Transcriptome Differences in Porcine Alveolar Macrophages from Tongcheng and Large White Pigs in Response to Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Infection
Abstract
:1. Introduction
2. Results
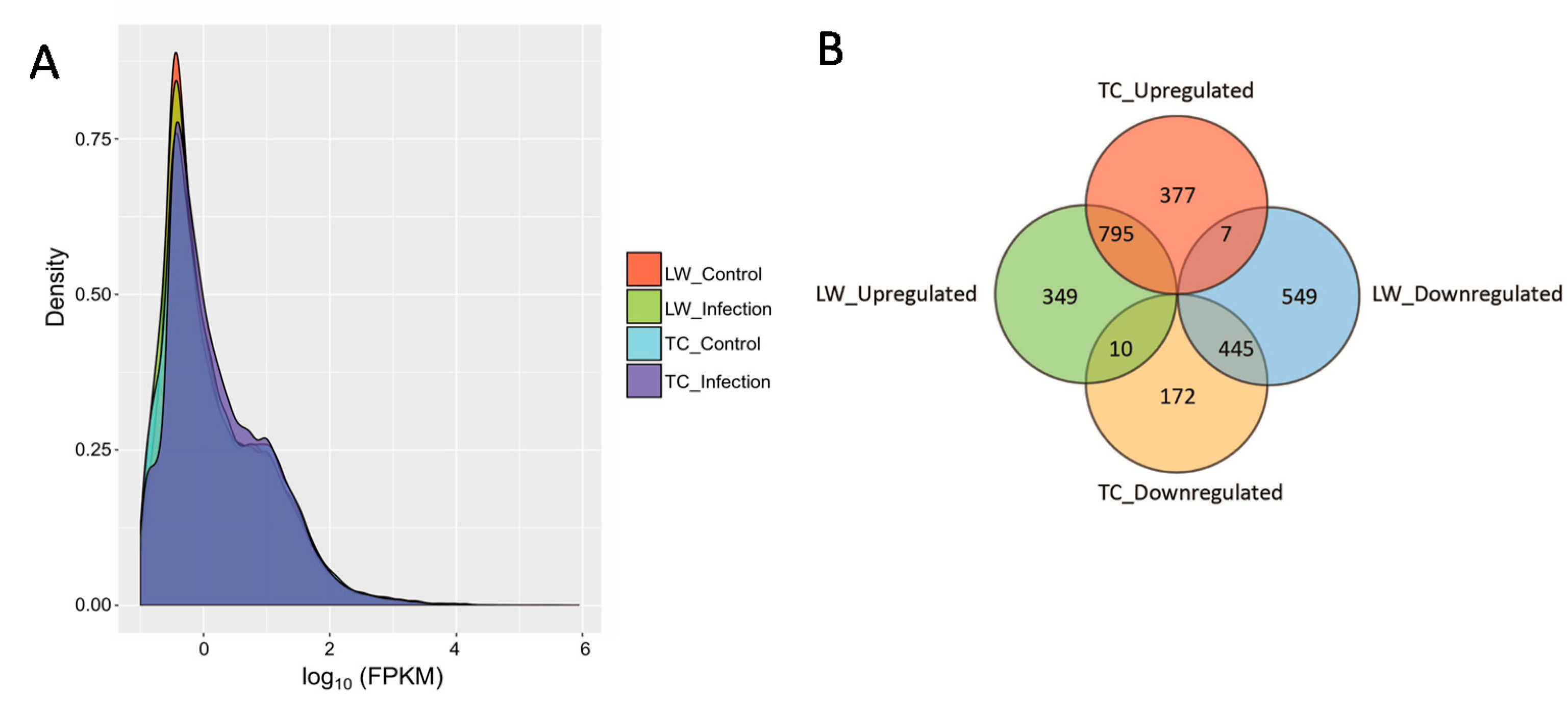
2.1. RNA Sequencing Data Mapping and Annotation
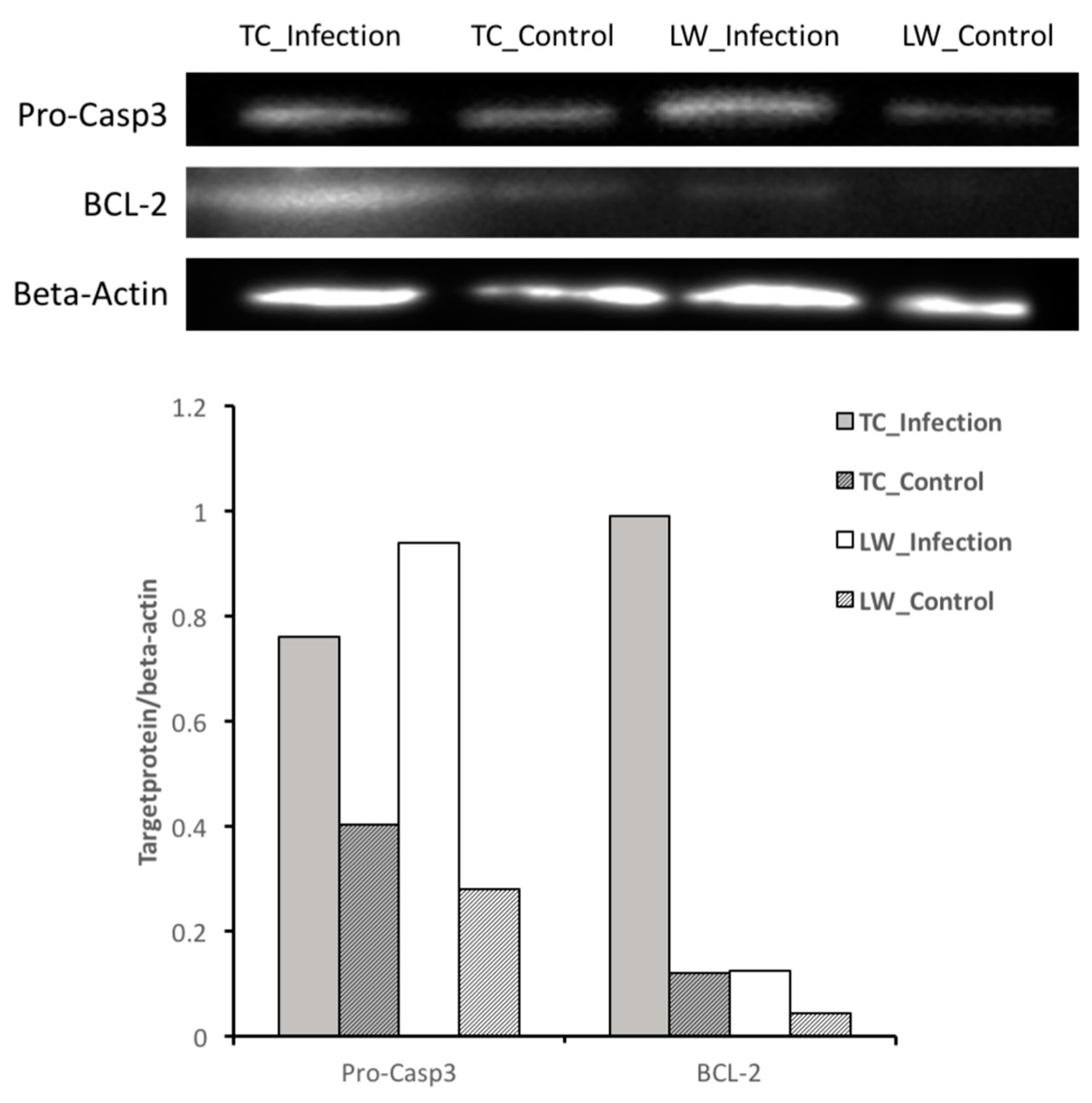
2.2. Differentially Expressed Genes Calling and Validation by RT-qPCR and Western-Blotting
2.3. The Basic Genetic Comparison of TC and LW Pigs Reveals Differences Among MHC Genes
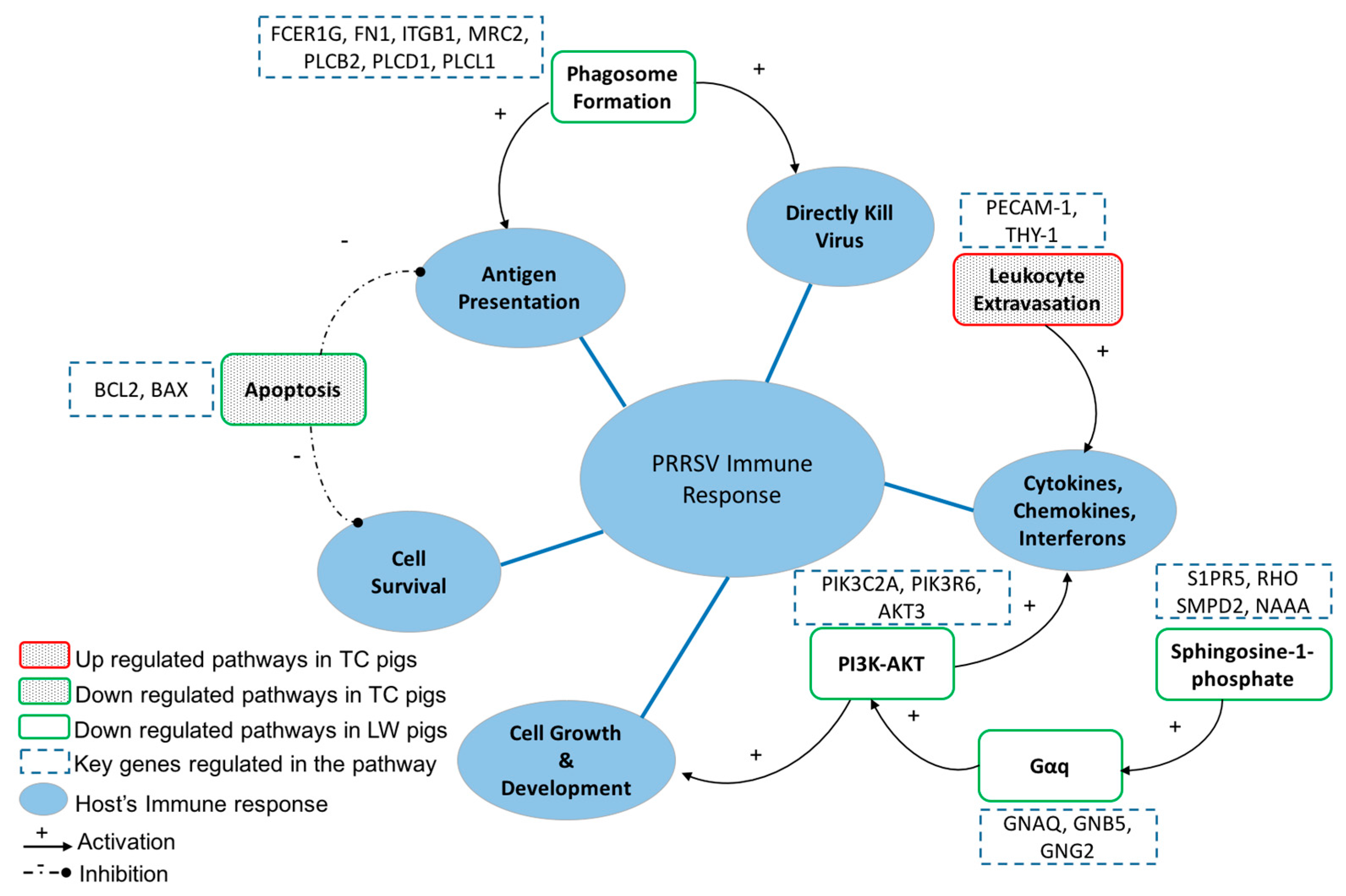
2.4. Host’s Response to PRRSV Infection Reveals the Activation of the Host Pathogen Recognition Receptors and the Production of Inflammatory Cytokines and Chemokines
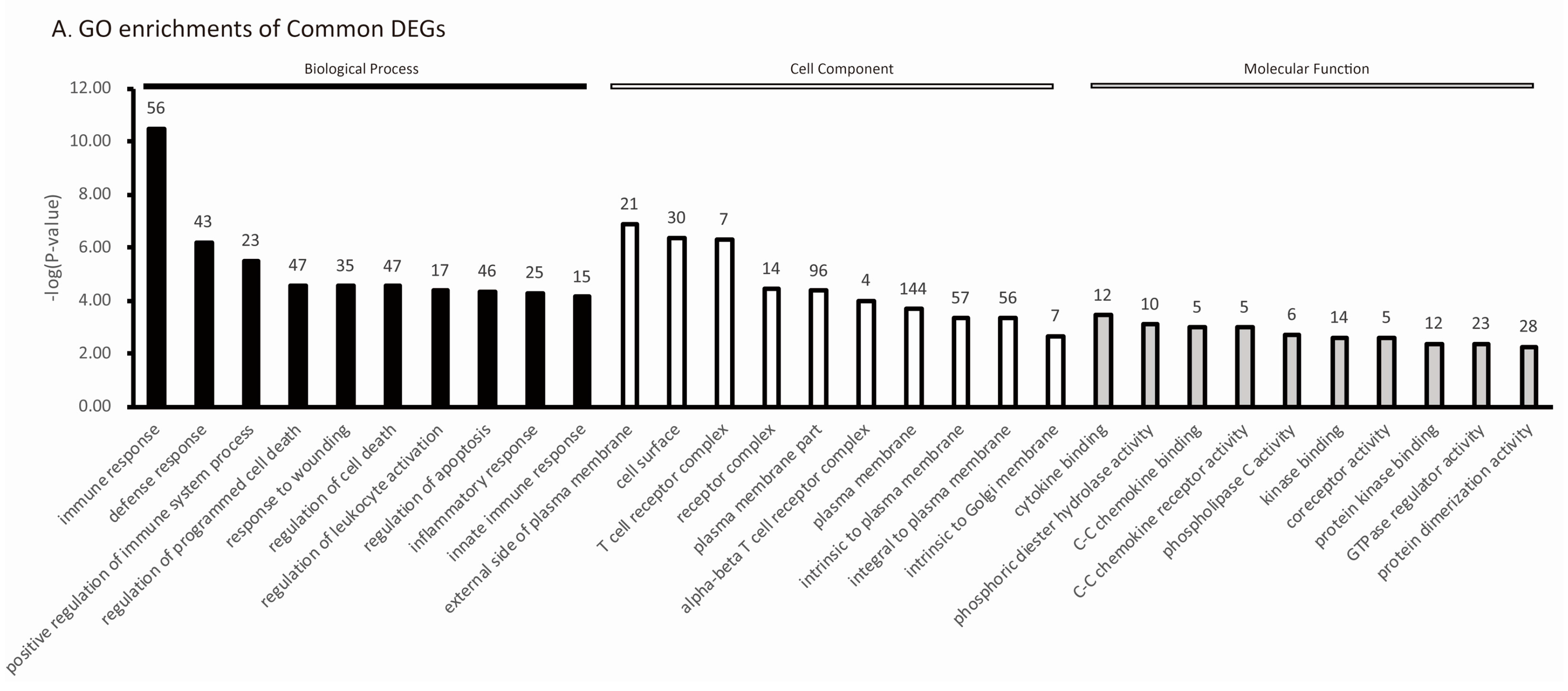
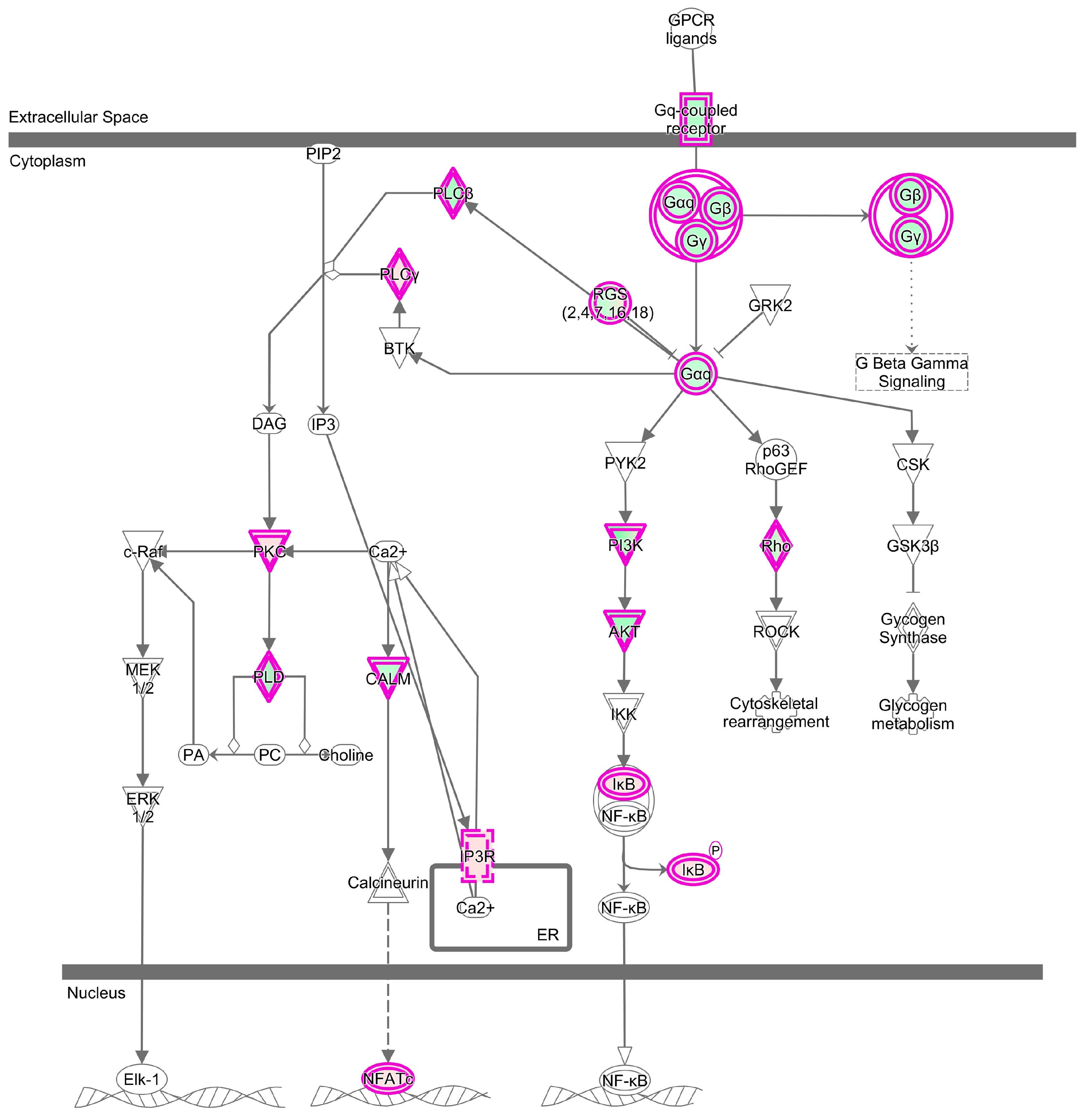
2.5. The Specific Enrichments of TC and LW Pigs’s DEGs
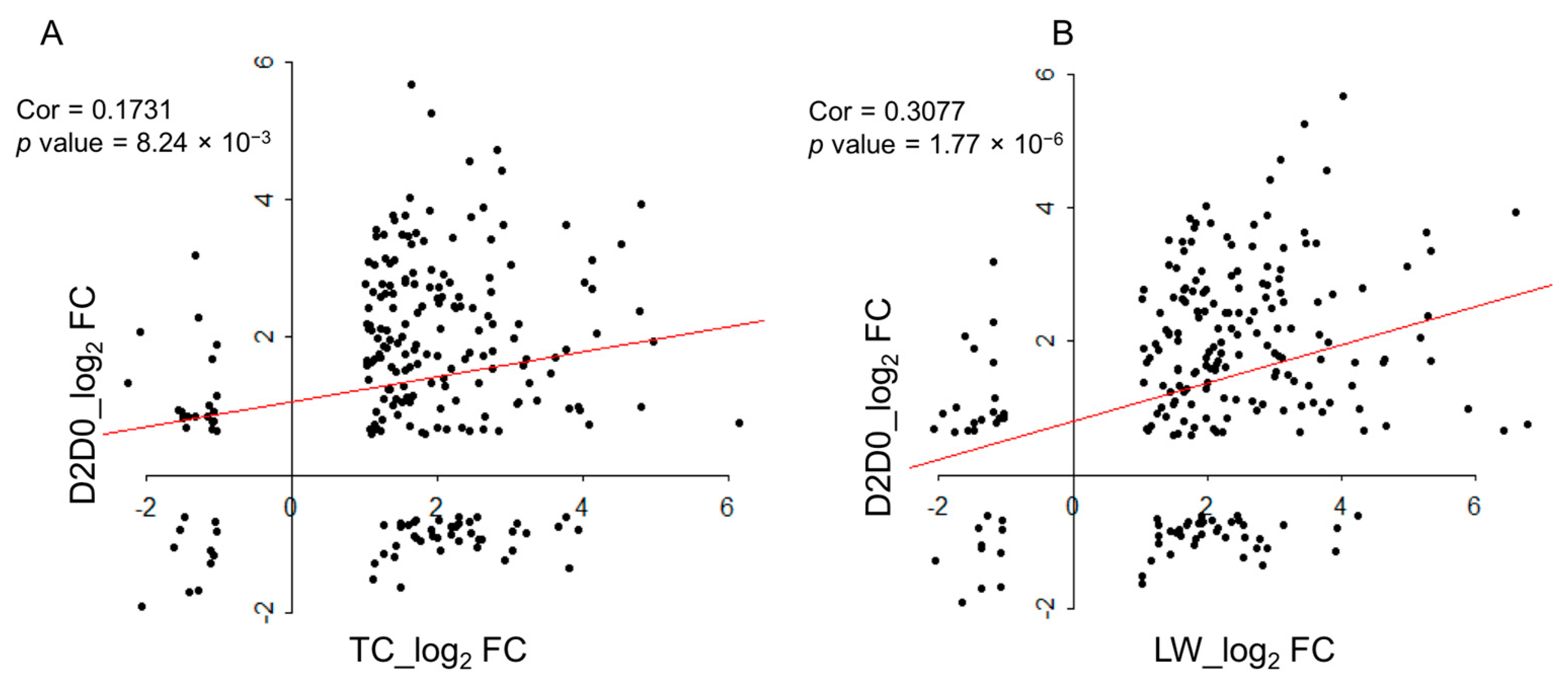
2.6 Comparison with the Published Data
3. Discussion
4. Materials and Methods
4.1. PRRSV Artificial Challenge and Sample Collection
4.2. RNA Preparation and Sequencing
4.3. RNA-Seq Data Analysis and DEGs Calling
4.4. Significant Functions and Gene Network Analysis
4.5. Validation of Differentially Expressed Genes with Quantitative Real Time RT-qPCR and Western-Blotting
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PRRS | Porcine Reproductive and Respiratory Syndrome |
| PRRSV | Porcine Reproductive and Respiratory Syndrome Virus |
| TC | Tongcheng |
| LW | Large White |
| PAMs | Porcine Alveolar Macrophages |
| DEGs | Differentially Expressed Genes |
| HR | High Response |
| LR | Low Response |
| ACHE | Acetylcholinesteras |
| XIST | X (inactive)-Specific Transcript |
| PHGC | PRRS Host Genetics Consortium |
| IFN | Interferon |
| TNF | Tumor Necrosis Factor |
| IL | Interleukin |
| MHC | Major Histocompatibility Complex |
| DPL | Dapulian |
| DLY | Duroc × Landrace × Yorkshire |
