Naturally Occurring Compounds: New Potential Weapons against Oxidative Stress in Chronic Kidney Disease
Abstract
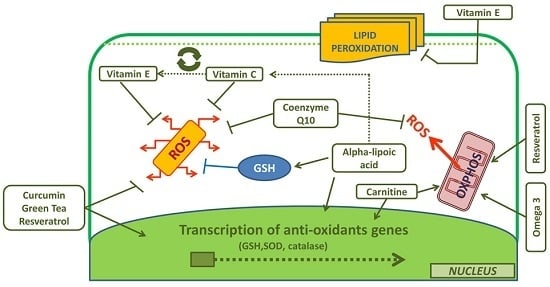
:1. Introduction
2. l-Carnitine
3. Vitamin E
4. Vitamin C
5. Coenzyme Q10
6. α-Lipoic Acid
7. Selenium
8. Green Tea
9. Resveratrol
10. Curcumin
11. Omega-3 Polyunsaturated Fatty Acids
12. Conclusions
Author Contributions
Conflicts of Interest
References
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. Suppl. 2008, 111, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. 2006, 48, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Dursun, B.; Dursun, E.; Suleymanlar, G.; Ozben, B.; Capraz, I.; Apaydin, A.; Ozben, T. Carotid artery intima-media thickness correlates with oxidative stress in chronic haemodialysis patients with accelerated atherosclerosis. Nephrol. Dial. Transplant. 2008, 23, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Odamaki, M.; Hishida, A. Blood 8-hydroxy-2′-deoxyguanosine is associated with erythropoietin resistance in hemodialysis patients. Nephrol. Dial. Transplant. 2003, 18, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, L.; Locatelli, F.; Carini, M. What we know about oxidative stress in patients with chronic kidney disease on dialysis—Clinical effects, potential treatment, and prevention. Semin Dial. 2011, 24, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Canaud, B.; Eckardt, K.; Stenvinkel, P.; Wanner, C.; Zoccali, C. Oxidative stress in end-stage renal disease: An emerging treat to patient outcome. Nephrol. Dial. Transplant. 2003, 18, 1272–1280. [Google Scholar] [CrossRef]
- Granata, S.; Zaza, G.; Simone, S.; Villani, G.; Latorre, D.; Pontrelli, P.; Carella, M.; Schena, F.P.; Grandaliano, G.; Pertosa, G. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genom. 2009, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- Zaza, G.; Granata, S.; Masola, V.; Rugiu, C.; Fantin, F.; Gesualdo, L.; Schena, F.P.; Lupo, A. Downregulation of nuclear-encoded genes of oxidative metabolism in dialyzed chronic kidney disease patients. PLoS ONE 2013, 8, e77847. [Google Scholar] [CrossRef]
- Granata, S.; Masola, V.; Zoratti, E.; Scupoli, M.T.; Baruzzi, A.; Messa, M.; Sallustio, F.; Gesualdo, L.; Lupo, A.; Zaza, G. NLRP3 inflammasome activation in dialyzed chronic kidney disease patients. PLoS ONE 2015, 10, e0122272. [Google Scholar] [CrossRef] [PubMed]
- Hajnóczky, G.; Csordás, G.; Das, S.; Garcia-Perez, C.; Saotome, M.; Sinha Roy, S. Mitochondrial calcium signalling and cell death: Approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium 2006, 40, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Ajioka, R.S.; Phillips, J.D.; Kushner, J.P. Biosynthesis of heme in mammals. Biochim. Biophys. Acta 2006, 1763, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Rossier, M.F. T channels and steroid biosynthesis: In search of a link with mitochondria. Cell Calcium 2006, 40, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. Apoptotic pathways: The roads to ruin. Cell 1998, 94, 695–698. [Google Scholar] [CrossRef]
- Gamboa, J.L.; Billings, F.T.; Bojanowski, M.T.; Gilliam, L.A.; Yu, C.; Roshanravan, B.; Roberts, L.J.; Himmelfarb, J.; Ikizler, T.A.; Brown, N.J. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol. Rep. 2016, 4, e12780. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Dhoopun, A.R.; Yuan, Y.; Huang, S.; Zhu, C.; Ding, G.; Liu, B.; Yang, T.; Zhang, A. Mitochondrial dysfunction is an early event in aldosterone-induced podocyte injury. Am. J. Physiol. Ren. Physiol. 2013, 305, F520–F531. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yuan, Y.; Bai, M.; Ding, G.; Jia, Z.; Huang, S.; Zhang, A. PGC-1α overexpression protects against aldosterone-induced podocyte depletion: Role of mitochondria. Oncotarget 2016, 7, 12150–12162. [Google Scholar] [PubMed]
- Yuan, Y.; Chen, Y.; Zhang, P.; Huang, S.; Zhu, C.; Ding, G.; Liu, B.; Yang, T.; Zhang, A. Mitochondrial dysfunction accounts for aldosterone-induced epithelial-to-mesenchymal transition of renal proximal tubular epithelial cells. Free Radic Biol. Med. 2012, 53, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Jia, Z.; Guo, X.; Yang, T. Aldosterone induces epithelial-mesenchymal transition via ROS of mitochondrial origin. Am. J. Physiol. Ren. Physiol. 2007, 293, F723–F731. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, H.A.; Wilmer, M.J.; Reijnders, D.; Jansen, J.; van den Broek, P.H.; Forkink, M.; Schepers, E.; Glorieux, G.; Vanholder, R.; van den Heuvel, L.P.; et al. Uremic toxins inhibit renal metabolic capacity through interference with glucuronidation and mitochondrial respiration. Biochim. Biophys. Acta 2013, 1832, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hébert, R.L. NADPH oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Dicus, M.; Ho, N.D.; Boroujerdi-Rad, L.; Sindhu, R.K. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int. 2003, 63, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Fortuño, A.; Beloqui, O.; San José, G.; Moreno, M.U.; Zalba, G.; Díez, J. Increased phagocytic nicotinamide adenine dinucleotide phosphate oxidase-dependent superoxide production in patients with early chronic kidney disease. Kidney Int. Suppl. 2005, 99, S71–S75. [Google Scholar] [CrossRef] [PubMed]
- Morena, M.; Cristol, J.P.; Senécal, L.; Leray-Moragues, H.; Krieter, D.; Canaud, B. Oxidative stress in hemodialysis patients: Is NADPH oxidase complex the culprit? Kidney Int. Suppl. 2002, 80, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Yoon, Y.J.; Choi, H.J.; Park, S.H.; Kim, C.D.; Kim, I.S.; Kwon, T.H.; Do, J.Y.; Kim, S.H.; Ryu, D.H.; et al. Dialysis modality-dependent changes in serum metabolites: Accumulation of inosine and hypoxanthine in patients on haemodialysis. Nephrol. Dial Transplant. 2011, 26, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Gondouin, B.; Jourde-Chiche, N.; Sallee, M.; Dou, L.; Cerini, C.; Loundou, A.; Morange, S.; Berland, Y.; Burtey, S.; Brunet, P.; et al. Plasma Xanthine Oxidase Activity Is Predictive of Cardiovascular Disease in Patients with Chronic Kidney Disease, Independently of Uric Acid Levels. Nephron 2015, 131, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Gobe, G.C. Oxidative Stress and Antioxidant Therapy in Chronic Kidney and Cardiovascular Disease. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; In Tech: Vienna, Austria, 2013. [Google Scholar]
