Determination of Sulfonamide Residues in Food by Capillary Zone Electrophoresis with On-Line Chemiluminescence Detection Based on an Ag(III) Complex
Abstract
:1. Introduction
2. Results
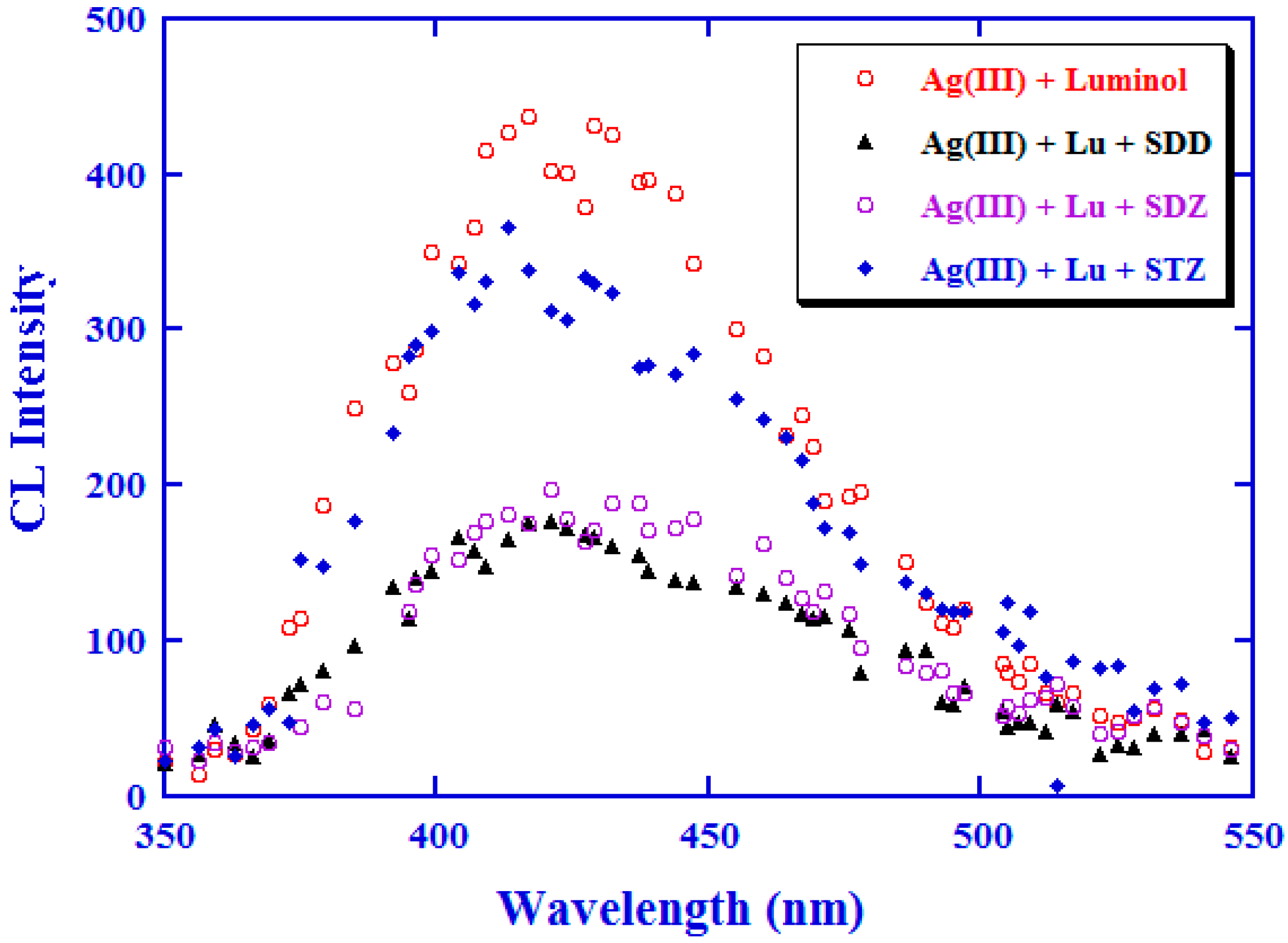
2.1. Optimization of the CL Detection System
2.2. Optimization of Capillary Electrophoresis (CE) Separation Conditions in the Presence of SAs
2.2.1. Selection of CE Buffer Solution and Its pH Value
2.2.2. Selection of Applied Voltage
2.2.3. Selection of Injection Mode and Injection Time
2.3. Linearity, Limit of Detection, and Reproducibility
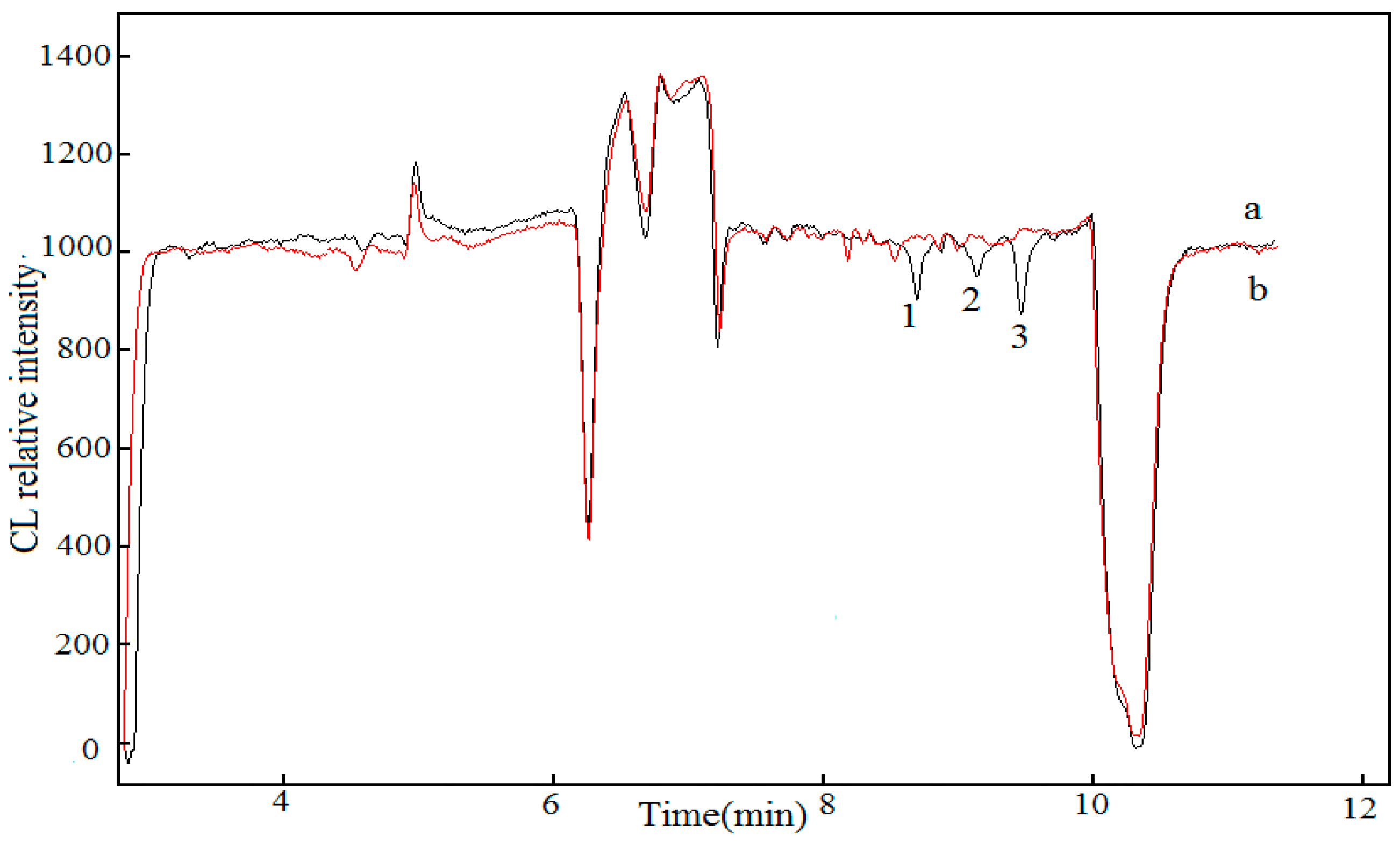
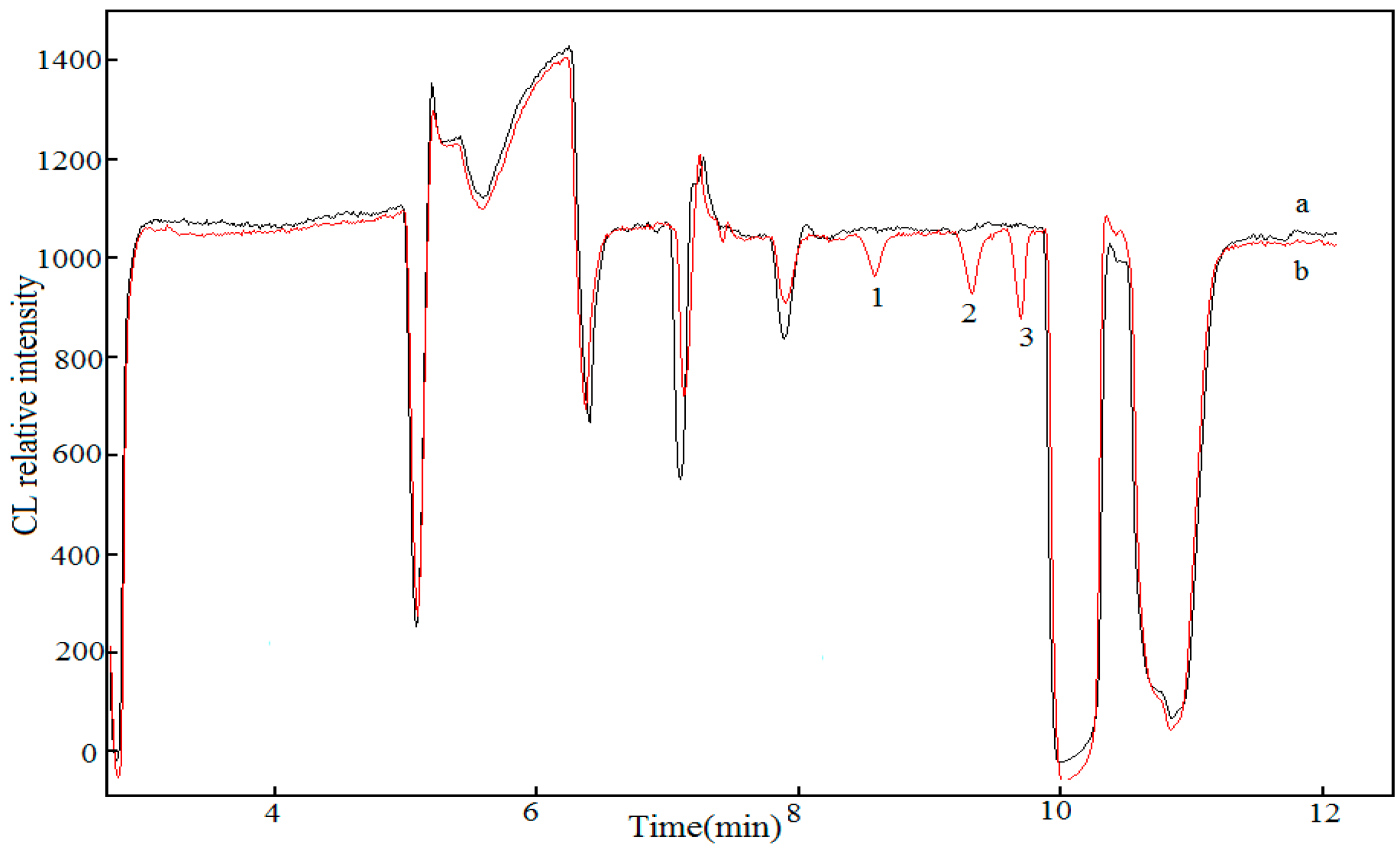
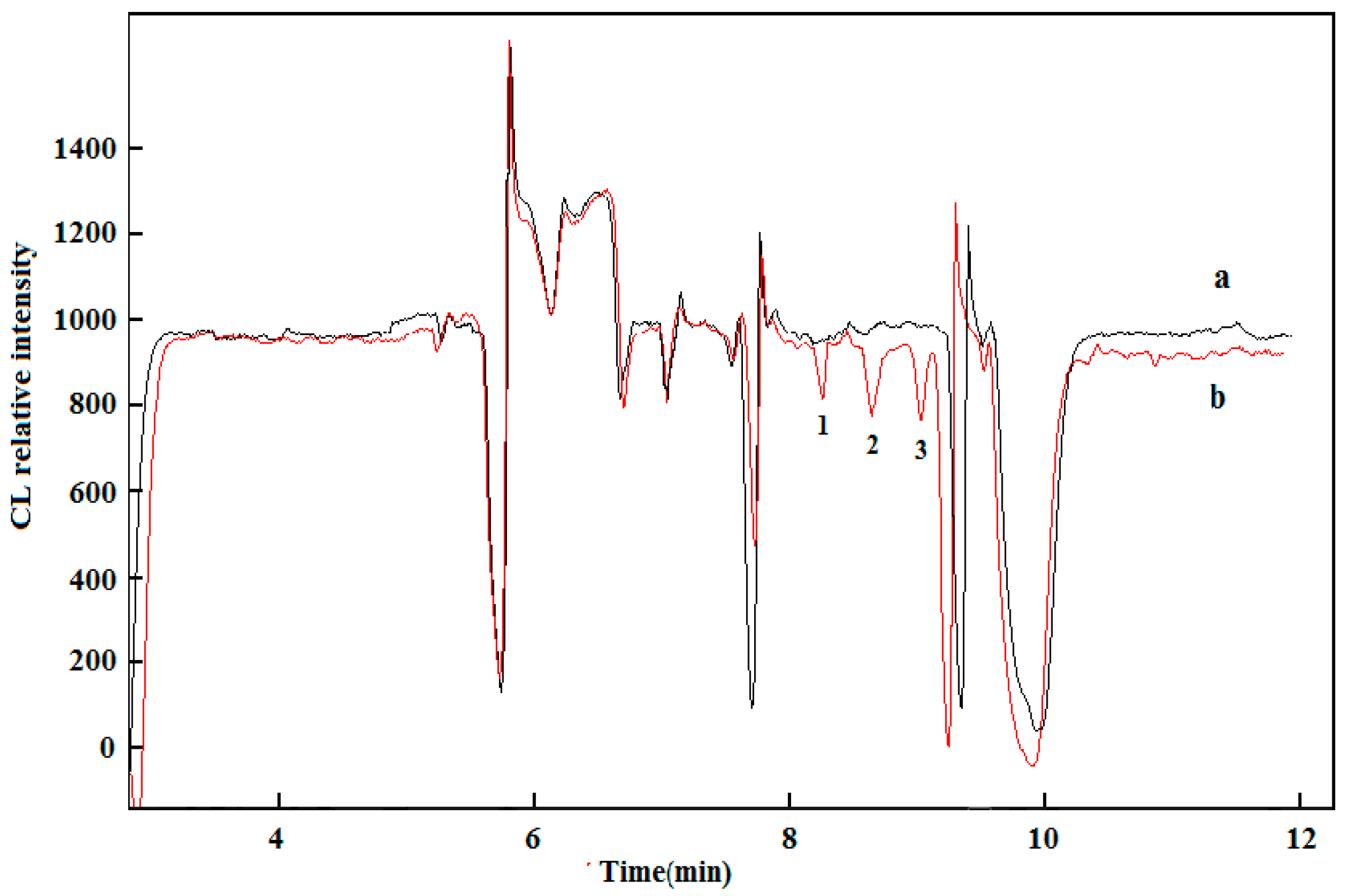
2.4. Sample Analysis
2.5. Recovery Experiments
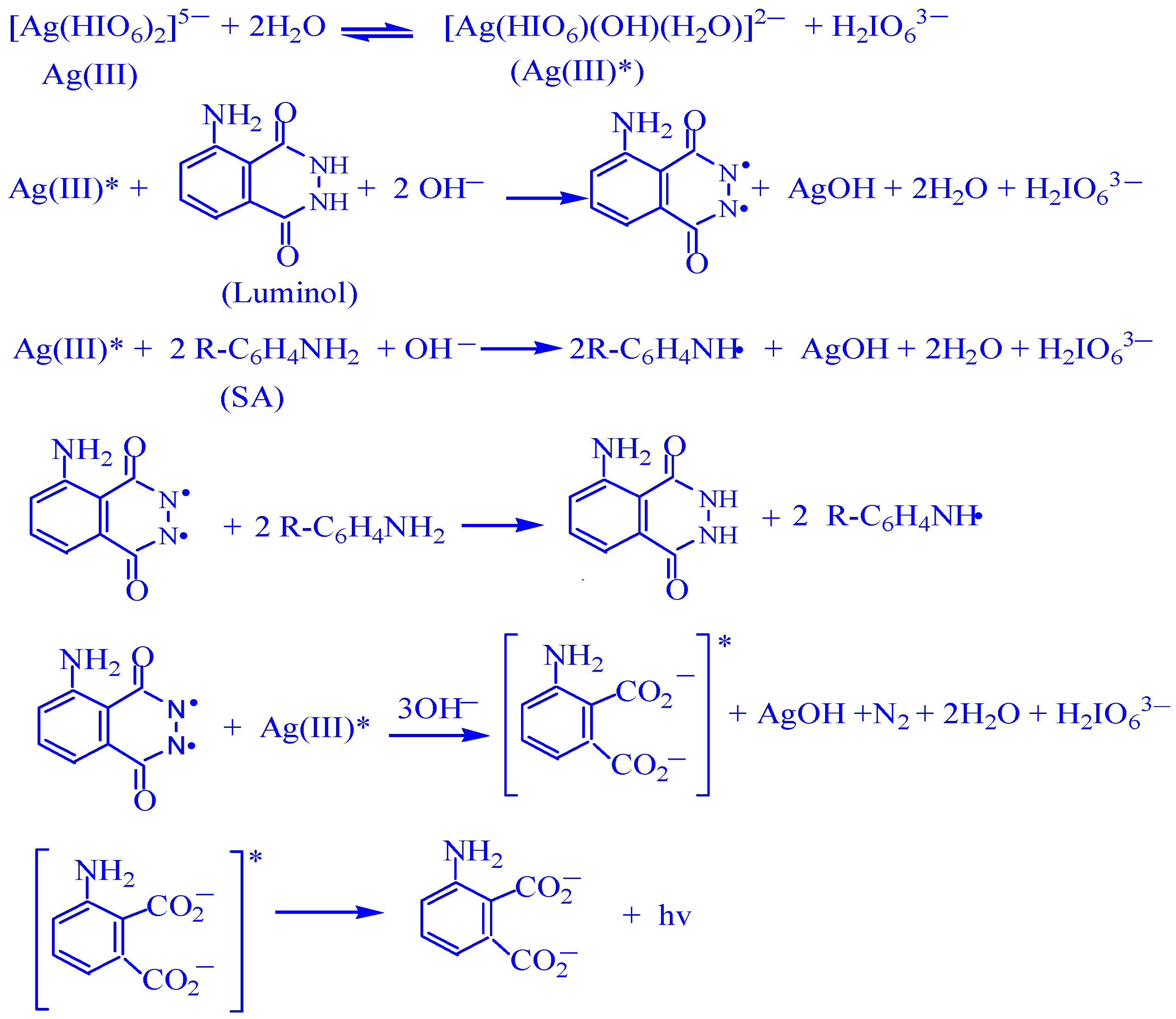
3. Discussion
4. Experiment
4.1. Reagents and Solutions
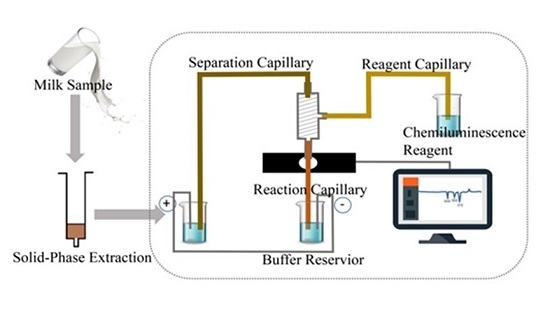
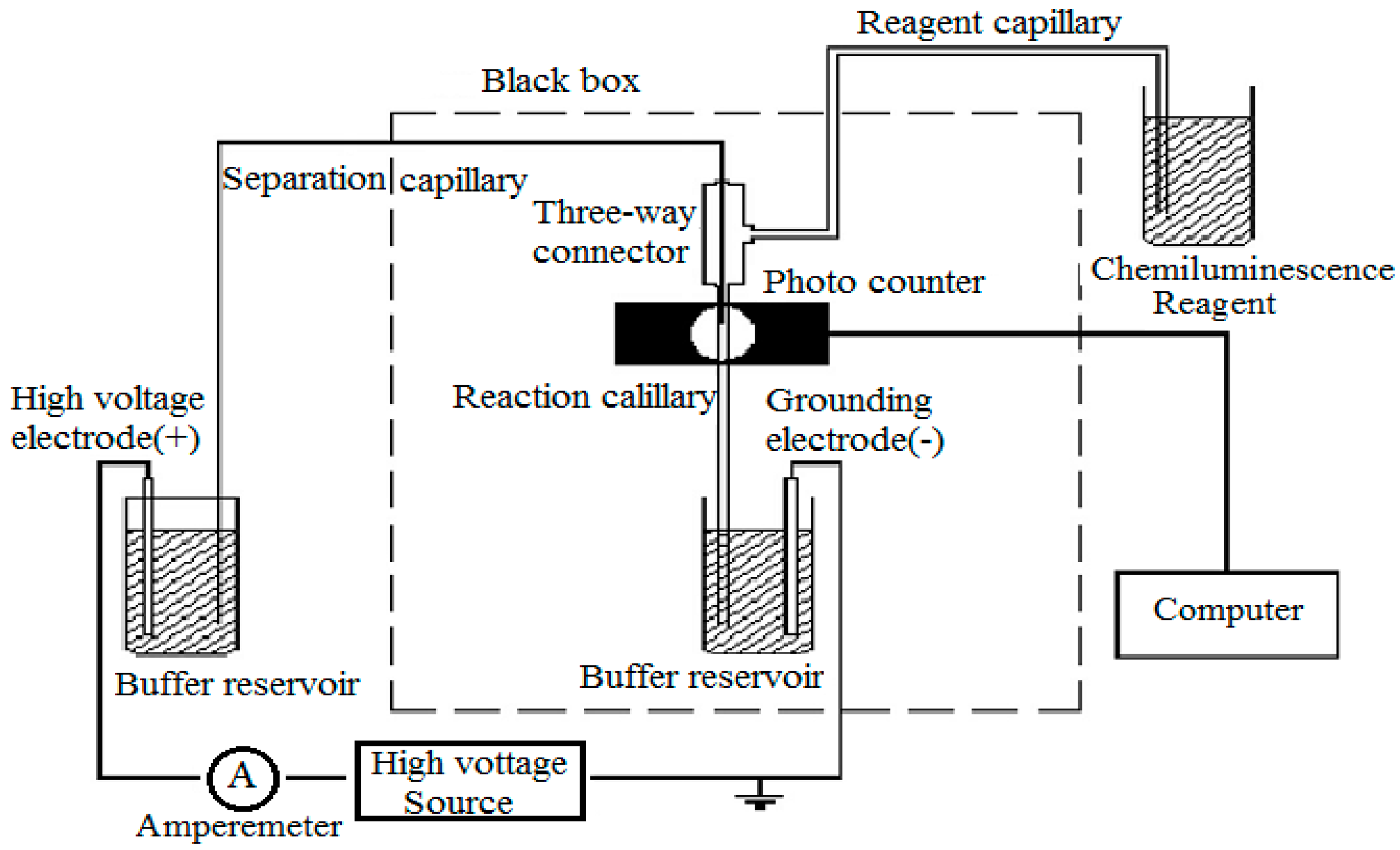
4.2. CE–CL Detection System and Electrophoresis Procedure
4.3. Samples Treatment and Extraction Procedure
4.4. Treatment and Application of SPE Column
- Condition: 2 mL methanol followed by 2 mL of 0.10 M HCl.
- Load: chicken/pork extraction solutions were applied to the cartridges.
- Wash: 1 mL of 0.10 M HCl, followed by 2 mL of a solvent mixture (50% methanol–50% acetonitrile).
- Elute: 4 mL of a solution mixture of 5% ammonia–95% methanol (V/V).
- Condition: 3 mL of methanol followed by 5 mL of perchloric acid solution (pH, 2.0).
- Load: the extracted solution from milk was applied to the cartridges.