| FPKM | Fragments per Kilobase of Exon per Million Fragments Mapped |
| GO | Gene Ontology |
| BP | Biological Processes |
| IPA | Ingenuity Pathway Analysis |
| PRRs | Pathogen Recognition Receptors |
References
- Benfield, D.; Nelson, J.; Rossow, K.; Nelson, C.; Steffen, M.; Rowland, R. Diagnosis of persistent or prolonged porcine reproductive and respiratory syndrome virus infections. Vet. Res. 2000, 31, 71. [Google Scholar] [CrossRef]
- Duan, X.; Nauwynck, H.; Pensaert, M. Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to porcine reproductive and respiratory syndrome virus (PRRSV). Arch. Virol. 1997, 142, 2483–2497. [Google Scholar] [CrossRef] [PubMed]
- Duinhof, T.; van Schaik, G.; van Esch, E.; Wellenberg, G. Detection of PRRSV circulation in herds without clinical signs of PRRS: Comparison of five age groups to assess the preferred age group and sample size. Vet. Microbiol. 2011, 150, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Mo, D.; Wang, Q.; Jia, J.; Qin, L.; Yu, X.; Niu, Y.; Zhao, X.; Liu, X.; Chen, Y. Aberrant host immune response induced by highly virulent PRRSV identified by digital gene expression tag profiling. BMC Genom. 2010, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Allende, R.; Lewis, T.; Lu, Z.; Rock, D.; Kutish, G.; Ali, A.; Doster, A.R.; Osorio, F. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J. Gen. Virol. 1999, 80, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Dea, S.; Gagnon, C.A.; Mardassi, H.; Pirzadeh, B.; Rogan, D. Current knowledge on the structural proteins of porcine reproductive and respiratory syndrome (PRRS) virus: Comparison of the North American and European isolates. Arch. Virol. 2000, 145, 659–688. [Google Scholar] [CrossRef] [PubMed]