- Goicoechea, M.; de Vinuesa, S.G.; Verdalles, U.; Ruiz-Caro, C.; Ampuero, J.; Rincón, A.; Arroyo, D.; Luño, J. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin. J. Am. Soc. Nephrol. 2010, 5, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Siu, Y.P.; Leung, K.T.; Tong, M.K.; Kwan, T.H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am. J. Kidney Dis. 2006, 47, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Ozkara, A.; Selcoki, Y.; Isik, B.; Turgut, F.; Bavbek, N.; Uz, E.; Akcay, A.; Yigitoglu, R.; Covic, A. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int. Urol. Nephrol. 2007, 39, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, M.; Garcia de Vinuesa, S.; Verdalles, U.; Verde, E.; Macias, N.; Santos, A.; Pérez de Jose, A.; Cedeño, S.; Linares, T.; Luño, J. Allopurinol and progression of CKD and cardiovascular events: Long-term follow-up of a randomized clinical trial. Am. J. Kidney Dis. 2015, 65, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; Schumacher, H.R., Jr.; Wortmann, R.L.; MacDonald, P.A.; Eustace, D.; Palo, W.A.; Streit, J.; Joseph-Ridge, N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N. Engl. J. Med. 2005, 353, 2450–2561. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Kumar, S.; Stamp, L.; Gow, P. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J. Rheumatol. 2006, 33, 1646–1650. [Google Scholar] [PubMed]
- Thurston, M.M.; Phillips, B.B.; Bourg, C.A. Safety and efficacy of allopurinol in chronic kidney disease. Ann. Pharmacother. 2013, 47, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Fleeman, N.; Pilkington, G.; Dundar, Y.; Dwan, K.; Boland, A.; Dickson, R.; Anijeet, H.; Kennedy, T.; Pyatt, J. Allopurinol for the treatment of chronic kidney disease: A systematic review. Health Technol. Assess. 2014, 18, 1–77. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, H.R., Jr.; Becker, M.A.; Wortmann, R.L.; Macdonald, P.A.; Hunt, B.; Streit, J.; Lademacher, C.; Joseph-Ridge, N. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: A 28-week, phase, I.I.I.; randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008, 59, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; Schumacher, H.R.; Espinoza, L.R.; Wells, A.F.; MacDonald, P.; Lloyd, E.; Lademacher, C. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: The CONFIRMS trial. Arthritis Res. Ther. 2010, 12, R63. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Harada, M.; Yamada, Y.; Hashimoto, K.; Kamijo, Y. Identification of chronic kidney disease patient characteristics influencing the renoprotective effects of febuxostat therapy: A retrospective follow-up study. BMC Nephrol. 2017, 18, 162. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.K.; Ehdaie, A.; Farmand, F.; Dhaliwal, K.K.; Nguyen, T.; Zhan, C.D.; Roberts, C.K.; Vaziri, N.D. Expression of catalase and glutathione peroxidase in renal insufficiency. Biochim. Biophys. Acta 2005, 1743, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.A.; Koo, L.C.; Moberly, J.B. Low whole blood and erythrocyte levels of glutathione in hemodialysis and peritoneal dialysis patients. Am. J. Kidney Dis. 1997, 30, 489–494. [Google Scholar] [CrossRef]
- Ceballos-Picot, I.; Witko-Sarsat, V.; Merad-Boudia, M.; Nguyen, A.T.; Thévenin, M.; Jaudon, M.C.; Zingraff, J.; Verger, C.; Jungers, P.; Descamps-Latscha, B. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic Biol. Med. 1996, 21, 845–853. [Google Scholar] [CrossRef]
- Tbahriti, H.F.; Kaddous, A.; Bouchenak, M.; Mekki, K. Effect of different stages of chronic kidney disease and renal replacement therapies on oxidant-antioxidant balance in uremic patients. Biochem. Res. Int. 2013, 2013, 358985. [Google Scholar] [CrossRef] [PubMed]
- Granata, S.; Dalla Gassa, A.; Tomei, P.; Lupo, A.; Zaza, G. Mitochondria: A new therapeutic target in chronic kidney disease. Nutr. Metab. 2015, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, G.; Situlin, R.; Biolo, G. Carnitine metabolism in uremia. Am. J. Kidney Dis. 2001, 38, S63–S67. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Black, S.M. Carnitine homeostasis, mitochondrial function, and cardiovascular disease. Drug Discov. Today Dis. Mech. 2009, 6, e31–e39. [Google Scholar] [CrossRef] [PubMed]
- Kerner, J.; Hoppel, C. Fatty acid import into mitochondria. Biochim. Biophys. Acta 2000, 1486, 1–17. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidant and antiradical activities of l-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Calò, L.A.; Pagnin, E.; Davis, P.A.; Semplicini, A.; Nicolai, R.; Calvani, M.; Pessina, A.C. Antioxidant effect of l-carnitine and its short chain esters: Relevance for the protection from oxidative stress related cardiovascular damage. Int. J. Cardiol. 2006, 107, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Arockia Rani, P.J.; Panneerselvam, C. Carnitine as a free radical scavenger in aging. Exp. Gerontol. 2001, 36, 1713–1726. [Google Scholar] [CrossRef]
- Rani, P.J.; Panneerselvam, C. Effect of l-carnitine on brain lipid peroxidation and antioxidant enzymes in old rats. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B134–B137. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, G. Carnitine in maintenance hemodialysis patients. J. Ren. Nutr. 2015, 25, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Di Liberato, L.; Arduini, A.; Rossi, C.; Di Castelnuovo, A.; Posari, C.; Sacchetta, P.; Urbani, A.; Bonomini, M. l-Carnitine status in end-stage renal disease patients on automated peritoneal dialysis. J. Nephrol. 2014, 27, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, I.G.; Douroudos, I.; Panagoutsos, S.; Pasadakis, P.; Nikolaidis, M.G.; Chatzinikolaou, A.; Sovatzidis, A.; Michailidis, Y.; Jamurtas, A.Z.; Mandalidis, D.; et al. Effects of l-carnitine on oxidative stress responses in patients with renal disease. Med. Sci. Sports Exerc. 2010, 42, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Pertosa, G.; Grandaliano, G.; Simone, S.; Soccio, M.; Schena, F.P. Inflammation and carnitine in hemodialysis patients. J. Ren. Nutr. 2005, 15, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Golper, T.A.; Goral, S.; Becker, B.N.; Langman, C.B. l-carnitine treatment of anemia. Am. J. Kidney Dis. 2003, 41, S27–S34. [Google Scholar] [CrossRef]
- Kletzmayr, J.; Mayer, G.; Legenstein, E.; Heinz-Peer, G.; Leitha, T.; Hörl, W.H.; Kovarik, J. Anemia and carnitine supplementation in hemodialyzed patients. Kidney Int. Suppl. 1999, 69, S93–S106. [Google Scholar] [CrossRef] [PubMed]
- Yee, J. l-carnitine for anemia in hemodialysis patients: A last resort. Clin. J. Am. Soc. Nephrol. 2012, 7, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Hurot, J.M.; Cucherat, M.; Haugh, M.; Fouque, D. Effects of l-carnitine supplementation in maintenance hemodialysis patients: A systematic review. J. Am. Soc. Nephrol. 2002, 13, 708–714. [Google Scholar] [PubMed]
- Mercadal, L.; Coudert, M.; Vassault, A.; Pieroni, L.; Debure, A.; Ouziala, M.; Depreneuf, H.; Fumeron, C.; Servais, A.; Bassilios, N.; et al. l-carnitine treatment in incident hemodialysis patients: The multicenter, randomized, double-blinded, placebo-controlled CARNIDIAL trial. Clin. J. Am. Soc. Nephrol. 2012, 7, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Eknoyan, G.; Latos, D.L.; Lindberg, J.; National Kidney Foundation Carnitine Consensus Conference. Practice recommendations for the use of l-carnitine in dialysis-related carnitine disorder. National Kidney Foundation Carnitine Consensus Conference. Am. J. Kidney Dis. 2003, 41, 868–876. [Google Scholar] [CrossRef]