- Wash: 5 mL water.
- Elute: 3 mL methanol.
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Gehring, R.; Davis, J.; Riviere, J.E. Health concerns and management of select veterinary drug residues. Food Chem. Toxicol. 2016, 88, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Baran, W.; Adamek, E.; Ziemiańska, J.; Sobczak, A. Effects of the presence of sulfonamides in the environment and their influence on human health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Catalano-Pons, C.; Bargy, S.; Schlecht, D.; Tabone, M.D.; Deschênes, G.; Bensman, A.; Ulinski, T. Sulfadiazine-induced nephrolithiasis in children. Pediatr. Nephrol. 2004, 19, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.M.; Ibrahim, I.T.; Attallah, K.M.; Ali, S.M. Tc-sulfadimidine as a potential radioligand for differentiation between septic and aseptic inflammations. Radiochemistry 2014, 56, 72–75. [Google Scholar] [CrossRef]
- Bourne, D.W.A.; Dittert, L.W.; Koritz, G.D.; Bevill, R.F. Disposition of sulfonamides in food-producing animals. VII. Disposition of sulfathiazole in tissue, urine, and plasma of swine following intravenous administration. J. Pharmacokinet. Biop. 1978, 6, 123–134. [Google Scholar] [CrossRef]
- Rodrigo, H.; Tânia, M.P.; Diaz-Cruz, M.S. Trends in sulfonamides and their by-products analysis in environmental samples using mass spectrometry techniques. Trends Environ. Anal. Chem. 2016, 9, 24–36. [Google Scholar]
