Sexually Transmitted Infections: A Novel Screening Strategy for Improving Women’s Health in Vulnerable Populations
Abstract
:1. Introduction
2. Results
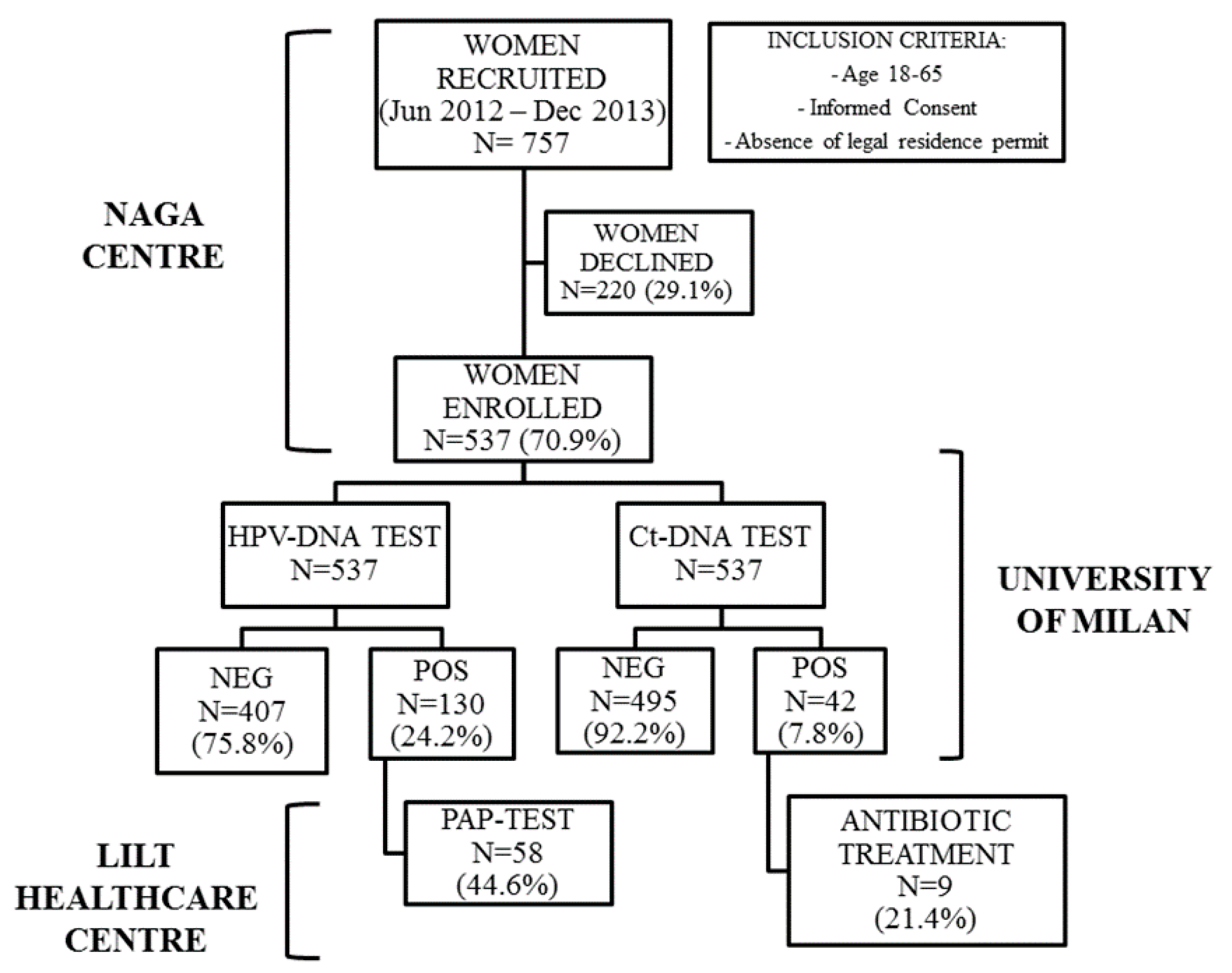
2.1. Study Population and Acceptability to the Study
2.2. Socio-Demographic and Sexual Health Characteristics
2.3. Evaluation of DNA Quality
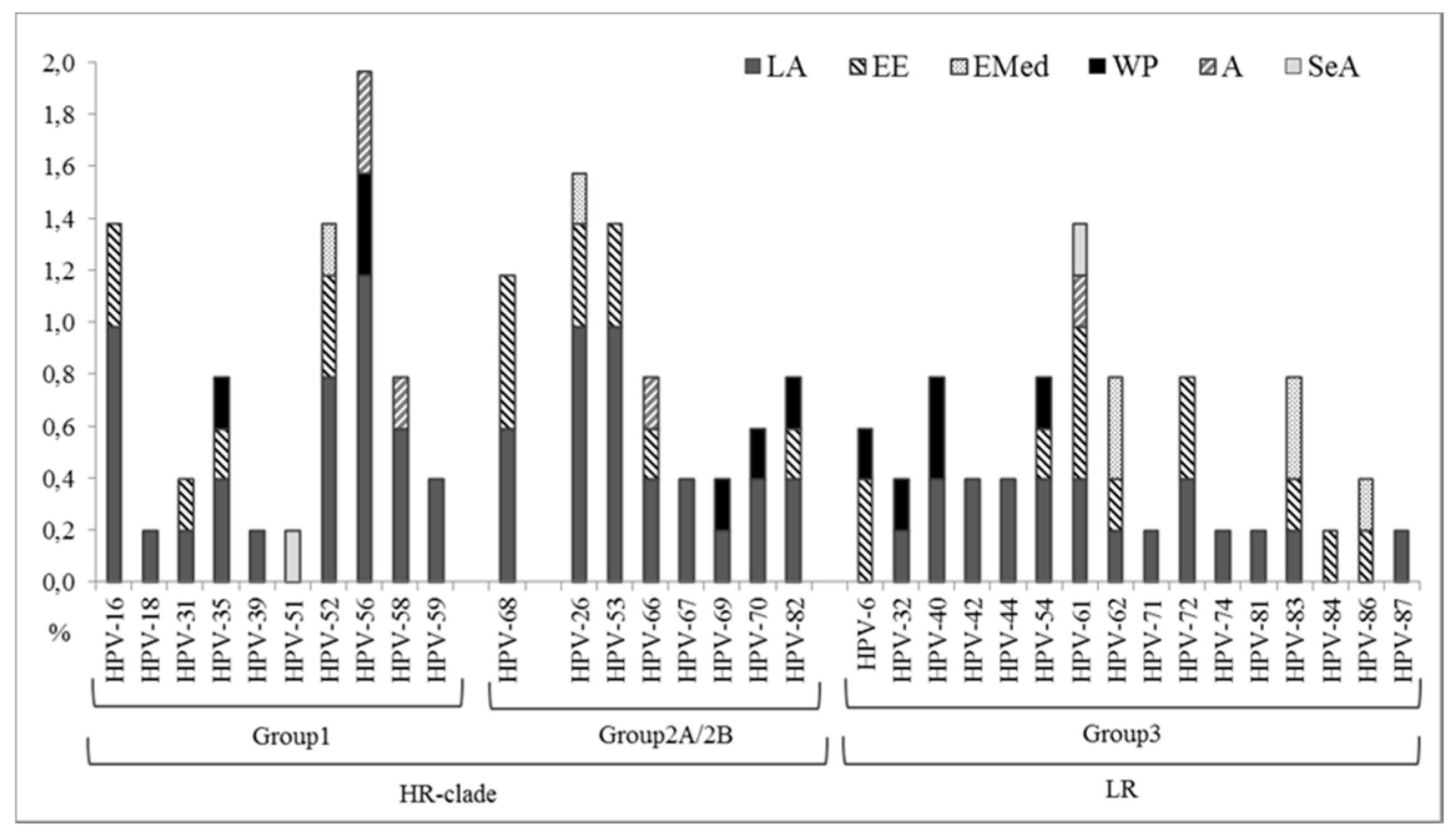
2.4. HPV Detection and Genotyping
2.5. C. trachomatis Detection and Genotyping
2.6. HPV/C. Trachomatis Co-Infections
2.7. Diagnostic and Therapeutic Follow-Up
2.8. N. gonorrhoeae and T. vaginalis Detection
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Sample Collection and DNA Extraction
4.3. Molecular Assays
4.3.1. HPV Detection and Genotyping
4.3.2. C. trachomatis Detection and Genotyping
4.3.3. N. gonorrhea and T. vaginalis Detection
4.4. Cytological Diagnosis
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United Nation Secretariat. Populations Facts, No 2013/2. Available online: http://www.un.org/en/development/desa/population (accessed on 3 April 2017).
