Propolis Diterpenes as a Remarkable Bio-Source for Drug Discovery Development: A Review
Abstract
:1. Introduction
2. Propolis Biological Activity
2.1. Antibacterial, Antiviral, Antifungal, and Antiparasite Activities
2.2. Anti-Inflammatory Effect
2.3. Cytotoxic Effect
2.4. Immunomodulatory Action
2.5. Toxicity
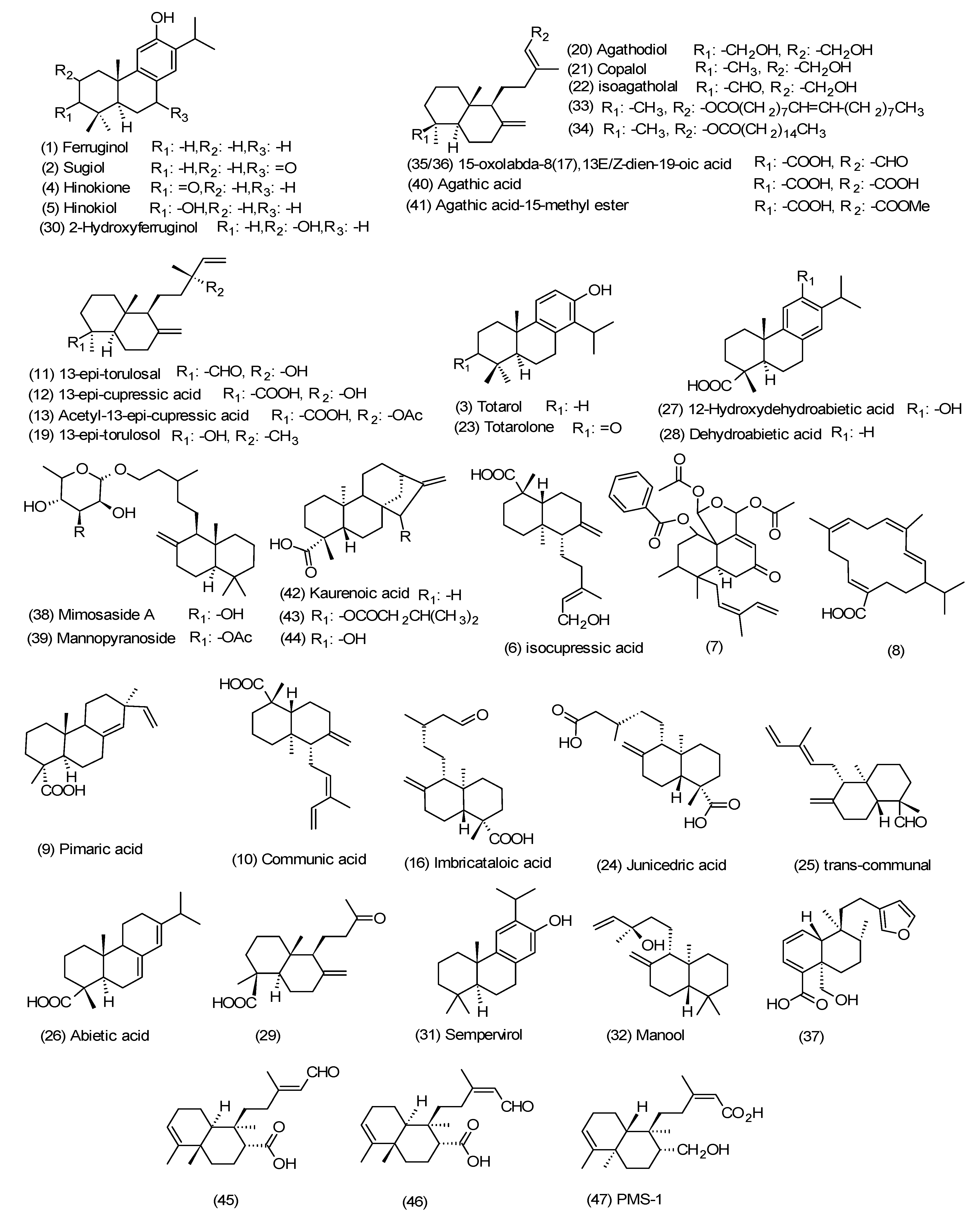
3. Diterpenes from the Propolis
4. Discussions and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Crane, E. A short history of knowledge about honey bees (Apis) up to 1,800. Bee world 2004, 85, 6–11. [Google Scholar] [CrossRef]
- Harissis, H.V.; Harissis, A.V. Apiculture in the Prehistoric Aegean: Minoan and Mycenaean Symbols Revisited; Hedges: Oxford, UK, 2009. [Google Scholar]
- Crane, E. Recent research on the world history of beekeeping. Bee World 1999, 80, 174–186. [Google Scholar] [CrossRef]
- Da Veiga, P.A.S. Health and Medicine in Ancient Egypt: Magic and Science; Archaeopress: Oxford, UK, 2009. [Google Scholar]
- Maruhashi, E. L-Mesitran® in the management of canine otitis externa. Ph.D. dissertation, Universidade de Lisboa, Faculdade de Medicina Veterinária, Portugal, 2015. [Google Scholar]
- Bankova, V.S.; Popov, S.S.; Marekov, N.L. Isopentenyl cinnamates from poplar buds and propolis. Phytochemistry 1989, 28, 871–873. [Google Scholar] [CrossRef]
- Ruttner, F. Biogeography and Taxonomy of Honeybees; Springer: Berlin, German, 1988. [Google Scholar]
- Bunney, M.H. Contact dermatitis in beekeepees due to propolis (bee glue). Br. J. Der. 1968, 80, 17–23. [Google Scholar] [CrossRef]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73 (Suppl. S1), S1–S6. [Google Scholar] [CrossRef]
- Meyer, W.; Ulrich, W. “Propolis Bees” and their activities. Bee World 2015, 37, 25–36. [Google Scholar] [CrossRef]
- Ghisalberti, E.L. Propolis: A review. Bee World 1979, 60, 59–84. [Google Scholar] [CrossRef]
- Starostensko, E.V. Propolization by bees of various races. Pchelovodsvo 1968, 88, 30. [Google Scholar]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid. Based Complement. Alternat. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Krell, R. Value-Added Products from Beekeeping; Food & Agriculture Org: Quebec city, QC, Canada, 1996. [Google Scholar]
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Bankova, V.S.; Castro, S.L.D.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Abu-Mellal, A.; Koolaji, N.; Duke, R.K.; Tran, V.H.; Duke, C.C. Prenylated cinnamate and stilbenes from Kangaroo Island propolis and their antioxidant activity. Phytochemistry 2012, 77, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Omar, R.; Siheri, W.; Al Mutairi, S.; Clements, C.; Fearnley, J.; Edrada-Ebel, R.; Watson, D. Chromatographic analysis with different detectors in the chemical characterisation and dereplication of African propolis. Talanta 2014, 120, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Trusheva, B.; Popova, M.; Bankova, V.; Simova, S.; Marcucci, M.C.; Miorin, P.L.; da Rocha Pasin, F.; Tsvetkova, I. Bioactive constituents of brazilian red propolis. Evid. Based Complement. Alternat. Med. 2006, 3, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; Braca, A.; de Tommasi, N.; Morelli, I.; Manunta, A.; Battinelli, L.; Mazzanti, G. Antimicrobial diterpenes from the seeds of Cephalotaxus harringtonia var. drupacea. Planta Med. 2003, 69, 468–470. [Google Scholar] [PubMed]
- Zeng, H.H.; Tu, P.F.; Zhou, K.; Wang, H.; Wang, B.H.; Lu, J.F. Antioxidant properties of phenolic diterpenes from Rosmarinus officinalis. Acta Pharmacol. Sin. 2001, 22, 1094–1098. [Google Scholar] [PubMed]