- Stanislava, G.D.; Elena, V.K.; Vladimir, V.A.; Veronika, V.T.; Yury, A.Z. Recent advances in sample preparation techniques and methods of sulfonamides detection–a review. Anal. Chim. Acta 2014, 850, 6–25. [Google Scholar]
- Natália, F.T.; Milena, G.M.; Caio, R.S.; Susanne, R. On-line solid phase extraction-ultra high performance liquid chromatography-tandem mass spectrometry as a powerful technique for the determination of sulfonamide residues in soils. J. Chromatogr. A 2016, 1452, 89–97. [Google Scholar]
- Worawa, J.; Suwanna, K.B.; Narin, B.; Chongrak, P.; Chau, T.D. Monitoring and determination of sulfonamide antibiotics (sulfamethoxydiazine, sulfamethazine, sulfamethoxazole and sulfadiazine) in imported Pangasius catfish products in Thailand using liquid chromatography coupled with tandem mass spectrometry. Food Chem. 2016, 212, 635–640. [Google Scholar]
- Kim, H.J.; Jeong, M.H.; Park, H.J.; Kim, W.C.; Kim, J.E. Development of an immunoaffinity chromatography and HPLC-UV method for determination of 16 sulfonamides in feed. Food Chem. 2016, 196, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, E.; Manousi, N.; Samanidou, V.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem. 2016, 196, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Natalia, A.M.; Laura, G.G.; Ana, G.C.M. Alternative sample treatments for the determination of sulfonamides in milk by HPLC with fluorescence detection. Food Chem. 2014, 143, 459–464. [Google Scholar]
- Stoev, G.; Michailova, A. Quantitative determination of sulfonamide residues in foods of animal origin by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A 2000, 871, 37–42. [Google Scholar] [CrossRef]