- International Labor Organization. Available online: http://www.ilo.org/global/lang--en/index.htm (accessed on 3 April 2017).
- Osservatorio Regionale per L’integrazione e la multi Etnicità Rapporto 2013. Gli Immigrati in Lombardia, Fondazione ISMU. 2013. Available online: http://www.orimregionelombardia.it (accessed on 3 April 2017).
- United Nations Children’s Fund (UNICEF), Policy, Advocacy and Knowledge Management, Division of Policy and Practice. The Rights of Children, Youth and Women in the Context of Migration. Social and Economic Policy Working Paper. New York, 2010. Available online: http://www.unicef.org/socialpolicy/files/The_Rights_of_Children_Youth_and_Women_in_the_Context_of_Migration_FINAL.pdf (accessed on 3 April 2017).
- World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 3 April 2017).
- Adanu, R.M.; Johnson, T.R. Migration and women’s health. Int. J. Gynaecol. Obstet. 2009, 106, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.A.; Basten, A.; Frattini, C. Migration: A Social Determinant of the Health of Migrants. Eurohealth 2009, 16, 10–12. [Google Scholar]
- Agudelo-Suarez, A.A.; Gil-Gonzalez, D.; Vives-Cases, C.; Love, J.G.; Wimpenny, P.; Ronda-Perez, E. A metasynthesis of qualitative studies regarding opinions and perceptions about barriers and determinants of health services’ accessibility in economic migrants. BMC Health Serv. Res. 2012, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Cuadra, C.B. Right of access to health care for undocumented migrants in EU: A comparative study of national policies. Eur. J. Public Health 2012, 22, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Keygnaert, I.; Guieu, A.; Ooms, G.; Vettenburg, N.; Temmerman, M.; Roelens, K. Sexual and reproductive health of migrants: Does the EU care? Health Policy 2014, 114, 215–225. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Sexually Transmitted Infections. Factsheet No. 110. WHO: Geneva, 2010. Available online: http://www.who.int/mediacentre/factsheets/fs110/en/index.html (accessed on 3 April 2017).
- Tornesello, M.L.; Duraturo, M.L.; Buonaguro, L.; Vallefuoco, G.; Piccoli, R.; Palmieri, S.; Buonaguro, F.M. Prevalence of human papillomavirus genotypes and their variants in high risk West Africa women immigrants in South Italy. Infect. Agents Cancer 2007, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, L.; Vassallo, R.; Matranga, D.; Affronti, M.; Caleca, M.P.; Bellavia, C.; Perino, A.; Ammatuna, P. Prevalence of cervical human papillomavirus infection and types among women immigrated to Sicily, Italy. Acta Obstet. Gynecol. Scand. 2009, 88, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Tornesello, M.L.; De Rosa, N.; Sarappa, F.; Buonaguro, L.; Piccoli, R.; Buonaguro, F.M. Assessment of Chlamydia trachomatis infection among Eastern European and West African women immigrants in South Italy. Sex. Trasm. Infect. 2012, 88, 70–71. [Google Scholar] [CrossRef]
- Associazione Volontaria di Assistenza Socio-Sanitaria e per i Diritti di Cittadini Stranieri, Rom e Sinti. Available online: http://www.naga.it/ (accessed on 3 April 2017).
- International Agency for Research on Cancer (IARC). Human Papillomaviruses. IARC Monogr Eval Carcinog Risks Hum; 100B:261-319. 2011. Available online: http://monographs.iarc.fr/ENG/Monographs/vol100B/mono100B-1.pdf (accessed on 3 April 2017).
