Centrosomal Protein 70 Is a Mediator of Paclitaxel Sensitivity
Abstract
:1. Introduction
2. Results
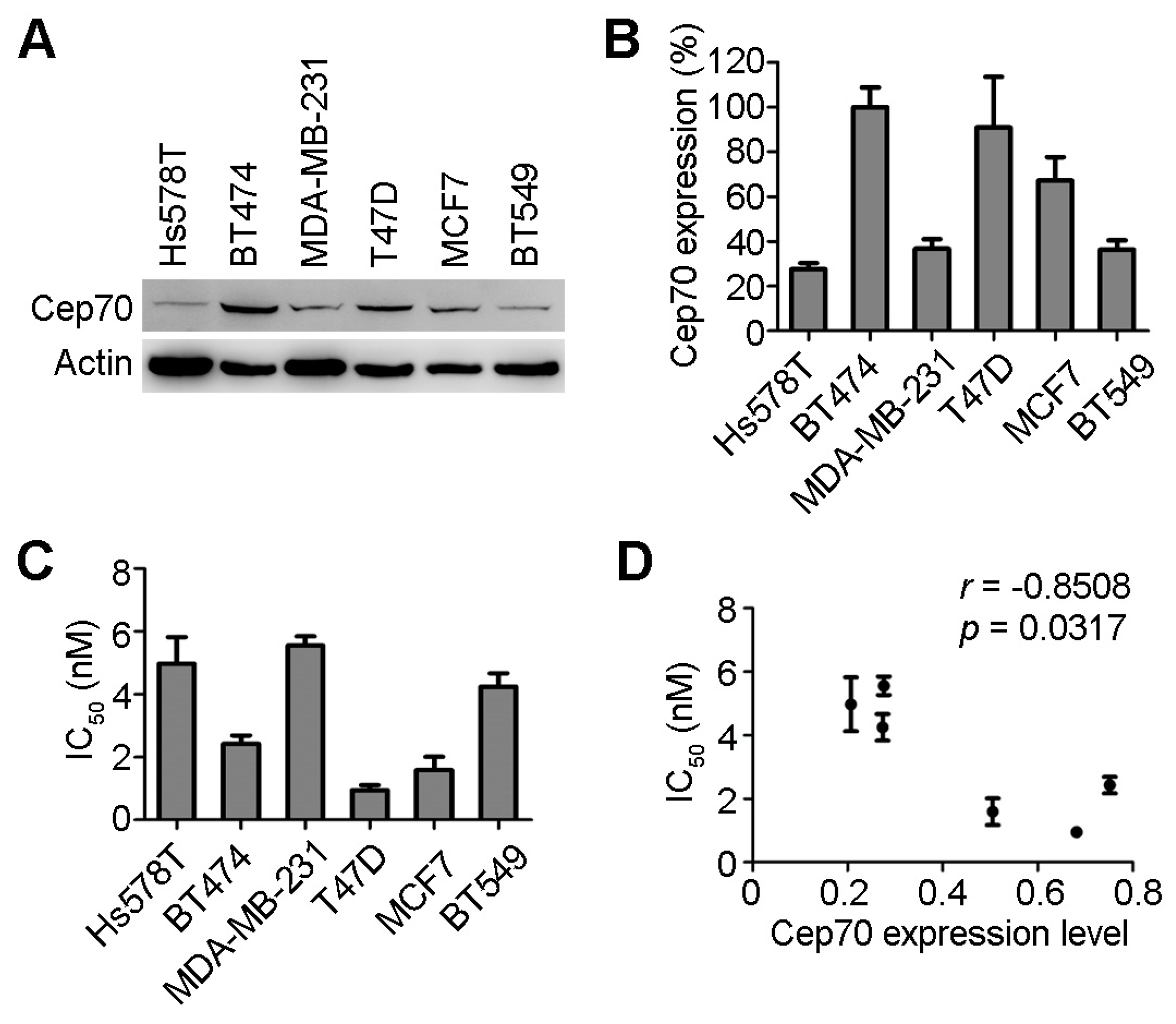
2.1. Centrosomal Protein 70 (Cep70) Expression and Paclitaxel Sensitivity in Breast Cancer Cells
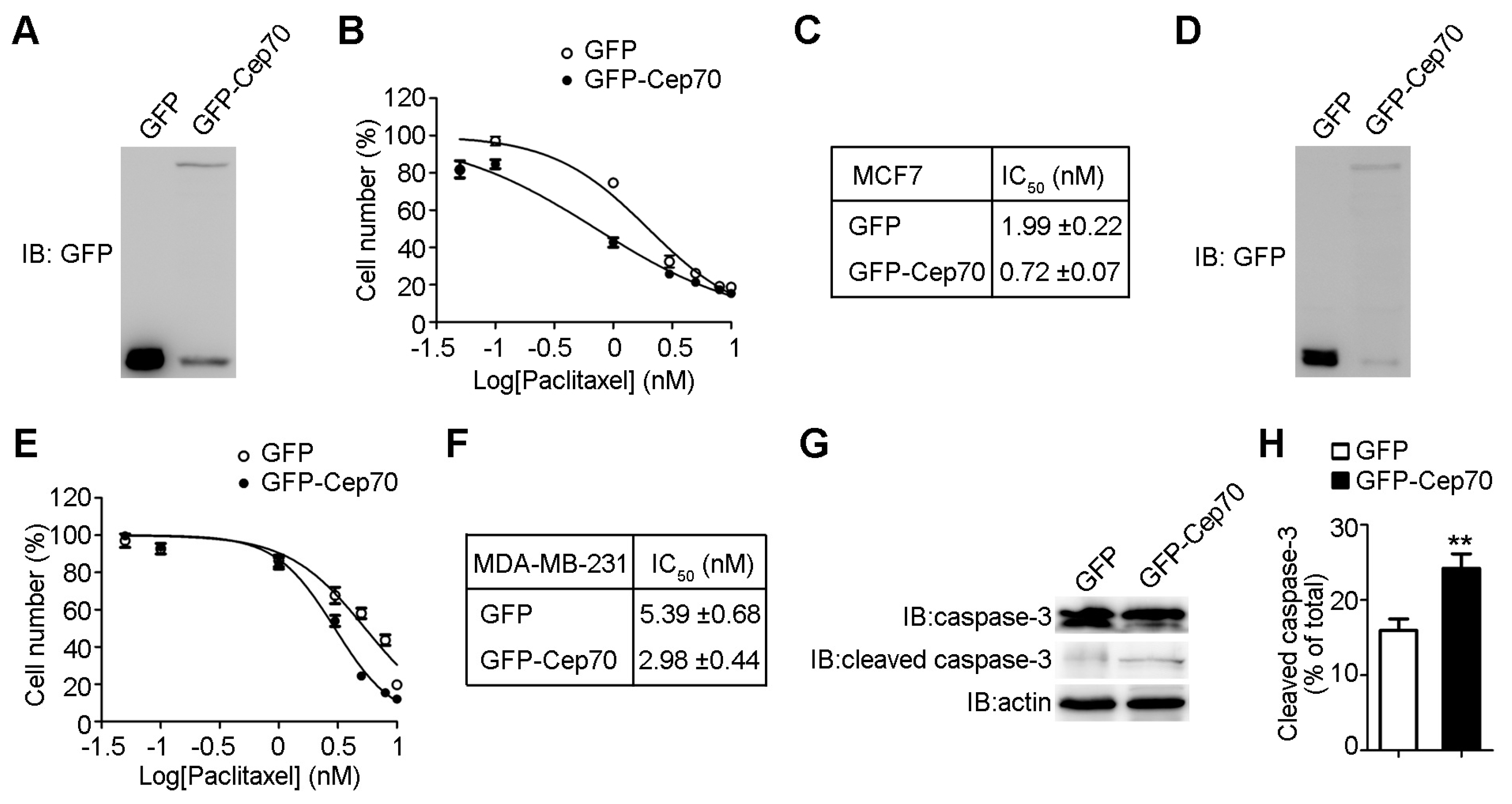
2.2. Cep70 Overexpression Enhances the Sensitivity of Breast Cancer Cells to Paclitaxel
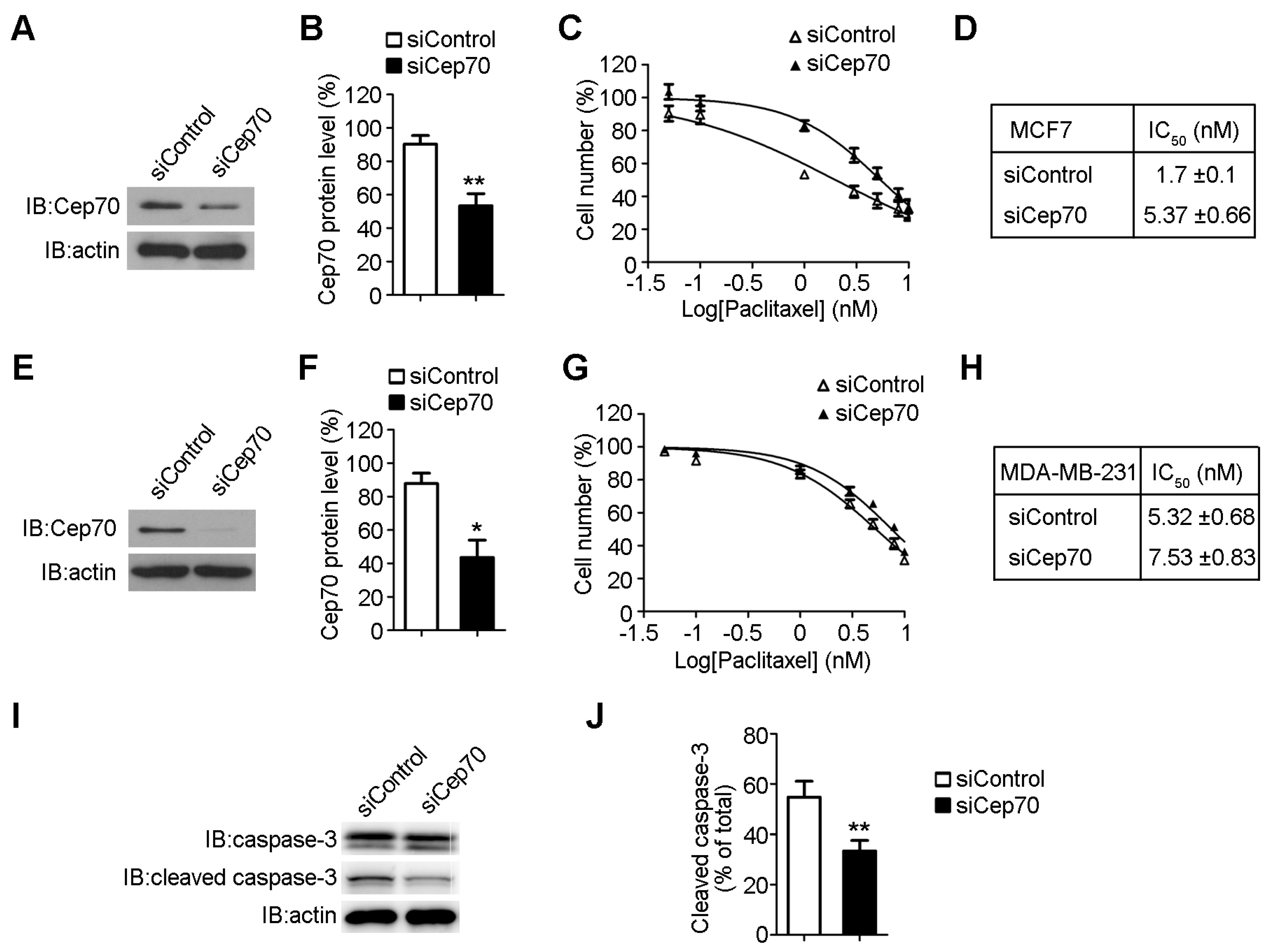
2.3. Knockdown of Cep70 Expression Reduces the Sensitivity to Paclitaxel of Breast Cancer Cells
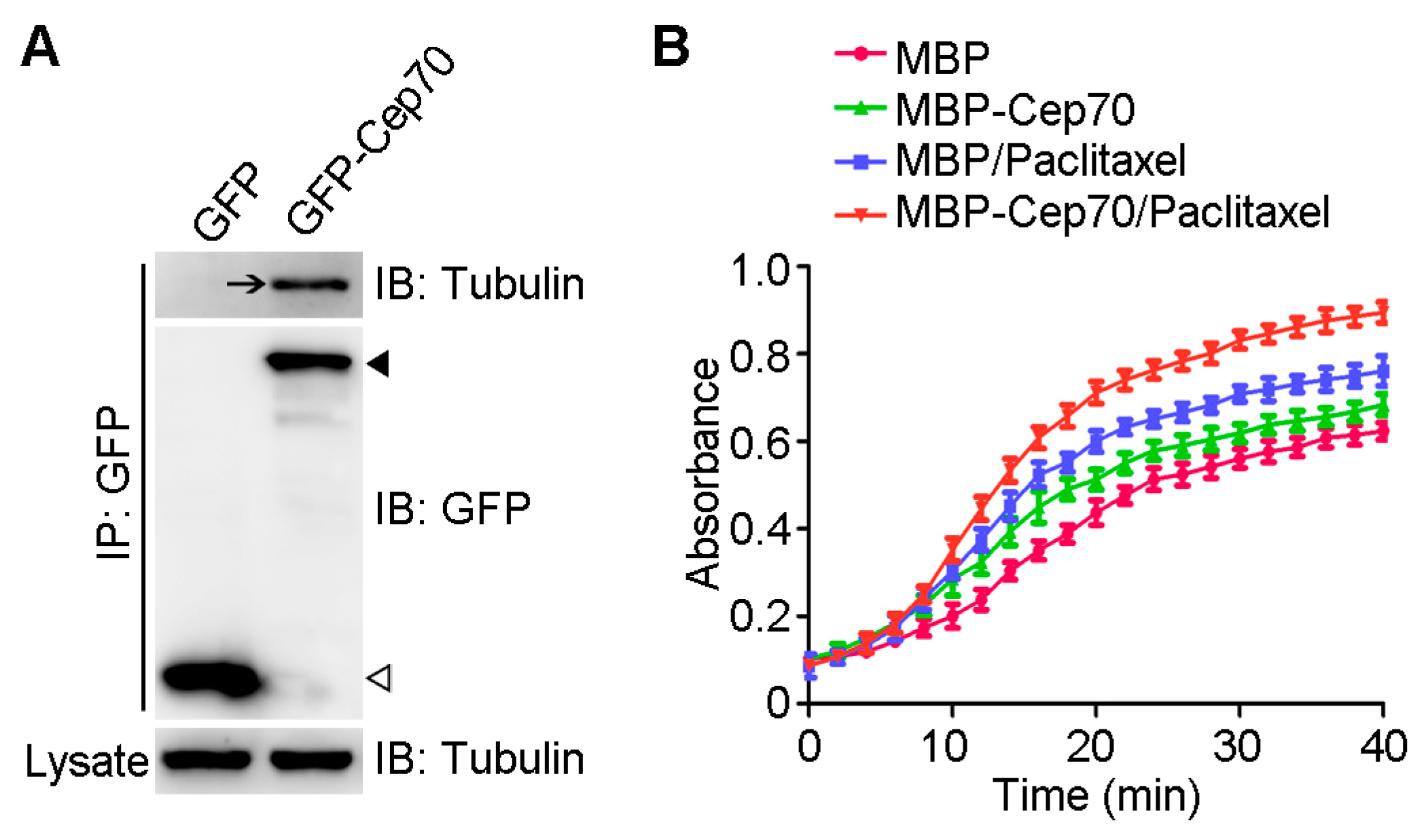
2.4. Cep70 Promotes the Capability of Paclitaxel to Induce Microtubule Assembly
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plasmids and Proteins
4.3. Cell Culture and Transfection
4.4. Immunoblot Analysis
4.5. Cell Proliferation Assay
4.6. Immunoprecipitation
4.7. In Vitro Tubulin Polymerization Assay
4.8. Statistics Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Q.; Ji, H.; Tao, X.; Xu, Y.; Zhang, Q. Quantitative assessment of HER2 amplification in HER2-positive breast cancer: Its association with clinical outcomes. Breast Cancer Res. Treat. 2015, 150, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Pan, C.; Ma, F.; Zhang, S. Prediction of poor prognosis in breast cancer patients based on microRNA-21 expression: A meta-analysis. PLoS ONE 2015, 10, e0118647. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu. Rev. Med. 1997, 48, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Giannakakou, P. Targeting microtubules for cancer chemotherapy. Curr. Med. Chem. Anti-Cancer Agents 2005, 5, 65–71. [Google Scholar] [CrossRef] [PubMed]
- McGrogan, B.T.; Gilmartin, B.; Carney, D.N.; McCann, A. Taxanes, microtubules and chemoresistant breast cancer. Biochim. Biophys. Acta 2008, 1785, 96–132. [Google Scholar] [CrossRef] [PubMed]
- Conduit, P.T.; Wainman, A.; Raff, J.W. Centrosome function and assembly in animal cells. Nat. Rev. Mol. Cell Biol. 2015, 16, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Godinho, S.A.; Pellman, D. Causes and consequences of centrosome abnormalities in cancer. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2014, 369, 20130467. [Google Scholar] [CrossRef] [PubMed]
- Rida, P.C.; Cantuaria, G.; Reid, M.D.; Kucuk, O.; Aneja, R. How to be good at being bad: Centrosome amplification and mitotic propensity drive intratumoral heterogeneity. Cancer Metastasis Rev. 2015, 34, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Song, Y.; Xu, B.; Zhan, Q. Overexpression of centrosomal protein Nlp confers breast carcinoma resistance to paclitaxel. Cancer Biol. Ther. 2012, 13, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zou, P.; Zhang, M.; Tian, L.; Liu, F. Effect of polo-like kinase 1 gene silence on cell cycle and drug resistance in K562/A02 cell. Chin. Med. J. 2006, 119, 605–608. [Google Scholar] [PubMed]