- Premprasert, C.; Tewtrakul, S.; Plubrukarn, A.; Wungsintaweekul, J. Anti-inflammatory activity of diterpenes from Croton stellatopilosus on LPS-induced RAW264.7 cells. J. Nat. Med. 2013, 67, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Rasoamiaranjanahary, L.; Marston, A.; Guilet, D.; Schenk, K.; Randimbivololona, F.; Hostettmann, K. Antifungal diterpenes from Hypoestes serpens (Acanthaceae). Phytochemistry 2003, 62, 333–337. [Google Scholar] [CrossRef]
- Lee, J.J.; Jin, Y.R.; Lee, J.H.; Yu, J.Y.; Han, X.H.; Oh, K.W.; Hong, J.T.; Kim, T.J.; Yun, Y.P. Antiplatelet activity of carnosic acid, a phenolic diterpene from Rosmarinus officinalis. Planta Med. 2007, 73, 121–127. [Google Scholar] [CrossRef]
- Greay, S.; Hammer, K. Recent developments in the bioactivity of mono- and diterpenes: Anticancer and antimicrobial activity. Phytochem. Rev. 2011, 14, 1–6. [Google Scholar] [CrossRef]
- Odek-Ogunde, M.; Rajab, M.S. Antihypertensive effect of the clerodane diterpene ajugarin I on experimentally hypertensive rats. East Afr. Med. J. 1994, 71, 587–590. [Google Scholar]
- Porto, T.S.; Rangel, R.; Furtado, N.A.; de Carvalho, T.C.; Martins, C.H.; Veneziani, R.C.; da Costa, F.B.; Vinholis, A.H.; Cunha, W.R.; Heleno, V.C.; et al. Pimarane-type diterpenes: Antimicrobial activity against oral pathogens. Molecules 2009, 14, 191–199. [Google Scholar] [CrossRef]
- Aceret, T.L.; Coll, J.C.; Uchio, Y.; Sammarco, P.W. Antimicrobial activity of the diterpenes flexibilide and sinulariolide derived from Sinularia flexibilis Quoy and Gaimard 1833 (Coelenterata: Alcyonacea, Octocorallia). Comp. Biochem. Physiol. C. Pharmacol. Toxicol. Endocrinol. 1998, 120, 121–126. [Google Scholar] [CrossRef]
- Reina, E.; Puentes, C.; Rojas, J.; Garcia, J.; Ramos, F.A.; Castellanos, L.; Aragon, M.; Ospina, L.F. Fuscoside E: A strong anti-inflammatory diterpene from Caribbean octocoral Eunicea fusca. Bioorg. Med. Chem. Lett. 2011, 21, 5888–5891. [Google Scholar] [CrossRef]
- Salah, M.A.; Bedir, E.; Toyang, N.J.; Khan, I.A.; Harries, M.D.; Wedge, D.E. Antifungal clerodane diterpenes from Macaranga monandra (L) Muell. et Arg. (Euphorbiaceae). J. Agric. Food Chem. 2003, 51, 7607–7610. [Google Scholar] [CrossRef]
- Tirapelli, C.R.; Ambrosio, S.R.; de Oliveira, A.M.; Tostes, R.C. Hypotensive action of naturally occurring diterpenes: A therapeutic promise for the treatment of hypertension. Fitoterapia 2010, 81, 690–702. [Google Scholar] [CrossRef]
- Martin, Y.C. A bioavailability score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef]
- Yoshida, F.; Topliss, J.G. QSAR model for drug human oral bioavailability. J. Med. Chem. 2000, 43, 2575–2585. [Google Scholar] [CrossRef]
- Boehm, M.; Fuenfschilling, P.C.; Krieger, M.; Kuesters, E.; Struber, F. An improved manufacturing process for the antimalaria drug coartem. Part I. Org. Process Res. Dev. 2007, 11, 336–340. [Google Scholar] [CrossRef]
- Nematollahi, A.; Aminimoghadamfarouj, N.; Wiart, C. Reviews on 1,4-naphthoquinones from Diospyros L. J. Asian Nat. Prod. Res. 2012, 14, 80–88. [Google Scholar] [CrossRef]
- Aminimoghadamfarouj, N.; Nematollahi, A.; Wiart, C. Annonaceae: Bio-resource for tomorrow’s drug discovery. J. Asian Nat. Prod. Res. 2011, 13, 465–476. [Google Scholar] [CrossRef]
- Meresta, L.; Meresta, T. Effect of pH on bactericidal activity of propolis. Bull. Veterina. Inst. Pulawy 1980, 24, 21–25. [Google Scholar]
- Gonzales, G.V.; Hernandez, N.R.; Vera, C.M. Comparative study of the antimicrobial activity of propolis and that of antibiotics and conventional disinfectants. Ciencia Y Tenica en la Agriculura Apiculura 1985, 1, 23–36. [Google Scholar]