- European Centre for Disease Prevention and Control (ECDC). Assessing the Burden of Key Infectious Diseases Affecting Migrant Populations in the EU/EEA. Stockholm, 2014. Available online: http://ecdc.europa.eu/en/publications/Publications/assessing-burden-disease-migrant-populations.pdf (accessed on 3 April 2017).
- Tanzi, E.; Bianchi, S.; Fasolo, M.M.; Frati, E.R.; Mazza, F.; Martinelli, M.; Colzani, D.; Beretta, R.; Zappa, A.; Orlando, G. High performance of a new PCR-based urine assay for HPV-DNA detection and genotyping. J. Med. Virol. 2013, 85, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Frati, E.R.; Panatto, D.; Martinelli, M.; Amicizia, D.; Zotti, C.M.; Martinese, M.; Bonanni, P.; Boccalini, S.; Coppola, R.C.; et al. Detection and Genotyping of Human Papillomavirus in Urine Samples from Unvaccinated Male and Female Adolescents in Italy. PLoS ONE 2013, 8, e79719. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Library. Comprehensive Cervical Cancer Control: A Guide to Essential Practice—2nd ed. Available online: http://www.who.int/reproductivehealth/publications/cancers/cervical-cancer-guide/en/ (accessed on 3 April 2017).
- Bruni, L.; Diaz, M.; Castellsagué, X.; Ferrer, E.; Bosch, F.X.; de Sanjosé, S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Borrego, M.J.; Nunes, B.; Florindo, C.; Gomes, J.P. Evolutionary dynamics of ompA, the gene encoding the Chlamydia trachomatis key Antigen. J. Bacteriol. 2009, 191, 7182–7192. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Barrionuevo-Rosas, L.; Albero, G.; Serrano, B.; Mena, M.; Gòmez, D.; Muñoz, J.; Bosch, F.X.; de Sanjosé, S.; ICO Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in Italy. Summary Report 15 December 2016. Available online: http://www.hpvcentre.net (accessed on 3 April 2017).
- Marcone, V.; Recine, N.; Gallinelli, C.; Nicosia, R.; Lichtner, M.; Degener, A.M.; Chiarini, F.; Calzolari, E.; Vullo, V. Epidemiology of Chlamydia trachomatis endocervical infection in a previously unscreened population in Rome, Italy, 2000 to 2009. Euro Surveill. 2012, 17, 20203. [Google Scholar] [PubMed]
- Newman, L.; Rowley, J.; Vander Hoorn, S.; Wijesooriya, N.S.; Unemo, M.; Low, N.; Stevens, G.; Gottlieb, S.; Kiarie, J.; Temmerman, M. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS ONE 2015, 10, e0143304. [Google Scholar] [CrossRef] [PubMed]
- “Donna Dovunque” Project. Lega Italiana per la Lotta Contro i Tumori (LILT). Available online: http://www.legatumori.mi.it/media/26773/donna_ovunque.pdf (accessed on 3 April 2017).
- Puranen, M.; Saarikoski, S.; Syrjänen, K.; Syrjänen, S. Polymerase chain reaction amplification of human papillomavirus DNA from archival, Papanicolaou-stained cervical smears. Acta Cytol. 1996, 40, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Jalal, H.; Al-Suwaine, A.; Sonnex, C.; Carne, C. The superiority of polymerase chain reaction over an amplified enzyme immunoassay for the detection of genital chlamydial infections. Sex. Transm. Infect. 2006, 82, 37–40. [Google Scholar] [CrossRef] [PubMed]
- BLAST-n. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 3 April 2017).
- Hjelmevoll, S.O.; Olsen, M.E.; Ericson Sollid, J.U.; Haaheim, H.; Unemo, M.; Skogen, V. A fast Real-Time Polymerase Chain Reaction Method for Sensitive and Specific Detection of the Neisseria gonorrhoeae porA pseudogene. J. Mol. Diagn. 2006, 8, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, A.M.; Jordan, J.A.; Green, A.M.; Ingersoll, J.; Diclemente, R.J.; Wingood, G.M. Real-time PCR improves detection of Trichomonas vaginalis infection compared with culture using self-collected vaginal swabs. Infect. Dis. Obstet. Gynecol. 2005, 13, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.; Davey, D.; Kurman, R.; Moriarty, A.; O’Connor, D.; Prey, M.; Raab, S.; Sherman, M.; Wilbur, D.; Wright, T.J.; et al. The 2001 Bethesda System: Terminology for reporting results of cervical cytology. JAMA 2002, 287, 2114–2119. [Google Scholar] [CrossRef] [PubMed]
- Open Source Epidemiologic Statistics for Public Health. Available online: http://www.openepi.com (accessed on 3 April 2017).