- Rowland, R.R.; Lunney, J.; Dekkers, J. Control of porcine reproductive and respiratory syndrome (PRRS) through genetic improvements in disease resistance and tolerance. Front. Genet. 2012, 3, 260. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.R.; Ait-Ali, T.; Clapperton, M.; Archibald, A.L.; Bishop, S. Genetic perspectives on host responses to porcine reproductive and respiratory syndrome (PRRS). Viral Immunol. 2007, 20, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Chen, H. Genetic control of host resistance to porcine reproductive and respiratory syndrome virus (PRRSV) infection. Virus Res. 2010, 154, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Petry, D.B.; Holl, J.W.; Weber, J.S.; Doster, A.R.; Osorio, F.A.; Johnson, R.K. Biological responses to porcine respiratory and reproductive syndrome virus in pigs of two genetic populations. J. Anim. Sci. 2005, 83, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.R.; Baldwin, D.A.; Channing, A.; Gifford, L.K.; Hsu, A.; Lu, P. Cooperativity of paired oligonucleotide probes for microarray hybridization assays. Anal. Biochem. 2005, 342, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, M.; Chen, H.; Steibel, J.P.; Kuhar, D.; Petry, D.; Bates, J.; Johnson, R.; Ernst, C.W.; Lunney, J.K. Identifying putative candidate genes and pathways involved in immune responses to porcine reproductive and respiratory syndrome virus (PRRSV) infection. Anim. Genet. 2012, 43, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Arceo, M.E.; Ernst, C.W.; Lunney, J.K.; Choi, I.; Raney, N.E.; Huang, T.; Tuggle, C.K.; Rowland, R.R.; Steibel, J.P. Characterizing differential individual response to porcine reproductive and respiratory syndrome virus infection through statistical and functional analysis of gene expression. Front. Genet. 2013, 3, 321. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Xing, F.; Zhang, C.; Zhang, Y.; Wang, N.; Li, Y.; Yang, L.; Jiang, C.; Zhang, C.; Wen, C.; et al. Genome-wide gene expression profiles in lung tissues of pig breeds differing in resistance to porcine reproductive and respiratory syndrome virus. PLoS ONE 2014, 9, e86101n. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Yu, X.; Zhao, T.; Feng, Y.; Cao, Z.; Wang, C.; Hu, Y.; Chen, X.; Hu, D.; Tian, X.; et al. Emergence of fatal PRRSV variants: Unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS ONE 2007, 2, e526. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhai, S.; Zhou, X.; Lin, P.; Jiang, T.; Hu, X.; Jiang, Y.; Wu, B.; Zhang, Q.; Xu, X.; et al. Molecular characterization of transcriptome-wide interactions between highly pathogenic porcine reproductive and respiratory syndrome virus and porcine alveolar macrophages in vivo. Int. J. Biol. Sci. 2011, 7, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Li, Z.; Wang, P.; Fan, P.; Zhang, Y.; Zhang, Q.; Wang, Y.; Xu, X.; Liu, B. Differences of immune responses between Tongcheng (Chinese local breed) and Large White pigs after artificial infection with highly pathogenic porcine reproductive and respiratory syndrome virus. Virus Res. 2016, 215, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2008, 37, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.M.; Ladinig, A.; Bao, H.; Kommadath, A.; Stothard, P.; Lunney, J.K.; Harding, J.C.; Plastow, G.S. Differences in whole blood gene expression associated with infection time-course and extent of fetal mortality in a reproductive model of type 2 porcine reproductive and respiratory syndrome virus (PRRSV) infection. PLoS ONE 2016, 11, e0153615. [Google Scholar]
- Middleton, J.; Patterson, A.M.; Gardner, L.; Schmutz, C.; Ashton, B.A. Leukocyte extravasation: Chemokine transport and presentation by the endothelium. Blood 2002, 100, 3853–3860. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. Molecular mechanisms that control leukocyte extravasation through endothelial cell contacts. Ernst Scher. Found. Symp. Proc. 2007, 3, 151–167. [Google Scholar] [CrossRef]