- Grange, J.M.; Davey, R.W. Antibacterial properties of propolis (bee glue). J. R. Soc. Med. 1990, 83, 159–160. [Google Scholar]
- Obregón Fuentes, A.; Rojas Hernández, N. Antimicrobial action of alcoholic extracts of propolis. Revista Cubana de Farmacia 1990, 24, 34–44. [Google Scholar]
- Ibragimova, A.; Pankratova, N. Combined antimicrobial effect of propolis and antibiotics on pathogenic staphylococci. Sbornik Nauchnykh Trudov, Kazanskii Veterinarnyi Institute 1983, 43, 46. [Google Scholar]
- Meresta, L.; Meresta, T. An attempt to use the extract from propolis in the treatment of mastitis of cows. Med. Weter. 1985, 41, 489–492. [Google Scholar]
- Maksimova-Todorova, V.; Manolova, N.; Gegova, G.; Serkedzhieva, I.; Uzunov, S. Antiviral action of fractions isolated from propolis. Acta Microbiol. Bulg. 1985, 17, 79–85. [Google Scholar]
- Amoros, M.; Sauvager, F.; Girre, L.; Cormier, M. In vitro antiviral activity of propolis. Apidologie 1992, 23, 231–240. [Google Scholar] [CrossRef]
- Bankova, V.; Galabov, A.S.; Antonova, D.; Vilhelmova, N.; di Perri, B. Chemical composition of propolis extract ACF(R) and activity against herpes simplex virus. Phytomedicine 2014, 21, 1432–1438. [Google Scholar] [CrossRef]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antiviral activity of Plantago major extracts and related compounds in vitro. Antiviral Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- Lyu, S.Y.; Rhim, J.Y.; Park, W.B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch. Pharm. Res. 2005, 28, 1293–1301. [Google Scholar] [CrossRef]
- Elias, D.; Beazely, M.; Kandepu, N. Bioactivities of chalcones. Curr. Med. Chem. 1999, 6, 1125. [Google Scholar]
- Suárez, B.; Álvarez, Á.L.; García, Y.D.; del Barrio, G.; Lobo, A.P.; Parra, F. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 2010, 120, 339–342. [Google Scholar] [CrossRef]
- Gravina, H.D.; Tafuri, N.F.; Silva Junior, A.; Fietto, J.L.; Oliveira, T.T.; Diaz, M.A.; Almeida, M.R. In vitro assessment of the antiviral potential of trans-cinnamic acid, quercetin and morin against equid herpesvirus 1. Res. Vet. Sci. 2011, 91, e158–e162. [Google Scholar] [CrossRef]
- Millet-Clerc, J.; Simeray, J.; Michel, D.; Chaumont, J. Antifungal properties of propolis against fungi causing mycoses. J. Mycol. Med. 1987, 15, 517–521. [Google Scholar]
- Holderna, E.; Kedzia, B. Investigation upon the combined action of propolis and antimycotic drugs on Candida albicans. Herba Polo. 1987, 33, 145–151. [Google Scholar]
- Silici, S.; Koc, N.A.; Ayangil, D.; Cankaya, S. Antifungal activities of propolis collected by different races of honeybees against yeasts isolated from patients with superficial mycoses. J. Pharmacol. Sci. 2005, 99, 39–44. [Google Scholar] [CrossRef]
- Kasote, D.; Ahmad, A.; Chen, W.; Combrinck, S.; Viljoen, A. HPTLC-MS as an efficient hyphenated technique for the rapid identification of antimicrobial compounds from propolis. Phytochem. Lett. 2015, 11, 326–331. [Google Scholar] [CrossRef]
- Lori, G. Fungicidal effect of propolis in bovine dermatomycosis. Industria Apícola 1990, 1, 38–43. [Google Scholar]
- Yang, S.Z.; Peng, L.T.; Su, X.J.; Chen, F.; Cheng, Y.J.; Fan, G.; Pan, S.Y. Bioassay-guided isolation and identification of antifungal components from propolis against Penicillium italicum. Food Chem. 2011, 127, 210–215. [Google Scholar] [CrossRef]