- Kliger, A.S.; Foley, R.N.; Goldfarb, D.S.; Goldstein, S.L.; Johansen, K.; Singh, A.; Szczech, L. KDOQI US commentary on the 2012 KDIGO Clinical Practice Guideline for Anemia in CKD. Am. J. Kidney Dis. 2013, 62, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Abbate, M.; Tang, L.; Cai, G.; Gong, Z.; Wei, R.; Zhou, J.; Chen, X. l-Carnitine supplementation for adults with end-stage kidney disease requiring maintenance hemodialysis: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 99, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Xiao, L.; Song, P.A.; Xu, X.; Liu, F.Y.; Sun, L. Effect of l-carnitine therapy on patients in maintenance hemodialysis: A systematic review and meta-analysis. J. Nephrol. 2014, 27, 317–329. [Google Scholar] [CrossRef] [PubMed]
- IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature of tocopherols and related compounds. Recommendations 1981. Eur. J. Biochem. 1982, 123, 473–475. [Google Scholar]
- Brigelius-Flohe, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [PubMed]
- Traber, M.G.; Atkinson, J. Vitamin E antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of α-tocopherol and α-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef]
- Princen, H.M.; van Duyvenvoorde, W.; Buytenhek, R.; van der Laarse, A.; van Poppel, G.; Gevers Leuven, J.A.; van Hinsbergh, V.W. Supplementation with low doses of vitamin E protects LDL from lipid peroxidation in men and women. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Cachia, O.; Benna, J.E.; Pedruzzi, E.; Descomps, B.; Gougerot-Pocidalo, M.A.; Leger, C.L. α-tocopherol inhibits the respiratory burst in human monocytes. Attenuation of p47(phox) membrane translocation and phosphorylation. J. Biol. Chem. 1998, 273, 32801–32805. [Google Scholar] [CrossRef] [PubMed]
- Bozaykut, P.; Karademir, B.; Yazgan, B.; Sozen, E.; Siow, R.C.; Mann, G.E.; Ozer, N.K. Effects of vitamin E on peroxisome proliferator-activated receptor γ and nuclear factor-erythroid 2-related factor 2 in hypercholesterolemia-induced atherosclerosis. Free Radic. Biol. Med. 2014, 70, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Stephens, N.G.; Parsons, A.; Schofield, P.M.; Kelly, F.; Cheeseman, K.; Mitchinson, M.J. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 1996, 347, 781–786. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Yoshida, N.; Manabe, H.; Terasawa, Y.; Takemura, T.; Kondo, M. α-Tocopherol protects against expression of adhesion molecules on neutrophils and endothelial cells. Biofactors 1998, 7, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Villacorta, L.; Graça-Souza, A.V.; Ricciarelli, R.; Zingg, J.M.; Azzi, A. α-Tocopherol induces expression of connective tissue growth factor and antagonizes tumor necrosis factor-α-mediated downregulation in human smooth muscle cells. Circ. Res. 2003, 92, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A.; Gysin, R.; Kempná, P.; Munteanu, A.; Negis, Y.; Villacorta, L.; Visarius, T.; Zingg, J.M. Vitamin E mediates cell signaling and regulation of gene expression. Ann. N. Y. Acad. Sci. 2004, 1031, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kuemerle, N.B.; Brandt, R.B.; Chan, W.; Krieg, R.J., Jr.; Chan, J.C. Inhibition of transforming growth factor β-1 induction by dietary vitamin E in unilateral obstruction in rats. Biochem. Mol. Med. 1997, 61, 82–96. [Google Scholar] [CrossRef]
- Boaz, M.; Smetana, S.; Weinstein, T.; Matas, Z.; Gafter, U.; Iaina, A.; Knecht, A.; Weissgarten, Y.; Brunner, D.; Fainaru, M.; et al. Secondary prevention with antioxidants of cardiovascular disease in end stage renal disease (SPACE): Randomised placebo-controlled trial. Lancet 2000, 356, 1213–1218. [Google Scholar] [CrossRef]
- Mann, J.F.; Lonn, E.M.; Yi, Q.; Gerstein, H.C.; Hoogwerf, B.J.; Pogue, J.; Bosch, J.; Dagenais, G.R.; Yusuf, S.; HOPE Investigators. Effects of vitamin E on cardiovascular outcomes in people with mild to moderate renal insufficiency: Results of the HOPE study. Kidney Int. 2004, 65, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Tasanarong, A.; Vohakiat, A.; Hutayanon, P.; Piyayotai, D. New strategy of α- and γ-tocopherol to prevent contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. Nephrol. Dial. Transplant. 2013, 28, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, Y.; Khademvatani, K.; Rahimi, B.; Khoshfetrat, M.; Arjmand, N.; Seyyed-Mohammadzad, M.H. Short-Term High-Dose Vitamin E to Prevent Contrast Medium-Induced Acute Kidney Injury in Patients With Chronic Kidney Disease Undergoing Elective Coronary Angiography: A Randomized Placebo-Controlled Trial. J. Am. Heart. Assoc. 2016, 5, e002919. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hsu, S.P.; Wu, M.S.; Hsu, S.M.; Chien, C.T. Effects of vitamin C infusion and vitamin E-coated membrane on hemodialysis-induced oxidative stress. Kidney Int. 2006, 69, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Takouli, L.; Hadjiyannakos, D.; Metaxaki, P.; Sideris, V.; Filiopoulos, V.; Anogiati, A.; Vlassopoulos, D. Vitamin E-coated cellulose acetate dialysis membrane: Long-term effect on inflammation and oxidative stress. Ren. Fail. 2010, 32, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Kirmizis, D.; Papagianni, A.; Belechri, A.M.; Memmos, D. Effects of vitamin E-coated membrane dialyser on markers of oxidative stress and inflammation in patients on chronic haemodialysis. Nephrol. Dial. Transplant. 2011, 26, 2296–2301. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; Rosati, A.; Paoletti, S.; Ferrandello, P.; Migliori, M.; Beati, S.; Bernabini, G.; Daini, R.; Casani, A.; Angelini, D.; et al. A vitamin E-coated polysulfone membrane reduces serum levels of inflammatory markers and resistance to erythropoietin-stimulating agents in hemodialysis patients: Results of a randomized cross-over multicenter trial. Blood Purif. 2011, 32, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Kamimura, K.; Yoshioka, N.; Hosotani, Y.; Tsuchida, K.; Koremoto, M.; Minakuchi, J. The effect of vitamin E-bonded polysulfone membrane dialyzer on a new oxidative lipid marker. J. Artif. Organ. 2013, 16, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Lines, S.W.; Carter, A.M.; Dunn, E.J.; Lindley, E.J.; Tattersall, J.E.; Wright, M.J. A randomized controlled trial evaluating the erythropoiesis stimulating agent sparing potential of a vitamin E-bonded polysulfone dialysis membrane. Nephrol. Dial. Transplant. 2014, 29, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Xiao, L.; Xu, B.; Xu, X.X.; Liu, F.Y.; Sun, L. Effects of vitamin E-coated dialyzer on oxidative stress and inflammation status in hemodialysis patients: A systematic review and meta-analysis. Ren. Fail. 2014, 36, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Veringa, S.J.; Nanayakkara, P.W.; van Ittersum, F.J.; Vegting, I.L.; van Guldener, C.; Smulders, Y.M.; ter Wee, P.M.; Stehouwer, C.D. Effect of a treatment strategy consisting of pravastatin, vitamin, E.; and homocysteine lowering on arterial compliance and distensibility in patients with mild to moderate chronic kidney disease. Clin. Nephrol. 2012, 78, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, P.W.; van Guldener, C.; ter Wee, P.M.; Scheffer, P.G.; van Ittersum, F.J.; Twisk, J.W.; Teerlink, T.; van Dorp, W.; Stehouwer, C.D. Effect of a treatment strategy consisting of pravastatin, vitamin, E.; and homocysteine lowering on carotid intima-media thickness, endothelial function, and renal function in patients with mild to moderate chronic kidney disease: Results from the Antioxidant Therapy in Chronic Renal Insufficiency (ATIC) Study. Arch. Intern. Med. 2007, 167, 1262–1270. [Google Scholar] [PubMed]