- Summa, S.; Magro, S.L.; Armentano, A.; Muscarella, M. Development and validation of an HPLC/DAD method for the determination of 13 sulphonamides in eggs. Food Chem. 2015, 187, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.R.; Okorodudu, A.O.; Mohammad, A.; Payne, D.A. Capillary electrophoresis and its application in the clinical laboratory. Clin. Chim. Acta 2003, 330, 1–30. [Google Scholar] [CrossRef]
- Ana, G.C.M.; Francisco, J.L.; Laura, G.G.; José, H.P.F. Chemiluminescence detection coupled to capillary electrophoresis. Trends Anal. Chem. 2009, 28, 973–986. [Google Scholar]
- Soto-Chinchilla, J.J.; García-Campaña, A.M.; Gámiz-Gracia, L.; Cruces-Blanco, C. Application of capillary zone electrophoresis with large-volume sample stacking to the sensitive determination of sulfonamides in meat and ground water. Electrophoresis 2006, 27, 4060–4068. [Google Scholar] [CrossRef] [PubMed]
- Hoff, R.B.; Barreto, F.; Ledur-Kist, T.B. Use of capillary electrophoresis with laser-induced fluorescence detection to screen and liquid chromatography-tandem mass spectrometry to confirm sulfonamide residues: Validation according to European Union 2002/657/EC. J. Chromatogr. A 2009, 1216, 8254–8261. [Google Scholar] [CrossRef] [PubMed]
- Font, G.; Juan-García, A.; Picó, Y. Pressurized liquid extraction combined with capillary electrophoresis-mass spectrometry as an improved methodology for the determination of sulfonamide residues in meat. J. Chromatogr. A 2007, 1159, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Campaña, G.; Ibáñez-Velasco, A. Advances and analytical applications in chemiluminescence coupled to capillary electrophoresis. Electrophoresis 2010, 31, 1998–2027. [Google Scholar]