- Almutairi, S.; Eapen, B.; Chundi, S.M.; Akhalil, A.; Siheri, W.; Clements, C.; Fearnley, J.; Watson, D.G.; Edrada-Ebel, R. New anti-trypanosomal active prenylated compounds from African propolis. Phytochem. Lett. 2014, 10, 35–39. [Google Scholar] [CrossRef]
- Hegazi, A.; Abd El Hady, F.; Shalaby, H. Egyptian propolis: 4—Chemical Composition of Siwa Oasis Propolis with Reference to its Effect on Fasciola Gigantica Eggs. In Proceedings of the 1st International Forum on Apitherapy, Athens, Greece, October 2006; pp. 31–32. [Google Scholar]
- Higashi, K.; de Castro, S. Propolis extracts are effective against Trypanosoma cruzi and have an impact on its interaction with host cells. J. Ethnopharmacol. 1994, 43, 149–155. [Google Scholar] [CrossRef]
- Miyares, C.; Hollands, I.; Castaneda, C.; Gonzalez, T.; Fragoso, T.; Curras, R.; Soria, C. Clinical trial with a preparation based on propolis “propolisina” in human giardiasis. Acta Gastroenterol. Latinoam. 1987, 18, 195–201. [Google Scholar]
- Starzyk, J.; Scheller, S.; Szaflarski, J.; Moskwa, M.; Stojko, A. Biological properties and clinical application of propolis. II. Studies on the antiprotozoan activity of ethanol extract of propolis. Arzneimittel-Forschung 1976, 27, 1198–1199. [Google Scholar]
- Torres, D.; Hollands, I.; Palacios, E. Effect of an alcoholic extract of propolis on the in vitro growth of Giardia lamblia. Rev. Cuba. Cienc. Vet. 1990, 21, 15–19. [Google Scholar]
- Salas, A.L.; Alberto, M.R.; Zampini, I.C.; Cuello, A.S.; Maldonado, L.; Ríos, J.L.; Schmeda-Hirschmann, G.; Isla, M.I. Biological activities of polyphenols-enriched propolis from Argentina arid regions. Phytomedicine 2016, 23, 27–31. [Google Scholar] [CrossRef]
- Bockmuehl, D.; Breves, R.; Weide, M.; Stumpe, S.; Heinzel, M. Adhesion inhibition of fungi. WO2003051125A1, 2003. [Google Scholar]
- Wang, K.; Hu, L.; Jin, X.L.; Ma, Q.X.; Marcucci, M.C.; Netto, A.A.L.; Sawaya, A.C.H.F.; Huang, S.; Ren, W.K.; Conlon, M.A.; et al. Polyphenol-rich propolis extracts from China and Brazil exert anti-inflammatory effects by modulating ubiquitination of TRAF6 during the activation of NF-κB. J. Funct. Foods 2015, 19, 464–478. [Google Scholar] [CrossRef]
- Cavendish, R.L.; de Souza Santos, J.; Neto, R.B.; Paixao, A.O.; Oliveira, J.V.; de Araujo, E.D.; Silva, A.A.B.E.; Thomazzi, S.M.; Cardoso, J.C.; Gomes, M.Z. Antinociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents. J. Ethnopharmacol. 2015, 173, 127–133. [Google Scholar]
- Valenzuela-Barra, G.; Castro, C.; Figueroa, C.; Barriga, A.; Silva, X.; de Las Heras, B.; Hortelano, S.; Delporte, C. Anti-inflammatory activity and phenolic profile of propolis from two locations in Region Metropolitana de Santiago, Chile. J. Ethnopharmacol. 2015, 168, 37–44. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Okamoto, K.; Izumi, R.; Tago, K.; Yanagisawa, K.; Narukawa, Y.; Kiuchi, F.; Kasahara, T.; Tamura, H. Anti-inflammatory activity of flavonoids in Nepalese propolis is attributed to inhibition of the IL-33 signaling pathway. Int. Immunopharmacol. 2015, 25, 189–198. [Google Scholar] [CrossRef]
- Li, F.; Awale, S.; Tezuka, Y.; Kadota, S. Cytotoxic constituents from Brazilian red propolis and their structure-activity relationship. Bioorg. Med. Chem. 2008, 16, 5434–5440. [Google Scholar] [CrossRef]
- Pratsinis, H.; Kletsas, D.; Melliou, E.; Chinou, I. Antiproliferative activity of Greek propolis. J. Med. Food 2010, 13, 286–290. [Google Scholar] [CrossRef]
- Matsuno, T.; Saito, M.; Matsumoto, Y.; Morikawa, J. A new benzo-γ-pyran derivative isolated from propolis. Z. Naturforsch. C 1998, 53, 1037–1039. [Google Scholar]