| Characteristics of Enrolled Women | Overall | Latin America | Eastern Europe | Eastern Mediterranean | Western Pacific | Africa | Southeast Asia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| 537 | 100 | 245 | 45.6 | 165 | 30.7 | 43 | 8.0 | 36 | 6.7 | 34 | 6.3 | 14 | 2.6 | |
| Socio-demographic characteristics | ||||||||||||||
| Migration time * | ||||||||||||||
| ≤1 year | 121 | 22.5 | 61 | 24.9 | 29 | 17.6 | 9 | 20.9 | 10 | 27.8 | 9 | 26.5 | 3 | 21.4 |
| 2–3 years | 112 | 20.9 | 55 | 22.4 | 27 | 16.4 | 10 | 23.3 | 10 | 27.8 | 7 | 20.6 | 3 | 21.4 |
| 4–5 years | 55 | 10.2 | 23 | 9.4 | 18 | 10.9 | 7 | 16.3 | 3 | 8.3 | 3 | 8.8 | 1 | 7.2 |
| >5 years | 249 | 46.4 | 106 | 43.3 | 91 | 55.1 | 17 | 39.5 | 13 | 36.1 | 15 | 44.1 | 7 | 50.0 |
| Marital status | ||||||||||||||
| Single or widow | 134 | 24.9 | 63 | 25.7 | 36 | 21.8 | 14 | 32.5 | 5 | 13.9 | 16 | 47.1 | 0 | 0.0 |
| Married | 308 | 57.4 | 138 | 56.3 | 95 | 57.6 | 18 | 41.9 | 27 | 75.0 | 17 | 50.0 | 13 | 92.9 |
| Divorced or separated | 95 | 17.7 | 44 | 18.0 | 34 | 20.6 | 11 | 25.6 | 4 | 11.1 | 1 | 2.9 | 1 | 7.1 |
| Educational background | ||||||||||||||
| No qualification | 29 | 5.4 | 2 | 0.8 | 20 | 12.1 | 5 | 11.6 | 0 | 0.0 | 3 | 8.8 | 0 | 0.0 |
| Elementary school | 83 | 15.4 | 31 | 12.7 | 24 | 14.6 | 11 | 25.5 | 5 | 13.9 | 6 | 17.6 | 5 | 35.7 |
| Secondary school | 47 | 8.8 | 24 | 9.8 | 14 | 8.5 | 6 | 14.0 | 1 | 2.8 | 1 | 3.0 | 1 | 7.2 |
| High school | 307 | 57.2 | 154 | 62.8 | 70 | 42.4 | 15 | 34.9 | 18 | 50.0 | 18 | 53.0 | 8 | 57.1 |
| Degree | 71 | 13.2 | 34 | 13.9 | 37 | 22.4 | 6 | 14.0 | 12 | 33.3 | 6 | 17.6 | 0 | 0.0 |
| Sexual and reproductive health characteristics | ||||||||||||||
| Age at first intercourse | ||||||||||||||
| Virgins | 7 | 1.3 | 0 | 0.0 | 3 | 1.8 | 3 | 7.0 | 0 | 0.0 | 1 | 3.0 | 0 | 0.0 |
| ≤15 years | 94 | 17.5 | 47 | 19.2 | 28 | 17.0 | 6 | 14.0 | 1 | 2.8 | 6 | 17.6 | 6 | 42.9 |
| 16–19 years | 238 | 44.3 | 117 | 47.8 | 83 | 50.3 | 13 | 30.2 | 14 | 38.9 | 10 | 29.4 | 1 | 7.1 |
| >19 years | 190 | 35.4 | 78 | 31.8 | 49 | 29.7 | 18 | 41.8 | 21 | 58.3 | 17 | 50.0 | 7 | 50.0 |
| Don’t remember | 8 | 1.5 | 3 | 1.2 | 2 | 1.2 | 3 | 7.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Sexual activity | ||||||||||||||
| No partner | 138 | 25.7 | 65 | 26.5 | 41 | 24.8 | 10 | 23.2 | 6 | 16.7 | 13 | 38.2 | 3 | 21.5 |
| Stable partner (>1 year) ** | 350 | 65.2 | 156 | 63.7 | 111 | 67.3 | 27 | 62.8 | 30 | 83.3 | 17 | 50.0 | 9 | 64.3 |