- Pearson, P.; Lewis, S.A.; Britton, J.; Young, I.S.; Fogarty, A. The pro-oxidant activity of high-dose vitamin E supplements in vivo. BioDrugs 2006, 20, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Antoniadi, G.; Eleftheriadis, T.; Liakopoulos, V.; Kakasi, E.; Kartsios, C.; Passadakis, P.; Vargemezis, V. Effect of one-year oral α-tocopherol administration on the antioxidant defense system in hemodialysis patients. Ther. Apher. Dial. 2008, 12, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Serbinova, E.A.; Forte, T.; Scita, G.; Packer, L. Recycling of vitamin E in human low density lipoproteins. J. Lipid Res. 1992, 33, 385–397. [Google Scholar] [PubMed]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Clase, C.M.; Ki, V.; Holden, R.M. Water-soluble vitamins in people with low glomerular filtration rate or on dialysis: A review. Semin. Dial. 2013, 26, 546–567. [Google Scholar] [CrossRef] [PubMed]
- Monsen, E.R. Dietary reference intakes for the antioxidant nutrients: Vitamin, C.; vitamin, E.; selenium, and carotenoids. J. Am. Diet Assoc. 2000, 100, 637–640. [Google Scholar] [CrossRef]
- Descamps-Latscha, B.; Drüeke, T.; Witko-Sarsat, V. Dialysis-induced oxidative stress: Biological aspects, clinical consequences, and therapy. Semin. Dial. 2001, 14, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Gruss-Fischer, T.; Fabian, I. Protection by ascorbic acid from denaturation and release of cytochrome c, alteration of mitochondrial membrane potential and activation of multiple caspases induced by H2O2, in human leukemia cells. Biochem. Pharmacol. 2002, 63, 1325–1335. [Google Scholar] [CrossRef]
- Perez-Cruz, I.; Carcamo, J.M.; Golde, D.W. Vitamin C inhibits FAS-induced apoptosis in monocytes and U937 cells. Blood 2003, 102, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Dhar-Mascareño, M.; Cárcamo, J.M.; Golde, D.W. Hypoxia-reoxygenation-induced mitochondrial damage and apoptosis in human endothelial cells are inhibited by vitamin C. Free Radic. Biol. Med. 2005, 38, 1311–1322. [Google Scholar]
- KC, S.; Cárcamo, J.M.; Golde, D.W. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J. 2005, 19, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Qu, Z.C.; Whitesell, R.R.; Cobb, C.E. Ascorbate recycling in human erythrocytes: Role of GSH in reducing dehydroascorbate. Free Radic Biol Med. 1996, 20, 543–551. [Google Scholar] [CrossRef]
- Nguyen-Khoa, T.; Massy, Z.A.; De Bandt, J.P.; Kebede, M.; Salama, L.; Lambrey, G.; Witko-Sarsat, V.; Drüeke, T.B.; Lacour, B.; Thévenin, M. Oxidative stress and haemodialysis: Role of inflammation and duration of dialysis treatment. Nephrol. Dial. Transplant. 2001, 16, 335–3540. [Google Scholar] [CrossRef] [PubMed]
- Deicher, R.; Horl, W.H. Vitamin C in chronic kidney disease and hemodialysis patients. Kidney Blood Press. Res. 2003, 26, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Deicher, R.; Ziai, F.; Bieglmayer, C.; Schillinger, M.; Hörl, W.H. Low total vitamin C plasma level is a risk factor for cardiovascular morbidity and mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Morimoto, S.; Okigaki, M.; Seo, M.; Someya, K.; Morita, T.; Matsubara, H.; Sugiura, T.; Iwasaka, T. Decreased plasma level of vitamin C in chronic kidney disease: Comparison between diabetic and non-diabetic patients. Nephrol. Dial. Transplant. 2011, 26, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Kamgar, M.; Zaldivar, F.; Vaziri, N.D.; Pahl, M.V. Antioxidant therapy does not ameliorate oxidative stress and inflammation in patients with end-stage renal disease. J. Natl. Med. Assoc. 2009, 101, 336–344. [Google Scholar] [CrossRef]
- Singer, R.F. Vitamin C supplementation in kidney failure: Effect on uraemic symptoms. Nephrol. Dial. Transplant. 2011, 26, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, Y.; Cheng, X.; Liu, L.; Bai, W.; Guo, W.; Wu, L.; Zuo, L. Cross-over study of influence of oral vitamin C supplementation on inflammatory status in maintenance hemodialysis patients. BMC Nephrol. 2013, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Deved, V.; Poyah, P.; James, M.T.; Tonelli, M.; Manns, B.J.; Walsh, M.; Hemmelgarn, B.R. Alberta Kidney Disease Network. Ascorbic acid for anemia management in hemodialysis patients: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 54, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Ahn, C.Y.; Ryu, B.K.; Shin, B.C.; Chung, J.H.; Kim, H.L. The effect of intravenous ascorbic acid in hemodialysis patients with normoferritinemic anemia. Kidney Res. Clin. Pract. 2012, 31, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Ernster, L.; Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta 1995, 1271, 195–204. [Google Scholar] [CrossRef]
- Pravst, I.; Zmitek, K.; Zmitek, J. Coenzyme Q10 contents in foods and fortification strategies. Crit. Rev. Food Sci. Nutr. 2010, 50, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Bentinger, M.; Tekle, M.; Dallner, G. Coenzyme Q—Biosynthesis and functions. Biochem. Biophys. Res. Commun. 2010, 396, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Bentinger, M.; Brismar, K.; Dallner, G. The antioxidant role of coenzyme Q. Mitochondrion 2007, 7, S41–S50. [Google Scholar] [CrossRef] [PubMed]
- Papucci, L.; Schiavone, N.; Witort, E.; Donnini, M.; Lapucci, A.; Tempestini, A.; Formigli, L.; Zecchi-Orlandini, S.; Orlandini, G.; et al. Coenzyme q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J. Biol. Chem. 2003, 278, 28220–28228. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, C.; Moruzzi, N.; Sblendido, A.; Lenaz, G.; Fato, R. A water soluble CoQ10 formulation improves intracellular distribution and promotes mitochondrial respiration in cultured cells. PLoS ONE 2012, 7, e33712. [Google Scholar] [CrossRef] [PubMed]
- Gazdíková, K.; Gvozdjáková, A.; Kucharská, J.; Spustová, V.; Braunová, Z.; Dzúrik, R. Oxidative stress and plasma concentrations of coenzyme Q10, α-tocopherol, and beta-carotene in patients with a mild to moderate decrease of kidney function. Nephron 2001, 88, 285. [Google Scholar] [CrossRef] [PubMed]
- Lippa, S.; Colacicco, L.; Callà, C.; Sagliaschi, G.; Angelitti, A.G. Coenzyme Q10 levels, plasma lipids and peroxidation extent in renal failure and in hemodialytic patients. Mol. Aspects Med. 1994, 15, S213–S219. [Google Scholar] [CrossRef]