- Mitamura, T.; Matsuno, T.; Sakamoto, S.; Maemura, M.; Kudo, H.; Suzuki, S.; Kuwa, K.; Yoshimura, S.; Sassa, S.; Nakayama, T. Effects of a new clerodane diterpenoid isolated from propolis on chemically induced skin tumors in mice. Anticancer Res. 1995, 16, 2669–2672. [Google Scholar]
- Conti, B.J.; Búfalo, M.C.; Golim, M.D.A.; Bankova, V.; Sforcin, J.M. Cinnamic acid is partially involved in propolis immunomodulatory action on human monocytes. Evid. Based Complement. Alternat. Med. 2013. [Google Scholar] [CrossRef]
- Orsatti, C.L.; Sforcin, J.M. Propolis immunomodulatory activity on TLR-2 and TLR-4 expression by chronically stressed mice. Nat. Prod. Res. 2012, 26, 446–453. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Sampietro Vattuone, M.M.; Vattuone, M.A. Immunomodulatory activity of Apis mellifera propolis from the North of Argentina. Food Sci. Tech. 2016, 70, 9–15. [Google Scholar] [CrossRef]
- Gritsenko, V.I.; Tikhonov, O.I.; Priakhin, O.R. Study of the polysaccharide preparation, propolis. Farm. Zh. 1977, 92–93. [Google Scholar]
- Ikeno, K.; Ikeno, T.; Miyazawa, C. Effects of propolis on dental-caries in rats. Caries Res. 1991, 25, 347–351. [Google Scholar] [CrossRef]
- Hay, K.D.; Greig, D.E. Propolis allergy: A cause of oral mucositis with ulceration. Oral Surg. Oral Med. Oral Pathol. 1990, 70, 584–586. [Google Scholar] [CrossRef]
- Rudzki, E.; Grzywa, Z.; Pomorski, Z. New data on dermatitis from propolis. Contact Derm. 1985, 13, 198–199. [Google Scholar]
- Rudzki, E.; Grzywa, Z. Dermatitis from propolis. Contact Derm. 1983, 9, 40–45. [Google Scholar] [CrossRef]
- Hausen, B.; Wollenweber, E.; Senff, H.; Post, B. Propolis allergy. Contact Derm. 1987, 17, 163–170. [Google Scholar] [CrossRef]
- Dickschat, J.S. Isoprenoids in three-dimensional space: The stereochemistry of terpene biosynthesis. Nat. Prod. Rep. 2011, 28, 1917–1936. [Google Scholar] [CrossRef]
- Gilbert, K. Plant secondary metabolism. Plant Growth Regul. 2001, 34, 149. [Google Scholar] [CrossRef]
- Pan, L.; Blanco, E.C.; Kinghorn, A.D. Plant-derived natural products as leads for drug discovery. In Plant-Derived Natural Products; Osbourn, A.E., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009; pp. 547–567. [Google Scholar]
- Righi, A.A.; Negri, G.; Salatino, A. Comparative chemistry of propolis from eight brazilian localities. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Araujo, M.J.; Mattar, N.S.; Reis, A.S.; Serra, I.C.; Fialho, E.M.; Assuncao, A.K.; Dutra, R.P.; Nogueira, A.M.; Liberio, S.A.; Guerra, R.N.; et al. Pharmacognostic and acute toxicological evaluation of Scaptotrigona aff. postica propolis extract in pre-clinical assays. Nat. Prod. Res. 2011, 25, 1037–1046. [Google Scholar] [CrossRef]
- Popova, M.P.; Graikou, K.; Chinou, I.; Bankova, V.S. GC-MS profiling of diterpene compounds in Mediterranean propolis from Greece. J. Agric. Food Chem. 2010, 58, 3167–3176. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Antonova, D.; Cutajar, S.; Mifsud, D.; Farrugia, C.; Tsvetkova, I.; Najdenski, H.; Bankova, V. The specific chemical profile of Mediterranean propolis from Malta. Food Chem. 2011, 126, 1431–1435. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Tsvetkova, I.; Naydenski, C.; Silva, M.V. The first glycosides isolated from propolis: Diterpene rhamnosides. Z. Naturforsch. C J. Biosci. 2001, 56, 1108–1111. [Google Scholar] [CrossRef]