- Lara, F.J.; Rodríguez, D.A.; González, D.M.; Pérez, J.F.H.; Campaña, A.M.G. Applications of capillary electrophoresis with chemiluminescense detection in clinical, environmental and food analysis. A review. Anal. Chim. Acta 2016, 913, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, S.; Huang, J.; Ye, F. Quantum dot-enhanced chemiluminescence detection for simultaneous determination of dopamine and epinephrine by capillary electrophoresis. Talanta 2011, 85, 2650–2654. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Huang, Y.; Shi, M.; Liu, Y.M. Quantification of biogenic amines by microchip electrophoresis with chemiluminescence detection. J. Chromatogr. A 2009, 1216, 5155–5159. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Shu, L.; Wu, M.; Wang, Z.; Wang, Q.; He, P.; Fang, Y. Development of a compact chemiluminescence system coupled with capillary electrophoresis for carbohydrate analysis. Talanta 2012, 93, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, K.; Saito, T.; Nakajima, R. Analysis of antioxidants by microchip capillary electrophoresis with chemiluminescence detection based on luminol reaction. Talanta 2008, 77, 514–517. [Google Scholar] [CrossRef]
- Shi, H.; Shen, S.; Sun, H.; Liu, Z.; Li, L. Oxidation of l-serine and l-threonine by bis(hydrogen periodato)argentate(III) complex anion. J. Inorg. Biochem. 2007, 101, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kang, W.; Shen, S.; Sun, H. Oxidation of 3-(4-methoxyphenoxy)-1,2-propanediol by bis(hydrogen periodato)argentate(III) complex anion: A kinetic study. Transit. Met. Chem. 2009, 34, 91–95. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, J.; Huo, S.; Shen, S.; Kang, W.; Shi, T. Kinetics and mechanism of oxidation of the drug intermediate 1-(2-hydroxyethyl)piperidine by bis(hydrogenperiodato)argentate(III). J. Brazil. Chem. Soc. 2013, 24, 1307–1313. [Google Scholar] [CrossRef]
- Blikungeri, A.; Pelletier, M.; Monnier, D. Contribution to the study of the complexes bis(dihydrogen tellurato)cuprate(III) and argentate(III), bis(hydrogen periodato)cuprate(III) and argentate(III). Inorg. Chim. Acta 1977, 22, 7–14. [Google Scholar] [CrossRef]
- Blikungeri, A.; Pelletier, M. Electronic spectra of bis(dihydorgen tellurato) and bis(hydrogen periodato) complexes of copper(III), silver(III) and gold(III). Inorg. Chim. Acta 1978, 29, 141–148. [Google Scholar] [CrossRef]
- Masse, R.; Simon, A. An inorganic complex of silver(III): K5Ag(IO5OH)2·8H2O. J. Solid State Chem. 1982, 44, 201–207. [Google Scholar] [CrossRef]
- Shi, H.; Xu, X.; Ding, Y.; Liu, S.; Li, L.; Kang, W. Determination of cortisol in human blood sera by a new Ag(III) complex-luminol chemiluminescent system. Anal. Biochem. 