activating the NF-κB Pathway in Mouse Aortic Endothelial Cells after Exposure to PM2.5
Abstract
:1. Introduction
2. Results
2.1. Characteristics of PM2.5 Components and Mass Concentration in Winter from a Beijing Urban Area
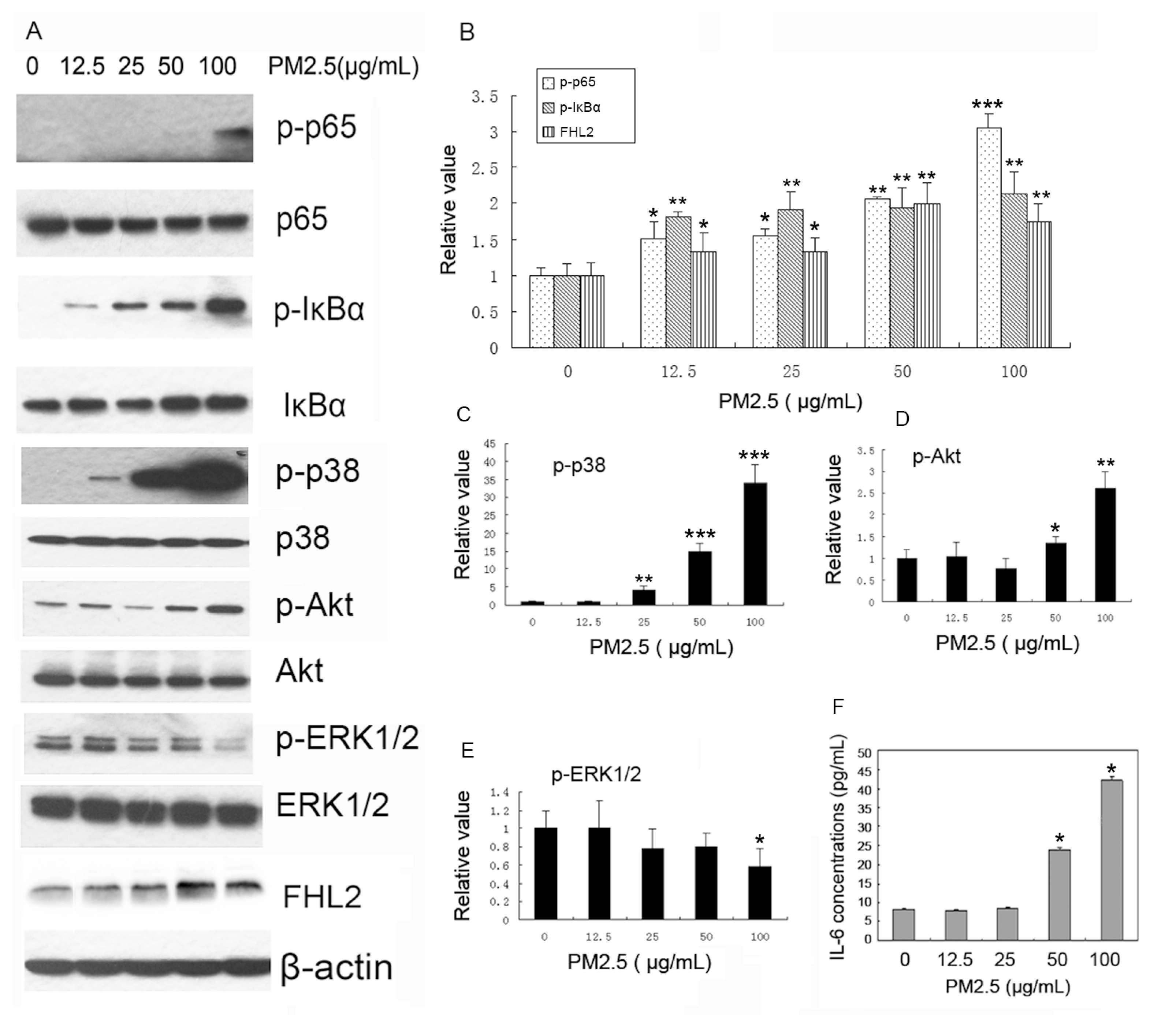
2.2. PM2.5 Exposure Promotes FHL2 Expression and Activates Signaling Pathways in MAECs
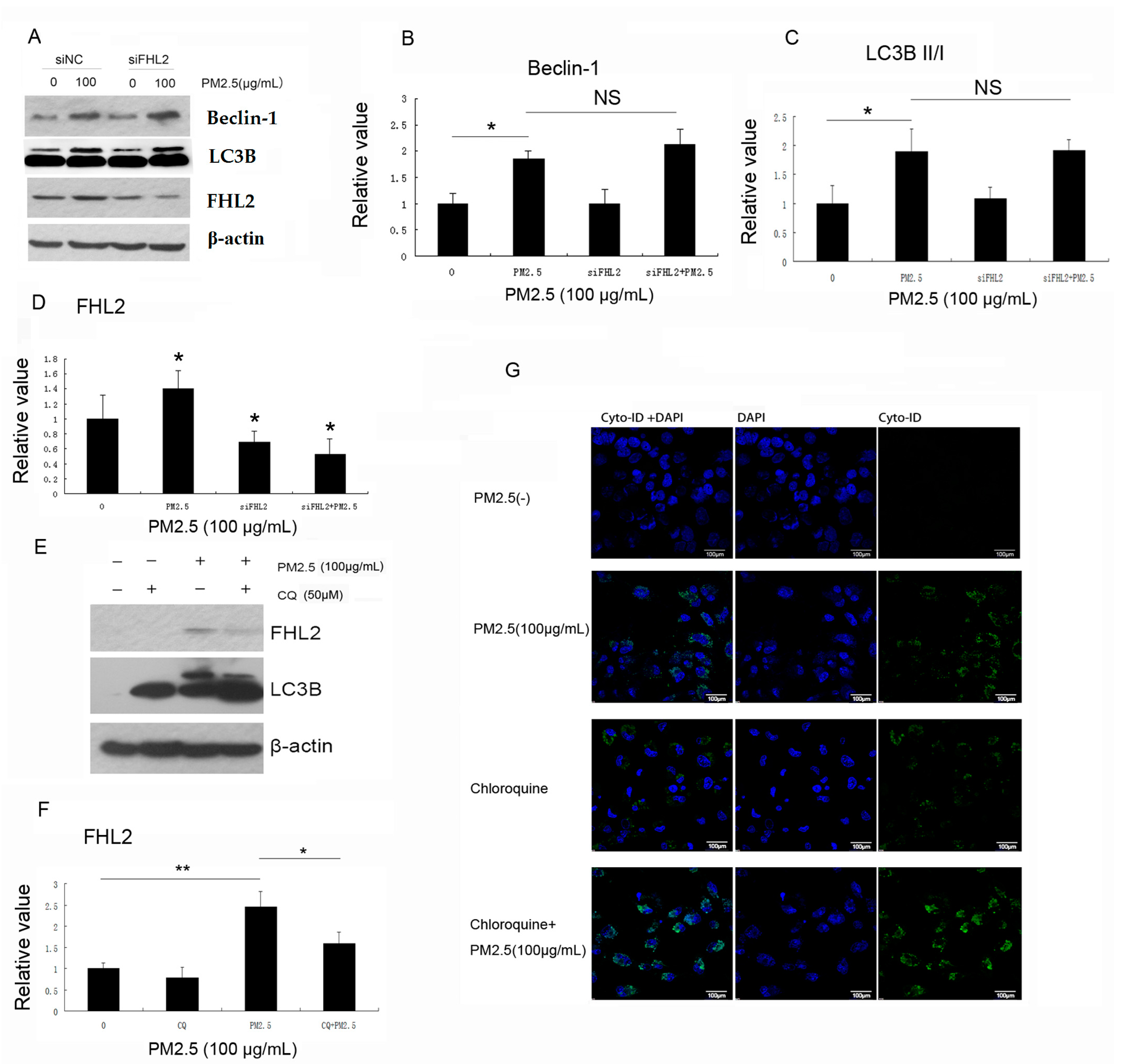
2.3. FHL2 Knockdown Inhibits PM2.5-Activated NF-κB Signaling
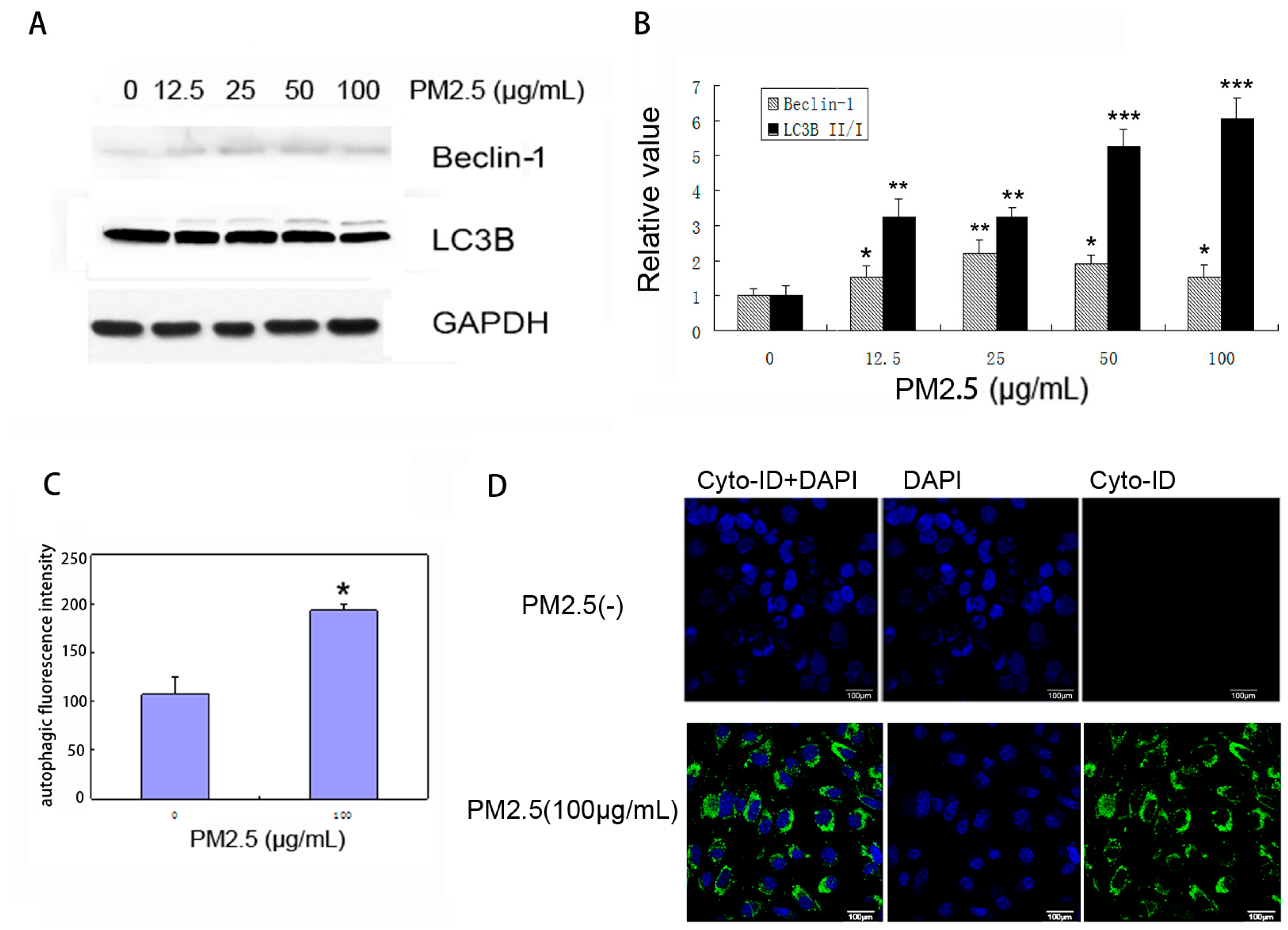
2.4. PM2.5 Exposure Induces Autophagy in MAECs
2.5. Inhibition of Autophagy Downregulates PM2.5-Induced FHL2 Expression
3. Discussion
4. Materials and Methods
4.1. PM2.5 Collection and Preparation
4.2. Chemical Analysis of PM2.5
4.3. MAEC Culture
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. PM2.5 Exposure and Cell Transfection
4.6. Autophagy Assay
4.7. Western Blot Assay
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FHL2 | Four and a half LIM domains 2 |
| NF-κB | Nuclear factor-κB |
| siRNA | Small interfering RNA |
| MAEC | Mouse aortic endothelial cells |
| PM2.5 | Particulate matter with an aerodynamic diameter less than or equal to 2.5 μm |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MIP-1α/β | Macrophage inflammatory protein 1α/β |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| sICAM-1 | Soluble intercellular adhesion molecule 1 |
| CVD | Cardiovascular diseases |
| PAH | Polycyclic aromatic hydrocarbon |
| FBS | Fetal bovine serum |
| LC3B | Microtubule-associated protein 1A/1B light-chain 3B |
| ERK1/2 | Extracellular signal-regulated kinases 1/2 |
References
- Wang, C.; Tu, Y.; Yu, Z.; Lu, R. PM2.5 and cardiovascular diseases in the elderly: An overview. Int. J. Environ. Res. Public Health 2015, 2, 8187–8197. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.J.; Staimer, N.; Tjoa, T.; Polidori, A.; Arhami, M.; Gillen, D.L.; Kleinman, M.T.; Vaziri, N.D.; Longhurst, J.; Zaldivar, F.; et al. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ. Health Perspect. 