- Macunluoglu, B.; Atakan, A.; Ari, E.; Kaya, Y.; Kaspar, C.; Demir, H.; Alp, H.H. Epicardial fat tissue thickness is correlated with diminished levels of co-enzyme Q10, a major antioxidant molecule among hemodialysis patients. Clin. Biochem. 2014, 47, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.R.; Silva, N.F.; Quinn, D.W.; Harte, A.L.; Pagano, D.; Bonser, R.S.; Kumar, S.; McTernan, P.G. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc. Diabetol. 2006, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Sacks, H.S.; Fain, J.N. Human epicardial adipose tissue: A review. Am. Heart J. 2007, 153, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, K.; Ozbek, O.; Kayikcioğlu, H.; Kayrak, M.; Solak, Y.; Nayman, A.; Anil, M.; Babur, H.; Tonbul, H.Z. The Relationship between Epicardial Adipose Tissue and Coronary Artery Calcification in Peritoneal Dialysis Patients. Cardiorenal. Med. 2012, 2, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Atakan, A.; Macunluoglu, B.; Kaya, Y.; Ari, E.; Demir, H.; Asicioglu, E.; Kaspar, C. Epicardial fat thickness is associated with impaired coronary flow reserve in hemodialysis patients. Hemodial. Int. 2014, 18, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Gokbel, H.; Turk, S.; Okudan, N.; Atalay, H.; Belviranli, M.; Gaipov, A.; Solak, Y. Effects of Coenzyme Q10 Supplementation on Exercise Performance and Markers of Oxidative Stress in Hemodialysis Patients: A Double-Blind Placebo-Controlled Crossover Trial. Am. J. Ther. 2016, 23, e1736–e1743. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; debusk, B.G.; Gunsalus, I.; Hornberger, C., Jr. Crystalline α-lipoic acid; A catalytic agent associated with pyruvate dehydrogenase. Science 1951, 114, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. α-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Sigel, H.; Prijs, B.; McCormick, D.B.; Shih, J.C. Stability of binary and ternary complexes of a-lipoate and lipoate derivatives with Mn2+, Cu2+, and Zn2+ in solution. Arch. Biochem. Biophys. 1978, 187, 208–214. [Google Scholar] [CrossRef]
- Liu, J. The effects and mechanisms of mitochondrial nutrient α-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: An overview. Neurochem. Res. 2008, 33, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Packer, L. α-Lipoic acid: A metabolic antioxidant which regulates NF-κB signal transduction and protects against oxidative injury. Drug Metab. Rev. 1998, 30, 245–275. [Google Scholar] [CrossRef] [PubMed]
- Petersen Shay, K.; Moreau, R.F.; Smith, E.J.; Hagen, T.M. Is α-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life 2008, 60, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.J.; Lee, K.; Kim, J.M.; Kim, H.S.; Kim, J.R.; Ha, C.M.; Choi, Y.K.; Lee, S.J.; Kim, J.Y.; Harris, R.A.; Jeong, D.; Lee, I.K. α-Lipoic acid attenuates vascular calcification via reversal of mitochondrial function and restoration of Gas6/Axl/Akt survival pathway. J. Cell. Mol. Med. 2012, 16, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, C.G.; Fang, C.Q.; Gao, J.; Liu, Y.Z.; Chen, Y.; Chen, Y.N.; Xu, Z.G. The protective effect of α-Lipoic acid on mitochondria in the kidney of diabetic rats. Int. J. Clin. Exp. Med. 2013, 6, 90–97. [Google Scholar] [PubMed]
- Melhem, M.F.; Craven, P.A.; Liachenko, J.; De Rubertis, F.R. α-lipoic acid attenuates hyperglycemia and prevents glomerular mesangial matrix expansion in diabetes. J. Am. Soc. Nephrol. 2002, 13, 16. [Google Scholar]
- Khabbazi, T.; Mahdavi, R.; Safa, J.; Pour-Abdollahi, P. Effects of α-lipoic acid supplementation on inflammation, oxidative stress, and serum lipid profile levels in patients with end-stage renal disease on hemodialysis. J. Ren. Nutr. 2012, 22, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Lee, E.K.; Kim, T.H.; Min, W.K.; Chun, S.; Lee, K.U.; Kim, S.B.; Park, J.S. Effects of α lipoic acid on the plasma levels of asymmetric dimethylarginine in diabetic end-stage renal disease patients on hemodialysis: A pilot study. Am. J. Nephrol. 2007, 27, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Safa, J.; Ardalan, M.R.; Rezazadehsaatlou, M.; Mesgari, M.; Mahdavi, R.; Jadid, M.P. Effects of α lipoic acid supplementation on serum levels of IL-8 and TNF-α in patient with ESRD undergoing hemodialysis. Int. Urol. Nephrol. 2014, 46, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.F.; Kane, J.; McMonagle, E.; Le, P.; Wu, P.; Shintani, A. Effects of combination tocopherols and α lipoic acid therapy on oxidative stress and inflammatory biomarkers in chronic kidney disease. J. Ren. Nutr. 2011, 21, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Ikizler, T.A.; Ellis, C.; Wu, P.; Shintani, A.; Dalal, S.; Kaplan, M.; Chonchol, M.; Hakim, R.M. Provision of antioxidant therapy in hemodialysis (PATH): A randomized clinical trial. J. Am. Soc. Nephrol. 2014, 25, 623–633. [Google Scholar] [CrossRef]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256. [Google Scholar] [CrossRef]
- Fujishima, Y.; Ohsawa, M.; Itai, K.; Kato, K.; Tanno, K.; Turin, T.C.; Onoda, T.; Endo, S.; Okayama, A.; Fujioka, T. Serum selenium levels are inversely associated with death risk among hemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 3331–3338. [Google Scholar] [CrossRef] [PubMed]
- Rucker, D.; Thadhani, R.; Tonelli, M. Trace element status in hemodialysis patients. Semin. Dial. 2010, 23, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Hemmelgarn, B.; Klarenbach, S.; Field, C.; Manns, B.; Thadhani, R.; Gill, J. Alberta Kidney Disease Network. Trace elements in hemodialysis patients: A systematic review and meta-analysis. BMC Med. 2009, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Pakfetrat, M.; Malekmakan, L.; Hasheminasab, M. Diminished selenium levels in hemodialysis and continuous ambulatory peritoneal dialysis patients. Biol. Trace Elem. Res. 2010, 137, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, O.; Zargari, M.; Varshi, G. Effect of selenium supplementation on glutathione peroxidase enzyme activity in patients with chronic kidney disease: A randomized clinical trial. Nephrourol. Mon. 2014, 6, e17945. [Google Scholar] [CrossRef] [PubMed]
- Zachara, B.A.; Koterska, D.; Manitius, J.; Sadowski, L.; Dziedziczko, A.; Salak, A.; Wasowicz, W. Selenium supplementation on plasma glutathione peroxidase activity in patients with end-stage chronic renal failure. Biol. Trace Elem Res. 2004, 97, 15–30. [Google Scholar] [CrossRef]
- Zachara, B.A.; Gromadzinska, J.; Palus, J.; Zbrog, Z.; Swiech, R.; Twardowska, E.; Wasowicz, W. The effect of selenium supplementation in the prevention of DNA damage in white blood cells of hemodialyzed patients: A pilot study. Biol. Trace Elem. Res. 2011, 142, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Sohrabi, Z.; Ekramzadeh, M.; Fallahzadeh, M.K.; Ayatollahi, M.; Geramizadeh, B.; Hassanzadeh, J.; Sagheb, M.M. Selenium supplementation improves the nutritional status of hemodialysis patients: A randomized, double-blind, placebo-controlled trial. Nephrol. Dial. Transplant. 2013, 28, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Omrani, H.R.; Rahimi, M.; Nikseresht, K. The effect of selenium supplementation on acute phase reactants and thyroid function tests in hemodialysis patients. Nephrourol. Mon. 2015, 7, e24781. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, M.R.; Tubbs, R.S.; Shoja, M.M. Vitamin E and selenium co-supplementation attenuates oxidative stress in haemodialysis patients receiving intra-dialysis iron infusion. Nephrol. Dial. Transplant. 2007, 22, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.A.B.; Tavares, J.F.D.; Carvalho, R.C.B. Comparison of catechins and aromas among different green teas using HPLC/SPME-GC. Food Res. Int. 1998, 31, 729–736. [Google Scholar] [CrossRef]