- Elnakady, Y.A.; Rushdi, A.I.; Franke, R.; Abutaha, N.; Ebaid, H.; Baabbad, M.; Omar, M.O.; Al Ghamdi, A.A. Characteristics, chemical compositions and biological activities of propolis from Al-Bahah, Saudi Arabia. Sci. Rep. 2017, 7, 41453. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Aazza, S.; Lyoussi, B.; Bankova, V.; Lourenco, J.P.; Costa, A.M.; Mariano, J.F.; Miguel, M.G.; Faleiro, M.L. Impact of biohybrid magnetite nanoparticles and Moroccan propolis on adherence of methicillin resistant strains of staphylococcus aureus. Molecules 2016, 21, 1208. [Google Scholar] [CrossRef]
- Popova, M.; Lyoussi, B.; Aazza, S.; Antunes, D.; Bankova, V.; Miguel, G. Antioxidant and α-glucosidase inhibitory properties and chemical profiles of Moroccan propolis. Nat. Prod. Commun. 2015, 10, 1961–1964. [Google Scholar]
- Tazawa, S.; Arai, Y.; Hotta, S.; Mitsui, T.; Nozaki, H.; Ichihara, K. Discovery of a novel diterpene in brown propolis from the State of Parana, Brazil. Nat. Prod. Commun. 2016, 11, 201–205. [Google Scholar]
- Tazawa, S.; Arai, Y.; Hotta, S.; Ichihara, K. Novel diterpene, its production method, antitumor agent containing the same, propolis extract, and method for evaluation of propolis extract. JP2015205824A, 2015. [Google Scholar]
- Nina, N.; Quispe, C.; Jimenez-Aspee, F.; Theoduloz, C.; Feresin, G.E.; Lima, B.; Leiva, E.; Schmeda-Hirschmann, G. Antibacterial activity, antioxidant effect and chemical composition of propolis from the region del maule, central Chile. Molecules 2015, 20, 18144–18167. [Google Scholar] [CrossRef]
- Graikou, K.; Popova, M.; Gortzi, O.; Bankova, V.; Chinou, I. Characterization and biological evaluation of selected Mediterranean propolis samples. Is it a new type? Lwt-Food Sci. Technol. 2016, 65, 261–267. [Google Scholar] [CrossRef]
- Siheri, W.; Igoli, J.O.; Gray, A.I.; Nasciemento, T.G.; Zhang, T.; Fearnley, J.; Clements, C.J.; Carter, K.C.; Carruthers, J.; Edrada-Ebel, R.; et al. The isolation of antiprotozoal compounds from Libyan propolis. Phytother. Res. 2014, 28, 1756–1760. [Google Scholar] [CrossRef]
- Almutairi, S.; Edrada-Ebel, R.; Fearnley, J.; Igoli, J.O.; Alotaibi, W.; Clements, C.J.; Gray, A.I.; Watson, D.G. Isolation of diterpenes and flavonoids from a new type of propolis from Saudi Arabia. Phytochem. Lett. 2014, 10, 160–163. [Google Scholar] [CrossRef]
- Sulaiman, G.M.; Al Sammarrae, K.W.; Ad’hiah, A.H.; Zucchetti, M.; Frapolli, R.; Bello, E.; Erba, E.; D’Incalci, M.; Bagnati, R. Chemical characterization of Iraqi propolis samples and assessing their antioxidant potentials. Food Chem. Toxicol. 2011, 49, 2415–2421. [Google Scholar] [CrossRef]
- Popova, M.P.; Chinou, I.B.; Marekov, I.N.; Bankova, V.S. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 2009, 70, 1262–1271. [Google Scholar] [CrossRef]
- Filho, A.A.D.S.; de Sousa, J.P.B.; Soares, S.; Furtado, N.A.J.C.; Silva, M.L.A.E.; Cunha, W.R.; Gregorio, L.E.; Nanayakkara, N.R.D.; Bastos, J.K. Antimicrobial activity of the extract and isolated compounds from Baccharis dracunculifolia D.C. (Asteraceae). Z. Naturforsch. C Bio. Sci. 2008, 63, 40–46. [Google Scholar]
- Midorikawa, K.; Banskota, A.H.; Tezuka, Y.; Matsushige, K.; Message, D.; Huertas, A.A.G.; Kadota, S. Buds of Baccharis dracunculifolia: Potent source of biologically active caffeoylquinic acids and labdane-type diterpenes of Brazilian propolis. Yakugaku Zasshi 2003, 20, 187–194. [Google Scholar]
- Midorikawa, K.; Banskota, A.H.; Tezuka, Y.; Nagaoka, T.; Matsushige, K.; Message, D.; Huertas, A.A.; Kadota, S. Liquid chromatography-mass spectrometry analysis of propolis. Phytochem. Anal. 2001, 12, 366–373. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, L. Chemical analysis and antimicrobial activity of Greek propolis. Planta Med. 2004, 70, 515–519. [Google Scholar] [CrossRef]