- Privratsky, J.R.; Newman, P.J. PECAM-1: Regulator of endothelial junctional integrity. Cell Tissue Res. 2014, 355, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Schubert, K.; Polte, T.; Bonisch, U.; Schader, S.; Holtappels, R.; Hildebrandt, G.; Lehmann, J.; Simon, J.C.; Anderegg, U.; Saalbach, A. Thy-1 (CD90) regulates the extravasation of leukocytes during inflammation. Eur. J. Immunol. 2011, 41, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Hofseth, L.J.; Hussain, S.P.; Harris, C.C. p53: 25 years after its discovery. Trends Pharmacol. Sci. 2004, 25, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, T.; Krajewski, S.; Krajewsk, M.; Wang, H.G.; Lin, H.K.; Liebermann, D.A.; Hoffman, B.; Reed, J.C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 1994, 9, 1799–1805. [Google Scholar] [PubMed]
- Cory, S.; Huang, D.C.; Adams, J.M. The Bcl-2 family: Roles in cell survival and oncogenesis. Oncogene 2003, 22, 8590–8607. [Google Scholar] [CrossRef] [PubMed]
- Blander, J.M.; Medzhitov, R. Regulation of phagosome maturation by signals from toll-like receptors. Science 2004, 304, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Tjelle, T.E.; Lovdal, T.; Berg, T. Phagosome dynamics and function. BioEssays 2000, 22, 255–263. [Google Scholar] [CrossRef]
- Klepac, K.; Kilić, A.; Gnad, T.; Brown, L.M.; Herrmann, B.; Wilderman, A.; Balkow, A.; Glöde, A.; Simon, K.; Lidell, M.E. The Gq signalling pathway inhibits brown and beige adipose tissue. Nat. Commun. 2016, 7, 10895. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Itoh, H. Functions and regulatory mechanisms of Gq-signaling pathways. Neurosignals 2009, 17, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Cooray, S. The pivotal role of phosphatidylinositol 3-kinase–Akt signal transduction in virus survival. J. Gen. Virol. 2004, 85, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Koyasu, S. The role of PI3K in immune cells. Nat. Immunol. 2003, 4, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol. Chem. 2002, 277, 25851–25854. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.; Goetzl, E.J. Sphingosine 1-phosphate and its receptors: An autocrine and paracrine network. Nat. Rev. Immunol. 2005, 5, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Wensvoort, G.; Terpstra, C.; Pol, J.M.; ter Laak, E.A.; Bloemraad, M.; de Kluyver, E.P.; Kragten, C.; van Buiten, L.D.; den Besten, A.; Wagenaar, F.; et al. Mystery swine disease in The Netherlands: The isolation of Lelystad virus. Vet. Q. 1991, 13, 121–130. [Google Scholar] [CrossRef] [PubMed]




| Group | Individual | Clean Reads | % Mapped Reads | Q20 (%) | Transcript Number (FPKM > 0.1) | Transcript Number (0.1 < FPKM < 1) |
|---|---|---|---|---|---|---|
| Tongcheng (TC)_Control | TC_C1 | 155,542,274 | 77.94 | 95.56 | 55,207 | 35,568 |
| TC_C2 | 144,420,088 | 76.08 | 95.93 | 72,765 | 54,856 | |
| TC_C3 | 159,784,438 | 78.84 | 95.96 | 54,234 | 35,805 | |
| TC_Infection | TC_I1 | 150,738,786 | 72.96 | 95.97 | 56,554 | 37,232 |
| TC_I2 | 158,394,072 | 60.85 | 95.38 | 60,754 | 41,058 | |
| TC_I3 | 147,835,638 | 76.54 | 96.45 | 54,838 | 35,665 | |
| Large White (LW)_Control | LW_C1 | 161,061,654 | 79.17 | 95.96 | 60,858 | 42,165 |
| LW_C2 | 110,922,288 | 77.54 | 95.70 | 75,926 | 56,881 | |
| LW_C3 | 146,633,740 | 78.62 | 95.95 | 69,040 | 51,313 | |
| LW_Infection | LW_I1 | 158,938,676 | 72.97 | 95.65 | 73,211 | 54,774 |
| LW_I2 | 136,571,286 | 70.36 | 95.49 | 66,589 | 49,258 | |
| LW_I3 | 140,256,430 | 75.98 | 95.92 | 72,837 | 54,384 |
| Category | LW | Count | −log10 (p Value) | TC | Count | −log10 (p Value) |
|---|---|---|---|---|---|---|
| Shared | Phospholipase C Signaling | 40 | 7.29 | Phospholipase C Signaling | 37 | 7.886 |
| Hepatic Fibrosis/Hepatic Stellate Cell Activation | 36 | 8.542 | Hepatic Fibrosis/Hepatic Stellate Cell Activation | 30 | 7.11 | |
| Granulocyte Adhesion and Diapedesis | 31 | 6.372 | Granulocyte Adhesion and Diapedesis | 27 | 5.923 | |
| T cell Receptor Signaling | 18 | 4.077 | T cell Receptor Signaling | 19 | 5.723 | |