- Yokozawa, T.; Dong, E.; Nakagawa Kashiwagi, H.; Nakagawa, H.; Takeuchi, S.; Chung, H.Y. In vitro and in vivo studies on the radical-scavenging activity of tea. J. Agric. Food Chem. 1998, 46, 2143–2150. [Google Scholar] [CrossRef]
- Nanjo, F.; Mori, M.; Goto, K.; Hara, Y. Radical scavenging activity of tea catechins and their related compounds. Biosci. Biotechnol. Biochem. 1999, 63, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Guo, Q.; Xin, W. Free radical scavenging by green tea polyphenols. Methods Enzymolol. 2001, 335, 217–231. [Google Scholar]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Peng, A. The Green Tea Polyphenol(−)-epigallocatechin-3-gallate and its beneficial roles in chronic kidney disease. J. Transl. Int. Med. 2016, 4, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Peng, A.; Ye, T.; Rakheja, D.; Tu, Y.; Wang, T.; Du, Y.; Zhou, J.K.; Vaziri, N.D.; Hu, Z.; Mohan, C.; Zhou, X.J. The green tea polyphenol (−)-epigallocatechin-3-gallate ameliorates experimental immune-mediated glomerulonephritis. Kidney Int. 2011, 80, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Zhen, J.; Du, Y.; Zhou, J.K.; Peng, A.; Vaziri, N.D.; Mohan, C.; Xu, Y.; Zhou, X.J. Green tea polyphenol (−)-epigallocatechin-3-gallate restores Nrf2 activity and ameliorates crescentic glomerulonephritis. PLoS ONE 2015, 10, e0119543. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.Y.; Ka, S.M.; Chang, J.M.; Chen, H.C.; Shui, H.A.; Li, C.Y.; Hua, K.F.; Chang, W.L.; Huang, J.J.; Yang, S.S.; et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic. Biol. Med. 2011, 51, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, N.; Yokozawa, T.; Oya, T.; Kim, M. Therapeutic potential of (−)-epigallocatechin 3-O-gallate on renal damage in diabetic nephropathy model rats. J. Pharmacol. Exp. Ther. 2006, 319, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yu, J.F.; Zhao, C.G.; Sui, F.X.; Teng, X.; Wu, Y.B. Therapeutic potential of EGCG on acute renal damage in a rat model of obstructive nephropathy. Mol. Med. Rep. 2013, 7, 1096–1102. [Google Scholar] [PubMed]
- Nakagawa, T.; Yokozawa, T.; Sano, M.; Takeuchi, S.; Kim, M.; Minamoto, S. Activity of (−)-epigallocatechin 3-O-gallate against oxidative stress in rats with adenine-induced renal failure. J. Agric. Food Chem. 2004, 52, 2103–2107. [Google Scholar] [CrossRef] [PubMed]
- Kakuta, Y.; Okumi, M.; Isaka, Y.; Tsutahara, K.; Abe, T.; Yazawa, K.; Ichimaru, N.; Matsumura, K.; Hyon, S.H.; Takahara, S.; Nonomura, N. Epigallocatechin-3-gallate protects kidneys from ischemia reperfusion injury by HO-1 upregulation and inhibition of macrophage infiltration. Transpl. Int. 2011, 24, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.A.; Das, D.K. Grapes, wines, resveratrol, and heart health. J. Cardiovasc. Pharmacol. 2009, 54, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.S.; Xia, C.; Jiang, B.H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Spanier, G.; Xu, H.; Xia, N.; Tobias, S.; Deng, S.; Wojnowski, L.; Forstermann, U.; Li, H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J. Physiol. Pharmacol. 2009, 60, 111–116. [Google Scholar] [PubMed]
- Hao, C.M.; Haase, V.H. Sirtuins and their relevance to the kidney. J. Am. Soc. Nephrol. 2010, 21, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Yang, F.; Jiang, K.; Ji, J.Y.; Watts, J.L.; Purushotham, A.; Boss, O.; Hirsch, M.L.; Ribich, S.; Smith, J.J.; et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010, 24, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Kume, S.; Takeda-Watanabe, A.; Kanasaki, K.; Koya, D. Sirtuins and renal diseases: Relationship with aging and diabetic nephropathy. Clin. Sci. 2013, 124, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Koya, D. Renal protective effects of resveratrol. Oxid. Med. Cell. Longev. 2013, 2013, 568093. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Anjaneyulu, M.; Kulkarni, S.K.; Chopra, K. Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology 2006, 76, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Palsamy, P.; Subramanian, S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim. Biophys. Acta 2011, 1812, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pang, S.; Deng, B.; Qian, L.; Chen, J.; Zou, J.; Zheng, J.; Yang, L.; Zhang, C.; Chen, X.; et al. High glucose induces renal mesangial cell proliferation and fibronectin expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is inhibited by resveratrol. Int. J. Biochem. Cell Biol. 2012, 44, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Ding, D.F.; Chen, S.; Dong, C.L.; Ye, X.L.; Yuan, Y.G.; Feng, Y.M.; You, N.; Xu, J.R.; Miao, H.; et al. Resveratrol protects podocytes against apoptosis via stimulation of autophagy in a mouse model of diabetic nephropathy. Sci. Rep. 2017, 7, 45692. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Ricardo, S.D.; Bertram, J.F.; Nikolic-Paterson, D.J. Resveratrol inhibits renal fibrosis in the obstructed kidney: Potential role in deacetylation of Smad3. Am. J. Pathol. 2010, 177, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Tian, S.; Han, J.; Xiong, P. Resveratrol as a therapeutic agent for renal fibrosis induced by unilateral ureteral obstruction. Ren. Fail. 2014, 36, 285–291. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Guan, X.; Wang, S.; Xiao, T.; Yang, K.; Xu, X.; Wang, J.; Zhao, J. Resveratrol prevents high glucose-induced epithelial-mesenchymal transition in renal tubular epithelial cells by inhibiting NADPH oxidase/ROS/ERK pathway. Mol. Cell Endocrinol. 2015, 402, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Wen, D.; Zhang, M.; Xie, Q.; Ma, L.; Guan, Y.; Ren, Y.; Chen, J.; Hao, C.M. Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-β/Smad3 pathway. J. Cell. Biochem. 2014, 115, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.J.; Sun, Y.N.; Chen, S.J.; Liu, S.; Jiang, G.R. Resveratrol attenuates skeletal muscle atrophy induced by chronic kidney disease via MuRF1 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 487, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Um, J.H.; Park, S.J.; Kang, H.; Yang, S.; Foretz, M.; McBurney, M.W.; Kim, M.K.; Viollet, B.; Chung, J.H. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes 2010, 59, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Huang, S.; Wang, W.; Wang, Y.; Zhang, P.; Zhu, C. Activation of peroxisome proliferator-activated receptor-γ coactivator 1α ameliorates mitochondrial dysfunction and protects podocytes from aldosterone-induced injury. Kidney Int. 2012, 82, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Do Amaral, C.L.; Francescato, H.D.; Coimbra, T.M.; Costa, R.S.; Darin, J.D.; Antunes, L.M.; Bianchi, M.L. Resveratrol attenuates cisplatin-induced nephrotoxicity in rats. Arch. Toxicol. 