2009, 387, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shi, H.; Ma, L.; Kang, W.; Li, S. Determination of trace amounts of dopamine by flow-injection analysis coupled with luminol-Ag(III) complex chemiluminescence detection. Luminescence 2011, 26, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, H.; Shi, H.; Ma, C.; Cong, B.; Kang, W. Determination of three major catecholamines in human urine by capillary zone electrophoresis with chemiluminescence detection. Anal. Biochem. 2012, 427, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Shi, H.; Lian, K.; Diao, Y.; Chen, Y.; Ma, C.; Kang, W. Highly selective and sensitive determination of several antioxidants in human breast milk using high-performance liquid chromatography based on Ag(III) complex chemiluminescence detection. Food Chem. 2017, 218, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Pérez, J.F.; Arroyo-Manzanares, N.; Havlíková, L.; Gámiz-Gracia, L.; Solich, P.; García-Campaña, A.M. Method optimization and validation for the determination of eight sulfonamides in chicken muscle and eggs by modified QuEChERS and liquid chromatography with fluorescence detection. J. Pharm. Biomed. Anal. 2016, 124, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Fuh, M.-R.S.; Chu, S.-Y. Quantitative determination of sulfonamide in meat by solid-phase extraction and capillary electrophoresis. Anal. Chim. Acta 2003, 499, 215–221. [Google Scholar] [CrossRef]
| Sample | Spiked (µg·mL−1) | Found (µg·mL−1) | Recovery (%, n = 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SDD | SDZ | STZ | SDD | SDZ | STZ | SDD | SDZ | STZ | |
| Milk | 40.0 | 40.0 | 10.0 | 37.7 | 40.7 | 10.6 | 94.2 | 101.7 | 106.0 |