| Calcium-induced T Lymphocyte Apoptosis | 13 | 3.455 | Calcium-induced T Lymphocyte Apoptosis | 15 | 5.596 | |
| Role of Macrophage, Fibroblasts and Endothelial Cells in Rheumatoid Arthritis | 39 | 4.564 | Role of Macrophage, Fibroblasts and Endothelial Cells in Rheumatoid Arthritis | 37 | 5.568 | |
| Molecular Mechanisms of Cancer | 42 | 3.508 | Molecular Mechanisms of Cancer | 42 | 5.281 | |
| Phagosome Formation | 21 | 5.265 | Phagosome Formation | 19 | 5.278 | |
| CCR5 Signaling in Macrophage | 15 | 4.201 | CCR5 Signaling in Macrophage | 15 | 5.114 | |
| Agranulocyte Adhesion and Diapedesis | 28 | 4.454 | Agranulocyte Adhesion and Diapedesis | 26 | 4.94 | |
| CTLA4 Signaling in Cytotoxic T lymphocytes | 16 | 3.848 | CTLA4 Signaling in Cytotoxic T lymphocytes | 16 | 4.78 | |
| Reelin Signaling in Neurons | 16 | 4.437 | Reelin Signaling in Neurons | 15 | 4.753 | |
| NF-κB Activation by Virus | 14 | 3.627 | NF-κB Activation by Virus | 14 | 4.458 | |
| Pathogenesis of Multiple Sclerosis | 5 | 3.952 | Pathogenesis of Multiple Sclerosis | 5 | 4.328 | |
| LPS/IL-1 Mediated Inhibition of RXR Function | 31 | 4.284 | LPS/IL-1 Mediated Inhibition of RXR Function | 27 | 4.081 | |
| Caveolar-mediated Endocytosis Signaling | 14 | 3.692 | Caveolar-mediated Endocytosis Signaling | 13 | 3.9 | |
| HER-2 Signaling in Breast Cancer | 14 | 3.501 | HER-2 Signaling in Breast Cancer | 13 | 3.717 | |
| Specific | Sphingosine-1-phosphate Signaling | 23 | 6.142 | Role of Tissue Factors in Cancer | 19 | 5.1 |
| Gαq Signaling | 24 | 4.254 | VDR/RXR Activation | 15 | 4.753 | |
| Differential Regulation of Cytokine Production in Intestinal Epithelial Cells by IL-17A and IL-17F | 8 | 4.225 | Cytotoxic T Lymphocyte-mediated Apoptosis of Target Cells | 9 | 4.475 | |
| Thrombin Signaling | 28 | 4.209 | Role of Pattern Recognition Receptor in Recognition of Bacteria and Viruses | 19 | 4.405 | |