2008, 82, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.A.; Migliori, M.; Panichi, V.; Origlia, N.; Filippi, C.; Das, D.K.; Giovannini, L. Resveratrol, a component of wine and grapes, in the prevention of kidney disease. Ann. N. Y. Acad. Sci. 2002, 957, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, S.; Zumbrun, E.E.; Guan, H.; Nagarkatti, P.S.; Nagarkatti, M. Resveratrol attenuates lipopolysaccharide-induced acute kidney injury by suppressing inflammation driven by macrophages. Mol. Nutr. Food Res. 2015, 59, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Smoliga, J.M.; Blanchard, O. Enhancing the delivery of resveratrol in humans: If low bioavailability is the problem, what is the solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.; Chirino, Y.I.; Molina-Jijón, E.; Andérica-Romero, A.C.; Tapia, E.; Pedraza-Chaverrí, J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol. 2013, 1, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. From exotic spice to modern drug? Cell 2007, 130, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Bates, T.E.; Mancuso, C.; Cornelius, C.; Ventimiglia, B.; Cambria, M.T. Curcumin and the cellular stress response in free radical-related diseases. Mol. Nutr. Food Res. 2008, 52, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Atsumi, T.; Ishihara, M.; Kadoma, Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res. 2004, 24, 563–569. [Google Scholar] [PubMed]
- Mun, S.H.; Joung, D.K.; Kim, Y.S.; Kang, O.H.; Kim, S.B.; Seo, Y.S.; Kim, Y.C.; Lee, D.S.; Shin, D.W.; Kweon, K.T.; Kwon, D.Y. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE 2011, 6, e26012. [Google Scholar] [CrossRef] [PubMed]
- Das, K.C.; Das, C.K. Curcumin (diferuloylmethane), a singlet oxygen ((1)O(2)) quencher. Biochem. Biophys. Res. Commun. 2002, 295, 62–66. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, A.R.; Chung, H.Y.; Han, S.Y.; Kim, B.S.; Choi, J.S. In vitro peroxynitrite scavenging activity of diarylheptanoids from Curcuma longa. Phytother. Res. 2003, 17, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; Oh, G.S.; Pae, H.O.; Jeong, S.O.; Kim, Y.C.; Shin, M.K.; Seo, B.Y.; Han, S.Y.; Lee, H.S.; Jeong, J.G.; et al. Comparative effects of curcuminoids on endothelial heme oxygenase-1 expression: Ortho-methoxy groups are essential to enhance heme oxygenase activity and protection. Exp. Mol. Med. 2006, 38, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.F.; Hou, Z.Q.; Zhong, L.M.; Zhang, Q.Q. Effect of curcumin on the induction of glutathione S-transferases and NADP(H):quinone oxidoreductase and its possible mechanism of action. Yao Xue Xue Bao 2007, 42, 376–380. [Google Scholar] [PubMed]
- Rushworth, S.A.; Ogborne, R.M.; Charalambos, C.A.; O’Connell, M.A. Role of protein kinase C delta in curcumin-induced antioxidant response element-mediated gene expression in human monocytes. Biochem. Biophys. Res. Commun. 2006, 341, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulkarni, S.K.; Chopra, K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Watanabe, K.; Sari, F.R.; Harima, M.; Thandavarayan, R.A.; Veeraveedu, P.T.; Arozal, W.; Sukumaran, V.; Lakshmanan, A.P.; Arumugam, S.; Suzuki, K. Curcumin attenuates diabetic nephropathy by inhibiting PKC-α and PKC-β1 activity in streptozotocin-induced type I diabetic rats. Mol. Nutr. Food Res. 2011, 55, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Sari, F.R.; Veeraveedu, P.T.; Thandavarayan, R.A.; Harima, M.; Sukumaran, V.; Lakshmanan, A.P.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr. Metab. 2011, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.; Khan, Z.A.; Farhangkhoee, H.; Chakrabarti, S. Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-κB. Nutrition 2009, 25, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yin, N.; Liu, W.; Cui, X.; Chen, S.; Wang, E. Curcumin Ameliorates Diabetic Nephropathy by Suppressing NLRP3 Inflammasome Signaling. BioMed Res. Int. 2017, 2017, 1516985. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Massey, H.D.; Krieg, R.; Fazelbhoy, Z.A.; Ghosh, S.; Sica, D.A.; Fakhry, I.; Gehr, T.W. Curcumin ameliorates renal failure in 5/6 nephrectomized rats: Role of inflammation. Am. J. Physiol. Ren. Physiol. 2009, 296, F1146–F1157. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Gehr, T.W.; Ghosh, S. Curcumin and chronic kidney disease (CKD): Major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules 2014, 19, 20139–20156. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Chaves, L.; Eadon, M.T.; Chang, A.; Quigg, R.J.; Alexander, J.J. Curcumin alleviates immune-complex-mediated glomerulonephritis in factor-H-deficient mice. Immunology 2013, 139, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Tapia, E.; Soto, V.; Ortiz-Vega, K.M.; Zarco-Márquez, G.; Molina-Jijón, E.; Cristóbal-García, M.; Santamaría, J.; García-Niño, W.R.; Correa, F.; Zazueta, C.; et al. Curcumin induces Nrf2 nuclear translocation and prevents glomerular hypertension, hyperfiltration, oxidant stress, and the decrease in antioxidant enzymes in 5/6 nephrectomized rats. Oxid. Med. Cell. Longev. 2012, 2012, 269039. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Sari, F.R.; Lakshmanan, A.P.; Arumugam, S.; Harima, M.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Mol. Nutr. Food Res. 2013, 57, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Tapia, E.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Zarco-Márquez, G.; Molina-Jijón, E.; Cristóbal-García, M.; Santamaría, J.; Pedraza-Chaverri, J. Curcumin reverses glomerular hemodynamic alterations and oxidant stress in 5/6 nephrectomized rats. Phytomedicine 2013, 20, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Trejo, O.E.; Tapia, E.; Molina-Jijón, E.; Medina-Campos, O.N.; Macías-Ruvalcaba, N.A.; León-Contreras, J.C.; Hernández-Pando, R.; García-Arroyo, F.E.; Cristóbal, M.; Sánchez-Lozada, L.G.; et al. Curcumin prevents mitochondrial dynamics disturbances in early 5/6 nephrectomy: Relation to oxidative stress and mitochondrial bioenergetics. Biofactors 2017, 43, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Bugyei-Twum, A.; Abadeh, A.; Thai, K.; Zhang, Y.; Mitchell, M.; Kabir, G.; Connelly, K.A. Suppression of NLRP3 Inflammasome Activation Ameliorates Chronic Kidney Disease-Induced Cardiac Fibrosis and Diastolic Dysfunction. Sci. Rep. 2016, 6, 39551. [Google Scholar] [CrossRef] [PubMed]