- Trusheva, B.; Popova, M.; Bankova, V.; Tsvetkova, I.; Naydenski, C.; Sabatini, A.G. A new type of European propolis, containing bioactive labdanes. Riv. Ital. EPPOS 2003, 36, 3–7. [Google Scholar]
- Bankova, V.; Popova, M.; Bogdanov, S.; Sabatini, A.G. Chemical composition of European propolis: Expected and unexpected results. Z. Naturforsch. C 2002, 57, 530–533. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Adnyana, I.K.; Ishii, E.; Midorikawa, K.; Matsushige, K.; Kadota, S. Hepatoprotective and anti-Helicobacter pylori activities of constituents from Brazilian propolis. Phytomedicine 2001, 8, 16–23. [Google Scholar] [CrossRef]
- Leist, M.; Gantner, F.; Bohlinger, I.; Germann, P.G.; Tiegs, C.; Wendel, A. Murine hepatocyte apoptosis induced in-vitro and in-vivo by TNF-α requires transcriptional arrest. J. Immunol. 1994, 153, 1778–1788. [Google Scholar]
- Gonzalez, R.; Corcho, I.; Remirez, D.; Rodriguez, S.; Ancheta, O.; Merino, N.; Gonzalez, A.; Pascual, C. Hepatoprotective effects of propolis extract on carbon tetrachloride-induced liver-injury in rats. Phytother. Res. 1995, 9, 114–117. [Google Scholar] [CrossRef]
- Velikova, M.; Bankova, V.; Marcucci, M.C.; Tsvetkova, I.; Kujumgiev, A. Chemical composition and biological activity of propolis from Brazilian meliponinae. Z. Naturforsch. C J. Biosci. 2000, 55, 785–789. [Google Scholar] [CrossRef]
- Velikova, M.; Bankova, V.; Tsvetkova, I.; Kujumgiev, A.; Marcucci, M.C. Antibacterial ent-kaurene from Brazilian propolis of native stingless bees. Fitoterapia 2000, 71, 693–696. [Google Scholar] [CrossRef]
- Matsuno, T.; Matsumoto, Y.; Saito, M.; Morikawa, J. Isolation and characterization of cytotoxic diterpenoid isomers from propolis. Z. Naturforsch. C Biosci. 1997, 52, 702–704. [Google Scholar]
- Matsuno, T. A new clerodane diterpenoid isolated from propolis. Z. Naturforsch. C Biosci. 1995, 50, 93–97. [Google Scholar]
- Yoshida, M.; Saito, Y.; Matsuno, T. Diterpenoid from propoles as antitumor agent. EP532156A1, 1993. [Google Scholar]
- Bankova, V.; Marcucci, M.C.; Simova, S.; Nikolova, N.; Kujumigiev, A. Antibacterial diterpenic acids from Brazilian propolis. Z. Naturforsch. C Biosci. 1996, 51, 227–280. [Google Scholar]
- Matsuno, T.; Shioda, M.; Kano, K. Tumoricidal Activity of a New Clerodane Diterpenoid Isolated from Propolis; Monduzzi Editore: Milano, Italy, 1994; pp. 691–694. [Google Scholar]
- Li, H.; Kapur, A.; Yang, J.X.; Srivastava, S.; McLeod, D.G.; Paredes-Guzman, J.F.; Daugsch, A.; Park, Y.K.; Rhim, J.S. Antiproliferation of human prostate cancer cells by ethanolic extracts of Brazilian propolis and its botanical origin. Int. J. Oncol. 2007, 31, 601–606. [Google Scholar] [CrossRef]
- Smith, E.C.; Kaatz, G.W.; Seo, S.M.; Wareham, N.; Williamson, E.M.; Gibbons, S. The phenolic diterpene totarol inhibits multidrug efflux pump activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 4480–4483. [Google Scholar] [CrossRef]
- Haraguchi, H.; Oike, S.; Muroi, H.; Kubo, I. Mode of antibacterial action of totarol, a diterpene from Podocarpus nagi. Planta Med. 1996, 62, 122–125. [Google Scholar] [CrossRef]
- Shapiro, S.; Guggenheim, B. Inhibition of oral bacteria by phenolic compounds. Part 1. QSAR analysis using molecular connectivity. Quant. Struct.-Act. Relat. 1998, 17, 327–337. [Google Scholar] [CrossRef]
- Kubo, I.; Muroi, H.; Himejima, M. Antibacterial activity of totarol and its potentiation. J. Nat. Prod. 1992, 55, 1436–1440. [Google Scholar] [CrossRef]