| Recently partner (<1 year) | 47 | 8.7 | 24 | 9.8 | 12 | 7.3 | 6 | 14.0 | 0 | 0.0 | 4 | 11.8 | 1 | 7.1 |
| More partners | 2 | 0.4 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 7.1 |
| Contraceptive methods | ||||||||||||||
| None | 348 | 64.8 | 143 | 58.3 | 119 | 72.1 | 27 | 62.8 | 27 | 75.0 | 20 | 58.8 | 12 | 85.8 |
| Condom | 45 | 8.4 | 24 | 9.8 | 11 | 6.7 | 0 | 0.0 | 2 | 5.6 | 7 | 20.6 | 1 | 7.1 |
| Pill | 107 | 19.9 | 58 | 23.7 | 21 | 12.7 | 14 | 32.6 | 6 | 16.7 | 7 | 20.6 | 1 | 7.1 |
| Others | 37 | 6.9 | 20 | 8.2 | 14 | 8.5 | 2 | 4.6 | 1 | 2.7 | 0 | 0.0 | 0 | 0.0 |
| Pregnancies | ||||||||||||||
| None | 161 | 30.0 | 78 | 31.8 | 38 | 23.0 | 20 | 46.5 | 4 | 11.1 | 18 | 52.9 | 3 | 21.5 |
| 1 | 140 | 26.1 | 61 | 24.9 | 44 | 26.7 | 10 | 23.2 | 13 | 36.1 | 7 | 20.6 | 5 | 35.7 |
| 2 | 121 | 22.5 | 48 | 19.6 | 48 | 29.1 | 9 | 20.9 | 12 | 33.3 | 2 | 5.9 | 2 | 14.2 |
| 3 | 70 | 13.0 | 33 | 13.5 | 23 | 13.9 | 2 | 4.7 | 5 | 13.9 | 3 | 8.8 | 4 | 28.6 |
| >3 | 45 | 8.4 | 25 | 10.2 | 12 | 7.3 | 2 | 4.7 | 2 | 5.6 | 4 | 11.8 | 0 | 0.0 |
| Past Sexually Transmitted Diseases (STD symptomatic cases) | ||||||||||||||
| Yes | 142 | 26.4 | 74 | 30.2 | 48 | 29.1 | 9 | 20.9 | 2 | 5.6 | 9 | 26.5 | 0 | 0.0 |
| Type of STDs | ||||||||||||||
| Condyloma | 5 | 0.9 | 3 | 1.2 | 2 | 1.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Syphilis | 1 | 0.2 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Gonorrhoea | 1 | 0.2 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Chlamydia | 3 | 0.6 | 1 | 0.4 | 2 | 1.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Genital Herpes | 4 | 0.7 | 3 | 1.2 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Bacterial vaginosis | 37 | 6.9 | 20 | 8.2 | 10 | 6.1 | 1 | 2.3 | 1 | 2.8 | 5 | 14.8 | 0 | 0.0 |
| Mycosis | 36 | 6.7 | 17 | 6.9 | 14 | 8.5 | 4 | 9.3 | 0 | 0.0 | 1 | 2.9 | 0 | 0.0 |
| Trichomoniasis | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1 | 2.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Bacterial vaginosis/mycosis | 21 | 3.9 | 10 | 4.2 | 8 | 4.8 | 2 | 4.7 | 0 | 0.0 | 1 | 2.9 | 0 | 0.0 |
| Others | 33 | 6.1 | 18 | 7.3 | 11 | 6.7 | 1 | 2.3 | 1 | 2.8 | 2 | 5.9 | 0 | 0.0 |
| Age-Stratified HPV Prevalence | Overall | Latin America | Eastern Europe | Eastern Mediterranean | Western Pacific | Africa | Southeast Asia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| <25 years | 27/83 | 32.5 | 17/42 | 40.5 | 9/32 | 28.1 | 1/7 | 14.3 | 0/0 | 0 | 0/2 | 0 | 0/0 | 0 |