2008, 116, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Siponen, T.; Yli-Tuomi, T.; Aurela, M.; Dufva, H.; Hillamo, R.; Hirvonen, M.R.; Huttunen, K.; Pekkanen, J.; Pennanen, A.; Salonen, I.; et al. Source-specific fine particulate air pollution and systemic inflammation in ischaemic heart disease patients. Occup. Environ. Med. 2015, 72, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Pai, J.K.; Pischon, T.; Ma, J.; Manson, J.E.; Hankinson, S.E.; Joshipura, K.; Curhan, G.C.; Rifai, N.; Cannuscio, C.C.; Stampfer, M.J.; et al. Inflammatory markers and the risk of coronary heart disease in men and women. N. Engl. J. Med. 2004, 351, 2599–2610. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., 3rd; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Toole, T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Kobayasi, R.; Akamine, E.H.; Davel, A.P.; Rodrigues, M.A.; Carvalho, C.R.; Rossoni, L.V. Oxidative stress and inflammatory mediators contribute to endothelial dysfunction in high-fat diet-induced obesity in mice. J. Hypertens. 2010, 28, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Mak, G.W.; Li, M.S.; Tsui, S.K. The LIM-only protein FHL2 regulates interleukin-6 expression through p38 MAPK mediated NF-κB pathway in muscle cells. Cytokine 2012, 59, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Wang, Y.; Pang, R.; Wang, J.; Dai, Y.; Ma, J.; Gu, Q.; Li, Z.; Zhang, Y.; Zou, B.; et al. Oncogene functions of FHL2 are independent from NF-κBIα in gastrointestinal cancer. Pathol. Oncol. Res. 2009, 15, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Dahan, J.; Nouët, Y.; Jouvion, G.; Levillayer, F.; Adib-Conquy, M.; Cassard-Doulcier, A.M.; Tebbi, A.; Blanc, F.; Remy, L.; Chen, J.; et al. LIM-only protein FHL2 activates NF-κB signaling in the control of liver regeneration and hepatocarcinogenesis. Mol. Cell. Biol. 2013, 33, 3299–3308. [Google Scholar] [CrossRef] [PubMed]