- Correa, F.; Buelna-Chontal, M.; Hernández-Reséndiz, S.; García-Niño, W.R.; Roldán, F.J.; Soto, V.; Silva-Palacios, A.; Amador, A.; Pedraza-Chaverrí, J.; Tapia, E.; et al. Curcumin maintains cardiac and mitochondrial function in chronic kidney disease. Free Radic. Biol. Med. 2013, 61, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Reséndiz, S.; Correa, F.; García-Niño, W.R.; Buelna-Chontal, M.; Roldán, F.J.; Ramírez-Camacho, I.; Delgado-Toral, C.; Carbó, R.; Pedraza-Chaverrí, J.; Tapia, E.; Zazueta, C. Cardioprotection by curcumin post-treatment in rats with established chronic kidney disease. Cardiovasc. Drugs Ther. 2015, 29, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P.; Pakfetrat, M.; Javidnia, K.; Azad, F.; Malekmakan, L.; Nasab, M.H.; Dehghanzadeh, G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-beta and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: A randomized, double-blind and placebo-controlled study. Scand. J. Urol. Nephrol. 2011, 45, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P.; Zanjaninejad, B.; Aflaki, E.; Nazarinia, M.; Azad, F.; Malekmakan, L. Oral supplementation of turmeric decreases proteinuria, hematuria, and systolic blood pressure in patients suffering from relapsing or refractory lupus nephritis: A randomized and placebo-controlled study. J. Ren. Nutr. 2012, 22, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, H. The roles of anabolic and catabolic reactions in the synthesis and recycling of polyunsaturated fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2002, 67, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.S.; Hassan, S.K.; Hijazi, E.G.; Khazim, K.O. Effects of omega-3 on lipid profile and inflammation markers in peritoneal dialysis patients. Ren. Fail. 2010, 32, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Shing, C.M.; Adams, M.J.; Fassett, R.G.; Coombes, J.S. Nutritional compounds influence tissue factor expression and inflammation of chronic kidney disease patients in vitro. Nutrition 2011, 27, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Novak, T.E.; Babcock, T.A.; Jho, D.H.; Helton, W.S.; Espat, N.J. NF-κB inhibition by omega-3 fatty acids modulates LPS-stimulated macrophage TNF-α transcription. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L84–L89. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Massaro, M. Omega-3 fatty acids and the regulation of expression of endothelial pro-atherogenic and pro-inflammatory genes. J. Membr. Biol. 2005, 206, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; Shih, C.; Cashman, J.R. Docosahexaenoic acid is a strong inhibitor of prostaglandin but not leukotriene biosynthesis. Proc. Natl. Acad. Sci. USA 1983, 80, 3581–3584. [Google Scholar] [CrossRef] [PubMed]
- Needleman, P.; Raz, A.; Minkes, M.S.; Ferrendelli, J.A.; Sprecher, H. Triene prostaglandins: Prostacyclin and thromboxane biosynthesis and unique biological properties. Proc. Natl. Acad. Sci. USA 1979, 76, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Seki, H.; Tani, Y.; Arita, M. Omega-3 PUFA derived anti-inflammatory lipid mediator resolvin E1. Prostaglandins Other Lipid Mediat. 2009, 89, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Wiktorowska-Owczarek, A.; Berezińska, M.; Nowak, J.Z. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Surette, M.E. The science behind dietary omega-3 fatty acids. CMA J. 2008, 178, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Yaqoob, P.; Thies, F.; Wallace, F.A.; Miles, E.A. Fatty acids and lymphocyte functions. Br. J. Nutr. 2002, 87, S31–S48. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Conn, C.A.; Trujillo, K.A. Conjugated linoleic acid or omega 3 fatty acids increase mitochondrial biosynthesis and metabolism in skeletal muscle cells. Lipids Health Dis. 2012, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Arab, K.; Rossary, A.; Flourié, F.; Tourneur, Y.; Steghens, J.P. Docosahexaenoic acid enhances the antioxidant response of human fibroblasts by upregulating gamma-glutamyl-cysteinyl ligase and glutathione reductase. Br. J. Nutr. 2006, 95, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Chung, H.Y. Antioxidative and anti-inflammatory actions of docosahexaenoic acid and eicosapentaenoic acid in renal epithelial cells and macrophages. J. Med. Food 2007, 10, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Gobe, G.C.; Fassett, R.G.; Coombes, J.S. The effects of dietary fish oil on inflammation, fibrosis and oxidative stress associated with obstructive renal injury in rats. Mol. Nutr. Food Res. 2011, 55, 400–410. [Google Scholar] [CrossRef] [PubMed]
- An, W.S.; Kim, H.J.; Cho, K.H.; Vaziri, N.D. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am. J. Physiol. Ren. Physiol. 2009, 297, F895–F903. [Google Scholar] [CrossRef] [PubMed]
- de Mattos, A.M.; da Costa, J.A.C.; Jordão Júnior, A.A.; Chiarello, P.G. Omega-3 Fatty Acid Supplementation is Associated with Oxidative Stress and Dyslipidemia, but Does not Contribute to Better Lipid and Oxidative Status on Hemodialysis Patients. J. Ren. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mas, E.; Barden, A.; Burke, V.; Beilin, L.J.; Watts, G.F.; Huang, R.C.; Puddey, I.B.; Irish, A.B.; Mori, T.A. A randomized controlled trial of the effects of n-3 fatty acids on resolvins in chronic kidney disease. Clin. Nutr. 2016, 35, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.M.; Booker, C.; Ellis, C.D.; Siew, E.D.; Graves, A.J.; Shintani, A.; Abumrad, N.N.; Himmelfarb, J.; Ikizler, T.A. Omega-3 fatty acids inhibit the up-regulation of endothelial chemokines in maintenance hemodialysis patients. Nephrol. Dial. Transplant. 2015, 30, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Dashti-Khavidaki, S.; Farnood, F.; Noshad, H.; Lotfi, M.; Gharekhani, A. Therapeutic Effects of Omega-3 Fatty Acids on Chronic Kidney Disease-Associated Pruritus: A Literature Review. Adv. Pharm. Bull. 2016, 6, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Burke, V.; Puddey, I.; Irish, A.; Cowpland, C.A.; Beilin, L.; Dogra, G.; Watts, G.F. The effects of omega-3 fatty acids and coenzyme Q10 on blood pressure and heart rate in chronic kidney disease: A randomized controlled trial. J. Hypertens. 2009, 27, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.E.; Burke, V.; Mas, E.; Beilin, L.J.; Puddey, I.B.; Watts, G.F.; Irish, A.B.; Mori, T.A. n-3 fatty acids reduce plasma 20-hydroxyeicosatetraenoic acid and blood pressure in patients with chronic kidney disease. J. Hypertens. 2015, 33, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Ferraccioli, G.F.; Gambaro, G.; Fulignati, P.; Costanzi, S. Combined treatment with renin-angiotensin system blockers and polyunsaturated fatty acids in proteinuric IgA nephropathy: A randomized controlled trial. Nephrol. Dial. Transplant. 2009, 24, 156–160. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorini, L.; Granata, S.; Lupo, A.; Zaza, G. Naturally Occurring Compounds: New Potential Weapons against Oxidative Stress in Chronic Kidney Disease. Int. J. Mol. Sci. 2017, 18, 1481. https://doi.org/10.3390/ijms18071481
Signorini L, Granata S, Lupo A, Zaza G. Naturally Occurring Compounds: New Potential Weapons against Oxidative Stress in Chronic Kidney Disease. International Journal of Molecular Sciences. 2017; 18(7):1481. https://doi.org/10.3390/ijms18071481
Chicago/Turabian StyleSignorini, Lorenzo, Simona Granata, Antonio Lupo, and Gianluigi Zaza. 2017. "Naturally Occurring Compounds: New Potential Weapons against Oxidative Stress in Chronic Kidney Disease" International Journal of Molecular Sciences 18, no. 7: 1481. https://doi.org/10.3390/ijms18071481