- Muroi, H.; Kubo, I. Antibacterial activity of anacardic acid and totarol, alone and in combination with methicillin, against methicillin-resistant Staphylococcus aureus. J. Appl. Bacteriol. 1996, 80, 387–394. [Google Scholar] [CrossRef]
- Nicolson, K.; Evans, G.; O’Toole, P.W. Potentiation of methicillin activity against methicillin-resistant Staphylococcus aureus by diterpenes. FEMS Microbiol. Lett. 1999, 179, 233–239. [Google Scholar] [CrossRef]
- Gibbons, S. Plants as a source of bacterial resistance modulators and anti-infective agents. Phytochem. Rev. 2005, 4, 63–78. [Google Scholar] [CrossRef]
- Evans, G.B.; Furneaux, R.H.; Gainsford, G.J.; Murphy, M.P. The synthesis and antibacterial activity of totarol derivatives. Part 3: Modification of ring-B. Bioorg. Med. Chem. 2000, 8, 1663–1675. [Google Scholar] [CrossRef]
- Micol, V.; Mateo, C.R.; Shapiro, S.; Aranda, F.J.; Villalain, J. Effects of (+)-totarol, a diterpenoid antibacterial agent, on phospholipid model membranes. Biochim. Biophys. Acta 2001, 1511, 281–290. [Google Scholar] [CrossRef]
- Bernabeu, A.; Shapiro, S.; Villalain, J. A MAS-NMR study of the location of (+)-totarol, a diterpenoid bioactive molecule, in phospholipid model membranes. Chem. Phys. Lipids 2002, 119, 33–39. [Google Scholar] [CrossRef]
- Mateo, C.R.; Prieto, M.; Micol, V.; Shapiro, S.; Villalain, J. A fluorescence study of the interaction and location of (+)-totarol, a diterpenoid bioactive molecule, in model membranes. Biochim. Biophys. Acta 2000, 1509, 167–175. [Google Scholar] [CrossRef]
- Osawa, M.; Anderson, D.E.; Erickson, H.P. Reconstitution of contractile FtsZ rings in liposomes. Science 2008, 320, 792–794. [Google Scholar] [CrossRef]
- Jaiswal, R.; Beuria, T.K.; Mohan, R.; Mahajan, S.K.; Panda, D. Totarol inhibits bacterial cytokinesis by perturbing the assembly dynamics of FtsZ. Biochemistry 2007, 46, 4211–4220. [Google Scholar] [CrossRef]
- Evans, G.B.; Furneaux, R.H.; Gravestock, M.B.; Lynch, G.P.; Scott, G.K. The synthesis and antibacterial activity of totarol derivatives. Part 1: Modifications of ring-C and pro-drugs. Bioorg. Med. Chem. 1999, 7, 1953–1964. [Google Scholar] [CrossRef]
- Evans, G.B.; Furneaux, R.H. The synthesis and antibacterial activity of totarol derivatives. Part 2: Modifications at C-12 and O-13. Bioorg. Med. Chem. 2000, 8, 1653–1662. [Google Scholar] [CrossRef]
- Salazar, F.J.; Quintero, A.; de Domínguez, N.G.; Concepción, J.L.; Acosta, M.E.; Tropper, E.; Villamizar, J.E. Synthesis, antimalarial and antileishmanial activity of novel 13-benzyl-15,16-bisnorlabdane derivatives. J. Chem. Res. 2013, 37, 657–661. [Google Scholar] [CrossRef]
- Simpson, B.S.; Claudie, D.J.; Gerber, J.P.; Pyke, S.M.; Wang, J.P.; McKinnon, R.A.; Semple, S.J. In vivo activity of benzoyl ester clerodane diterpenoid derivatives from dodonaea polyandra. J. Nat. Prod. 2011, 650–657. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminimoghadamfarouj, N.; Nematollahi, A. Propolis Diterpenes as a Remarkable Bio-Source for Drug Discovery Development: A Review. Int. J. Mol. Sci. 2017, 18, 1290. https://doi.org/10.3390/ijms18061290
Aminimoghadamfarouj N, Nematollahi A. Propolis Diterpenes as a Remarkable Bio-Source for Drug Discovery Development: A Review. International Journal of Molecular Sciences. 2017; 18(6):1290. https://doi.org/10.3390/ijms18061290
Chicago/Turabian StyleAminimoghadamfarouj, Noushin, and Alireza Nematollahi. 2017. "Propolis Diterpenes as a Remarkable Bio-Source for Drug Discovery Development: A Review" International Journal of Molecular Sciences 18, no. 6: 1290. https://doi.org/10.3390/ijms18061290