| Role of JAK1 and JAK3 in γC Cytokine Signaling | 13 | 3.875 | Virus Entry via Endocytic Pathways | 16 | 4.353 | |
| VDR/RXR Activation | 15 | 3.862 | Micropinocytosis Signaling | 13 | 4.231 | |
| P70S6K Signaling | 20 | 3.845 | Role of Osteoblasts, Osteoblasts and Chondrocytes in Rheumatoid Arthritis | 27 | 4.081 | |
| Role of NFAT in Regulation of the Immune Response | 25 | 3.746 | CD28 Signaling in T Helper Cells | 18 | 3.986 | |
| Human Embryonic Stem Cell Pluripotency | 21 | 3.648 | Leukocyte Extravasation Signaling | 25 | 3.971 | |
| Tec Kinase Signaling | 23 | 3.609 | Signaling by Pho Family GTPases | 27 | 3.733 | |
| Type I Diabetes Mellitus Signaling | 18 | 3.595 | IL-8 Signaling | 23 | 3.692 | |
| Glioma Invasiveness Signaling | 12 | 3.564 | HGF Signaling | 16 | 3.673 | |
| Colorectal Cancer Metastasis Signaling | 30 | 3.381 | p53 Signaling | 15 | 3.638 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Ji, L.; Zhang, Y.; Zhen, Y.; Zhang, Q.; Xu, X.; Liu, B. Transcriptome Differences in Porcine Alveolar Macrophages from Tongcheng and Large White Pigs in Response to Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Infection. Int. J. Mol. Sci. 2017, 18, 1475. https://doi.org/10.3390/ijms18071475
Liang W, Ji L, Zhang Y, Zhen Y, Zhang Q, Xu X, Liu B. Transcriptome Differences in Porcine Alveolar Macrophages from Tongcheng and Large White Pigs in Response to Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Infection. International Journal of Molecular Sciences. 2017; 18(7):1475. https://doi.org/10.3390/ijms18071475
Chicago/Turabian StyleLiang, Wan, Likai Ji, Yu Zhang, Yueran Zhen, Qingde Zhang, Xuewen Xu, and Bang Liu. 2017. "Transcriptome Differences in Porcine Alveolar Macrophages from Tongcheng and Large White Pigs in Response to Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Infection" International Journal of Molecular Sciences 18, no. 7: 1475. https://doi.org/10.3390/ijms18071475