| 25–34 years | 35/161 | 21.7 | 20/75 | 26.7 | 7/48 | 14.6 | 3/15 | 20 | 2/4 | 50 | 3/15 | 20 | 0/4 | 0 |
| 35–44 years | 34/131 | 26 | 16/60 | 26.7 | 5/32 | 15.6 | 2/12 | 16.7 | 5/12 | 41.7 | 3/11 | 27.3 | 3/4 | 75 |
| 45–54 years | 22/103 | 21.3 | 11/44 | 25 | 6/32 | 18.7 | 2/8 | 25 | 3/13 | 23.1 | 0/2 | 0 | 0/4 | 0 |
| >55 years | 12/59 | 20.3 | 8/24 | 33.5 | 3/21 | 14.3 | 1/1 | 100 | 0/7 | 0 | 0/4 | 0 | 0/2 | 0 |
| TOT | 130/537 | 24.2 | 72/245 | 29.4 | 30/165 | 18.2 | 9/43 | 21 | 10/36 | 27.8 | 6/34 | 17.6 | 3/14 | 21.4 |
| Age-Stratified Ct Prevalence | Overall | Latin America | Eastern Europe | Eastern Mediterranean | Western Pacific | Africa | Southeast Asia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| <25 years | 11/83 | 13.2 | 10/42 | 23.8 | 0/32 | 0 | 1/7 | 14.3 | 0/0 | 0 | 0/2 | 0 | 0/0 | 0 |
| 25–34 years | 18/161 | 11.2 | 8/75 | 10.7 | 2/48 | 4.2 | 5/15 | 33.4 | 1/4 | 25 | 2/15 | 13.4 | 0/4 | 0 |
| 35–44 years | 6/131 | 4.6 | 2/60 | 3.3 | 2/32 | 6.2 | 1/12 | 8.3 | 0/12 | 0 | 1/11 | 9.1 | 0/4 | 0 |
| 45–54 years | 4/103 | 3.9 | 3/44 | 6.8 | 0/32 | 0 | 0/8 | 0 | 1/13 | 7.7 | 0/2 | 0 | 0/4 | 0 |
| >55 years | 3/59 | 5 | 1/24 | 4.2 | 2/21 | 9.5 | 0/1 | 0 | 0/7 | 0 | 0/4 | 0 | 0/2 | 0 |
| TOT | 42/537 | 7.8 | 24/245 | 9.8 | 6/165 | 3.6 | 7/43 | 16.3 | 2/36 | 5.6 | 3/34 | 8.8 | 0/14 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frati, E.R.; Fasoli, E.; Martinelli, M.; Colzani, D.; Bianchi, S.; Carnelli, L.; Amendola, A.; Olivani, P.; Tanzi, E. Sexually Transmitted Infections: A Novel Screening Strategy for Improving Women’s Health in Vulnerable Populations. Int. J. Mol. Sci. 2017, 18, 1311. https://doi.org/10.3390/ijms18061311
Frati ER, Fasoli E, Martinelli M, Colzani D, Bianchi S, Carnelli L, Amendola A, Olivani P, Tanzi E. Sexually Transmitted Infections: A Novel Screening Strategy for Improving Women’s Health in Vulnerable Populations. International Journal of Molecular Sciences. 2017; 18(6):1311. https://doi.org/10.3390/ijms18061311
Chicago/Turabian StyleFrati, Elena R., Ester Fasoli, Marianna Martinelli, Daniela Colzani, Silvia Bianchi, Luciana Carnelli, Antonella Amendola, Pierfranco Olivani, and Elisabetta Tanzi. 2017. "Sexually Transmitted Infections: A Novel Screening Strategy for Improving Women’s Health in Vulnerable Populations" International Journal of Molecular Sciences 18, no. 6: 1311. https://doi.org/10.3390/ijms18061311