- Nouët, Y.; Dahan, J.; Labalette, C.; Levillayer, F.; Julien, B.; Jouvion, G.; Cairo, S.; Vives, F.L.; Ribeiro, A.; Huerre, M.; et al. The four and a half LIM-only protein 2 regulates liver homeostasis and contributes to carcinogenesis. J. Hepatol. 2012, 57, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, Z.; Yan, J.; Yang, X.; Liu, A.; Qiu, W.; Zhu, J.; Han, J.; Zhang, H.; Lin, J.; et al. Human four-and-a-half LIM family members suppress tumor cell growth through a TGF-β-like signaling pathway. J. Clin. Investig. 2009, 119, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, T.; Simon, D.; Lemarié, C.A.; Simeone, S.; Heidari, M.; Mann, K.K.; Wassmann, S.; Lehoux, S. Absence of four-and-a-half LIM domain protein 2 decreases atherosclerosis in ApoE−/− Mice. Arterioscler. Thromb. Vasc. Biol. 2015, 3, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.H.; Yeh, H.I.; Wu, H.H.; Hong, R.C.; Shiu, T.F.; Yang, C.M. Deletion of the FHL2 gene attenuates the formation of atherosclerotic lesions after a cholesterol-enriched diet. Life Sci. 2010, 86, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Shelton, J.M.; Rothermel, B.; Li, X.; Richardson, J.A.; Bassel-Duby, R.; Williams, R.S. Cardiac-specific LIM protein FHL2 modifies the hypertrophic response to β-adrenergic stimulation. Circulation 2001, 103, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, F.W.; Reischmann, S.; Schwalm, A.; Unger, A.; Ramanujam, D.; Münch, J.; Müller, O.J.; Hengstenberg, C.; Galve, E.; Charron, P.; et al. FHL2 expression and variants in hypertrophic cardiomyopathy. Basic Res. Cardiol. 2014, 109, 451. [Google Scholar] [CrossRef] [PubMed]
- Arimura, T.; Hayashi, T.; Matsumoto, Y.; Shibata, H.; Hiroi, S.; Nakamura, T.; Inagaki, N.; Hinohara, K.; Takahashi, M.; Manatsu, S.I.; et al. Structural analysis of four and half LIM protein-2 in dilated cardiomyopathy. Biochem. Biophys. Res. Commun. 2007, 357, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, K.; Vos, M.; Otermin Rubio, I.; Marinković, G.; Buettner, R.; Heukamp, L.C.; Stap, J.; de Waard, V.; van Tiel, C.M.; de Vries, C.J. The LIM-only protein FHL2 reduces vascular lesion formation involving inhibition of proliferation and migration of smooth muscle cells. PLoS ONE 2014, 9, e94931. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Feng, N.; Zheng, M.; Ye, X.; Lin, H.; Yu, X.; Gan, Z.; Fang, Z.; Zhang, H.; Gao, M.; et al. PM2.5 exposure-induced autophagy is mediated by lncRNA loc146880 which also promotes the migration and invasion of lung cancer cells. Biochim. Biophys. Acta 2016, 1861, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Jin, X.; Zhang, W.; Li, Z.; Liu, X.; Ren, J. Particulate matter exposure induces the autophagy of macrophages via oxidative stress-mediated PI3K/AKT/mTOR pathway. Chemosphere 2016, 167, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, H.; Liu, S.; Xing, C.; Liu, Y.; Aodengqimuge; Zhou, W.; Yuan, X.; Ma, Y.; Hu, M.; et al. TP53-dependent autophagy links the ATR-CHEK1 axis activation to proinflammatory VEGFA production in human bronchial epithelial cells exposed to fine particulate matter (PM2.5). Autophagy 2016, 12, 1832–1848. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Z.; Huang, H.; He, H.; Yang, L.; Chen, T.; Yang, T.; Ren, N.; Jiang, Y.; Xu, W.; et al. AMPK is required for PM2.5-induced autophagy in human lung epithelial A549 cells. Int. J. Clin. Exp. Med. 2015, 8, 58–72. [Google Scholar] [PubMed]
- Aulin, J.; Siegbahn, A.; Hijazi, Z.; Ezekowitz, M.D.; Andersson, U.; Connolly, S.J.; Huber, K.; Reilly, P.A.; Wallentin, L.; Oldgren, J. Interleukin-6 and C-reactive protein and risk for death and cardiovascular events in patients with atrial fibrillation. Am. Heart J. 2015, 170, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Pabois, A.; Pagie, S.; Gérard, N.; Laboisse, C.; Pattier, S.; Hulin, P.; Nedellec, S.; Toquet, C.; Charreau, B. Notch signaling mediates crosstalk between endothelial cells and macrophages via Dll4 and IL-6 in cardiac microvascular inflammation. Biochem. Pharmacol. 