| 60.0 | 60.0 | 20.0 | 51.5 | 67.4 | 18.0 | 85.8 | 112.4 | 89.8 | |
| 100.0 | 100.0 | 40.0 | 107.2 | 112.4 | 35.5 | 107.2 | 112.4 | 88.8 | |
| Pork | 40.0 | 40.0 | 10.0 | 35.2 | 38.8 | 8.1 | 88.1 | 96.9 | 80.9 |
| 60.0 | 60.0 | 20.0 | 65.3 | 65.5 | 18.6 | 108.7 | 109.2 | 93.2 | |
| 100.0 | 100.0 | 40.0 | 112.4 | 112.4 | 33.3 | 112.4 | 112.4 | 83.3 | |
| Chicken | 40.0 | 40.0 | 10.0 | 36.8 | 37.2 | 7.9 | 92.3 | 93.1 | 79.5 |
| 60.0 | 60.0 | 20.0 | 62.3 | 64.6 | 18.2 | 103.8 | 107.7 | 91.3 | |
| 100.0 | 100.0 | 40.0 | 108.5 | 110.5 | 32.8 | 108.5 | 110.5 | 82.4 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, T.; Duan, J.; Li, X.; Xu, X.; Shi, H.; Kang, W. Determination of Sulfonamide Residues in Food by Capillary Zone Electrophoresis with On-Line Chemiluminescence Detection Based on an Ag(III) Complex. Int. J. Mol. Sci. 2017, 18, 1286. https://doi.org/10.3390/ijms18061286
Dai T, Duan J, Li X, Xu X, Shi H, Kang W. Determination of Sulfonamide Residues in Food by Capillary Zone Electrophoresis with On-Line Chemiluminescence Detection Based on an Ag(III) Complex. International Journal of Molecular Sciences. 2017; 18(6):1286. https://doi.org/10.3390/ijms18061286
Chicago/Turabian StyleDai, Tingting, Jie Duan, Xinghua Li, Xiangdong Xu, Hongmei Shi, and Weijun Kang. 2017. "Determination of Sulfonamide Residues in Food by Capillary Zone Electrophoresis with On-Line Chemiluminescence Detection Based on an Ag(III) Complex" International Journal of Molecular Sciences 18, no. 6: 1286. https://doi.org/10.3390/ijms18061286