- Ro, S.; Yang, L.X. Centrosomes—Their role in tumors and cancer therapy. Anticancer Res. 2004, 24, 3269–7323. [Google Scholar] [PubMed]
- Anand, S.; Penrhyn-Lowe, S.; Venkitaraman, A.R. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell 2003, 3, 51–62. [Google Scholar] [CrossRef]
- Shi, X.; Li, D.; Wang, Y.; Liu, S.; Qin, J.; Wang, J.; Ran, J.; Zhang, Y.; Huang, Q.; Liu, X.; et al. Discovery of centrosomal protein 70 as an important player in the development and progression of breast cancer. Am. J. Pathol. 2017, 187, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Lazo, P.A. Is centrosomal protein 70, a centrosomal protein with new roles in breast cancer dissemination and metastasis, a facilitator of epithelial-mesenchymal transition? Am. J. Pathol. 2017, 187, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. The use and action of drugs in analyzing mitosis. Methods Cell Biol. 1999, 61, 267–295. [Google Scholar] [PubMed]
- Gascoigne, K.E.; Taylor, S.S. How do anti-mitotic drugs kill cancer cells? J. Cell Sci. 2009, 122, 2579–2585. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sun, X.; Liu, M.; Li, D.; Aneja, R.; Zhou, J. Cep70 protein interacts with γ-tubulin to localize at the centrosome and is critical for mitotic spindle assembly. J. Biol. Chem. 2011, 286, 33401–33408. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yao, Y.; Wang, Y.; Zhang, Y.; Huang, Q.; Zhou, J.; Liu, M.; Li, D. Cep70 regulates microtubule stability by interacting with HDAC6. FEBS Lett. 2015, 589, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Rouzier, R.; Rajan, R.; Wagner, P.; Hess, K.R.; Gold, D.L.; Stec, J.; Ayers, M.; Ross, J.S.; Zhang, P.; Buchholz, T.A.; et al. Microtubule-associated protein tau: A marker of paclitaxel sensitivity in breast cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 8315–8320. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, D.; Yang, Y.; Ren, Y.; Li, J.; Wang, Z.; Dong, B.; Liu, M.; Zhou, J. Microtubule-binding protein CLIP-170 is a mediator of paclitaxel sensitivity. J. Pathol. 2012, 226, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, M.; Li, D.; Wang, J.; Aneja, R.; Zhou, J. Cep70 contributes to angiogenesis by modulating microtubule rearrangement and stimulating cell polarization and migration. Cell Cycle 2012, 11, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, J.; Yang, Y.; Ren, Y.; Zhou, J.; Li, D. Cep70 promotes microtubule assembly in vitro by increasing microtubule elongation. Acta Biochim. Biophys. Sin. 2012, 44, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Qin, J.; Liu, S.; Zhang, Y.; Wang, J.; Shi, X.; Li, D.; Zhou, J.; Liu, M. Cep70 overexpression stimulates pancreatic cancer by inducing centrosome abnormality and microtubule disorganization. Sci. Rep. 2016, 6, 21263. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protocols 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Huo, L.; Sun, X.; Liu, M.; Li, D.; Dong, J.T.; Zhou, J. The tumor suppressor CYLD regulates microtubule dynamics and plays a role in cell migration. J. Biol. Chem. 2008, 283, 8802–8809. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Wang, Y.; Sun, X.; Wang, C.; Jiang, P.; Zhang, Y.; Huang, Q.; Liu, X.; Li, D.; Zhou, J.; et al. Centrosomal Protein 70 Is a Mediator of Paclitaxel Sensitivity. Int. J. Mol. Sci. 2017, 18, 1267. https://doi.org/10.3390/ijms18061267
Shi X, Wang Y, Sun X, Wang C, Jiang P, Zhang Y, Huang Q, Liu X, Li D, Zhou J, et al. Centrosomal Protein 70 Is a Mediator of Paclitaxel Sensitivity. International Journal of Molecular Sciences. 2017; 18(6):1267. https://doi.org/10.3390/ijms18061267
Chicago/Turabian StyleShi, Xingjuan, Yujue Wang, Xiaoou Sun, Chan Wang, Peng Jiang, Yu Zhang, Qinghai Huang, Xiangdong Liu, Dengwen Li, Jun Zhou, and et al. 2017. "Centrosomal Protein 70 Is a Mediator of Paclitaxel Sensitivity" International Journal of Molecular Sciences 18, no. 6: 1267. https://doi.org/10.3390/ijms18061267