2016, 104, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Gao, C.; Cui, Z. Ginkgolide A reduces inflammatory response in high-glucose-stimulated human umbilical vein endothelial cells through STAT3-mediated pathway. Int. Immunopharmacol. 2015, 25, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhao, L.; Tong, J.; Yan, Y.; Xu, C. Fine particulate matter and sulfur dioxide coexposures induce rat lung pathological injury and inflammatory responses via TLR4/p38/NF-κB pathway. Int. J. Toxicol. 2017, 36, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Rui, W.; Guan, L.; Zhang, F.; Zhang, W.; Ding, W. PM2.5-induced oxidative stress increases adhesion molecules expression in human endothelial cells through the ERK/AKT/NF-κB-dependent pathway. J. Appl. Toxicol. 2016, 36, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bai, Y.; Xu, X.; Sun, L.; Wang, A.; Wang, T.Y.; Maurya, S.K.; Periasamy, M.; Morishita, M.; Harkema, J.; et al. Exaggerated effects of particulate matter air pollution in genetic type II diabetes mellitus. Part. Fibre Toxicol. 2014, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Lapaquette, P.; Guzzo, J.; Bretillon, L.; Bringer, M.A. Cellular and molecular connections between autophagy and inflammation. Mediat. Inflamm. 2015, 2015, 398483. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Tseng, C.Y.; Chao, M.W. Diesel exhaust particles contribute to endothelia apoptosis via autophagy pathway. Toxicol. Sci. 2017, 156, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.X.; Wong, S.H.; Chan, M.T.; Yu, L.; Yu, S.S.; Wu, F.; Xiao, Z.; Wang, X.; Zhang, L.; Cheng, A.S.; et al. Autophagy mediates HBx-induced nuclear factor-κB activation and release of IL-6, IL-8, and CXCL2 in hepatocytes. J. Cell. Physiol. 2015, 230, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Y.; Wang, L.; Diao, Z.; Liu, W. The role of autophagy in kidney inflammatory injury via the NF-κB route induced by LPS. Int. J. Med. Sci. 2015, 12, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, M.; Inoue, M.; Danzaki, K.; Hammer, G.; He, Y.W.; Shinohara, M.L. Autophagy enhances NF-κB activity in specific tissue macrophages by sequestering A20 to boost antifungal immunity. Nat. Commun. 2015, 6, 5779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copetti, T.; Bertoli, C.; Dalla, E.; Demarchi, F.; Schneider, C. p65/RelA modulates BECN1 transcription and autophagy. Mol. Cell. Biol. 2009, 29, 2594–2608. [Google Scholar] [CrossRef] [PubMed]
- Harris, J. Autophagy and cytokines. Cytokine 2011, 56, 140–144. [Google Scholar] [CrossRef] [PubMed]

| Pollutant | Range | |
|---|---|---|
| Low Concentration | High Concentration | |
| PM2.5 | 32.3 μg/m3 | 296.3 μg/m3 |
| Al | 248.1 ng/m3 | 265.6 ng/m3 |
| Se | 3.8 ng/m3 | 13.8 ng/m3 |
| Fe | 324 ng/m3 | 704.4 ng/m3 |
| Mn | 17 ng/m3 | 55.1 ng/m3 |
| Hg | 0.7 ng/m3 | 1.2 ng/m3 |
| As | 3.7 ng/m3 | 12.5 ng/m3 |
| Zn | 101.1 ng/m3 | 318.4 ng/m3 |
| Cu | 12.4 ng/m3 | 34.2 ng/m3 |
| Cd | 0.7 ng/m3 | 3.6 ng/m3 |
| Pb | 39 ng/m3 | 157.4 ng/m3 |
| Ni | 1.0 ng/m3 | 3.1 ng/m3 |
| Cr | 1.8 ng/m3 | 4.9 ng/m3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, W.-R.; Fu, W.; Wang, Q.; Zhu, X.; Xing, W.-W.; Wang, M.; Xu, D.-Q.; Xu, D.-G. Autophagy Induced FHL2 Upregulation Promotes IL-6 Production by Activating the NF-κB Pathway in Mouse Aortic Endothelial Cells after Exposure to PM2.5. Int. J. Mol. Sci. 2017, 18, 1484. https://doi.org/10.3390/ijms18071484
Xia W-R, Fu W, Wang Q, Zhu X, Xing W-W, Wang M, Xu D-Q, Xu D-G. Autophagy Induced FHL2 Upregulation Promotes IL-6 Production by Activating the NF-κB Pathway in Mouse Aortic Endothelial Cells after Exposure to PM2.5. International Journal of Molecular Sciences. 2017; 18(7):1484. https://doi.org/10.3390/ijms18071484
Chicago/Turabian StyleXia, Wen-Rong, Wenliang Fu, Qin Wang, Xiaoming Zhu, Wei-Wei Xing, Min Wang, Dong-Qun Xu, and Dong-Gang Xu. 2017. "Autophagy Induced FHL2 Upregulation Promotes IL-6 Production by Activating the NF-κB Pathway in Mouse Aortic Endothelial Cells after Exposure to PM2.5" International Journal of Molecular Sciences 18, no. 7: 1484. https://doi.org/10.3390/ijms18071484




