Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): An Interplay among Drugs, Viruses, and Immune System
Abstract
:1. Introduction
2. Clinical Features
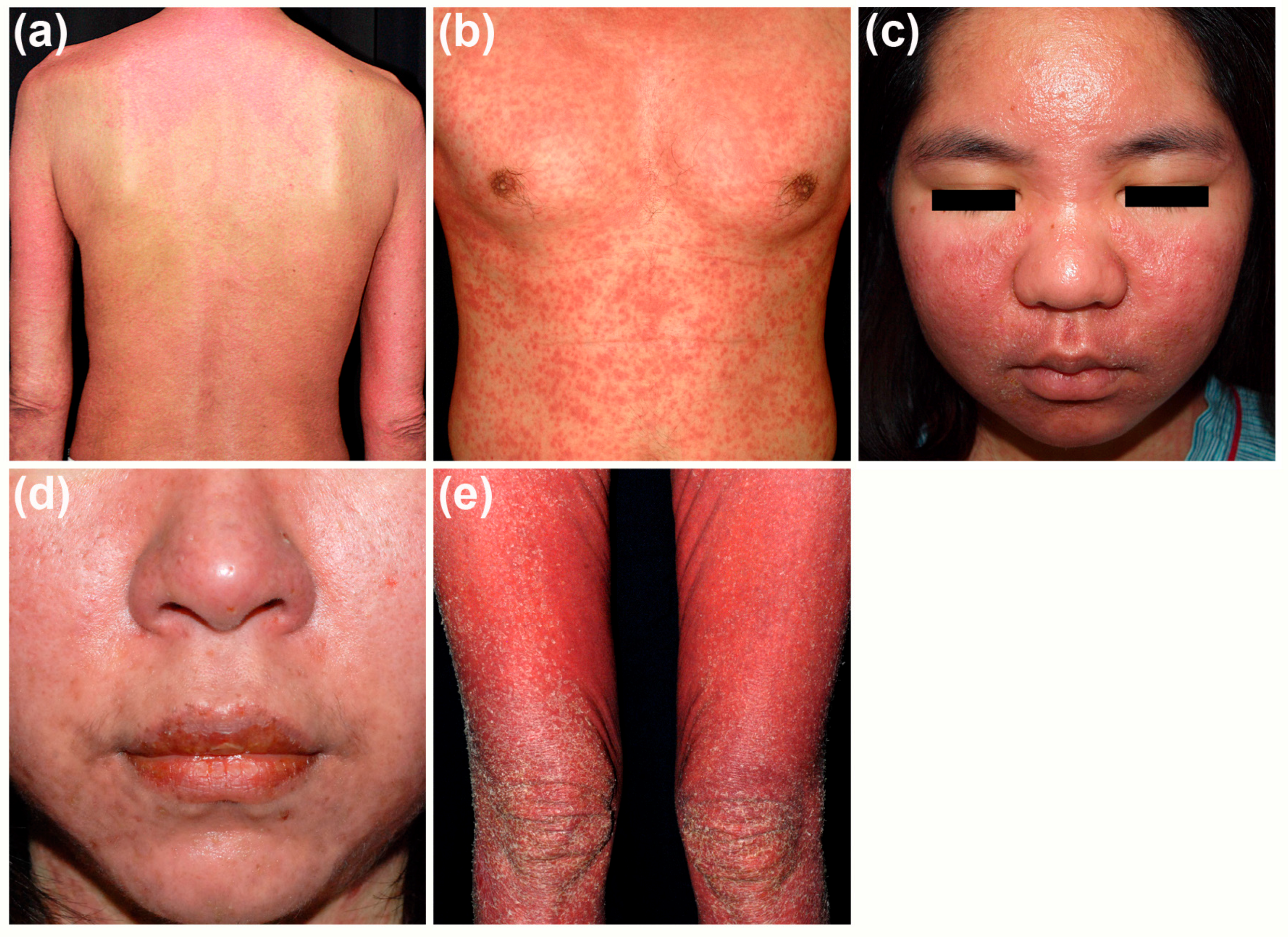
2.1. Cutaneous Manifestations
2.2. Internal Organ Involvement
2.3. Culprit Drugs
2.4. Clinical Courses
2.5. Diagnostic Criteria
2.6. Prognosis and Long-Term Sequelae
3. Histopathology
4. Pathomechanisms
4.1. Genetic Factors
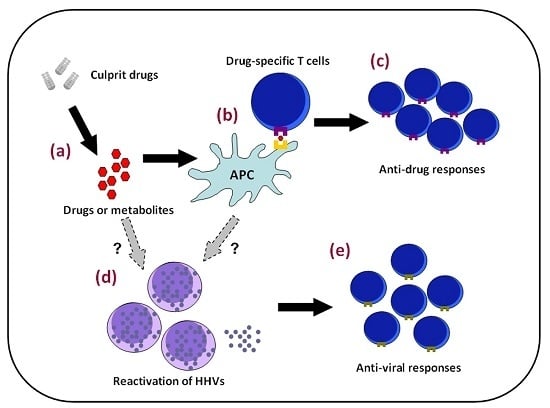
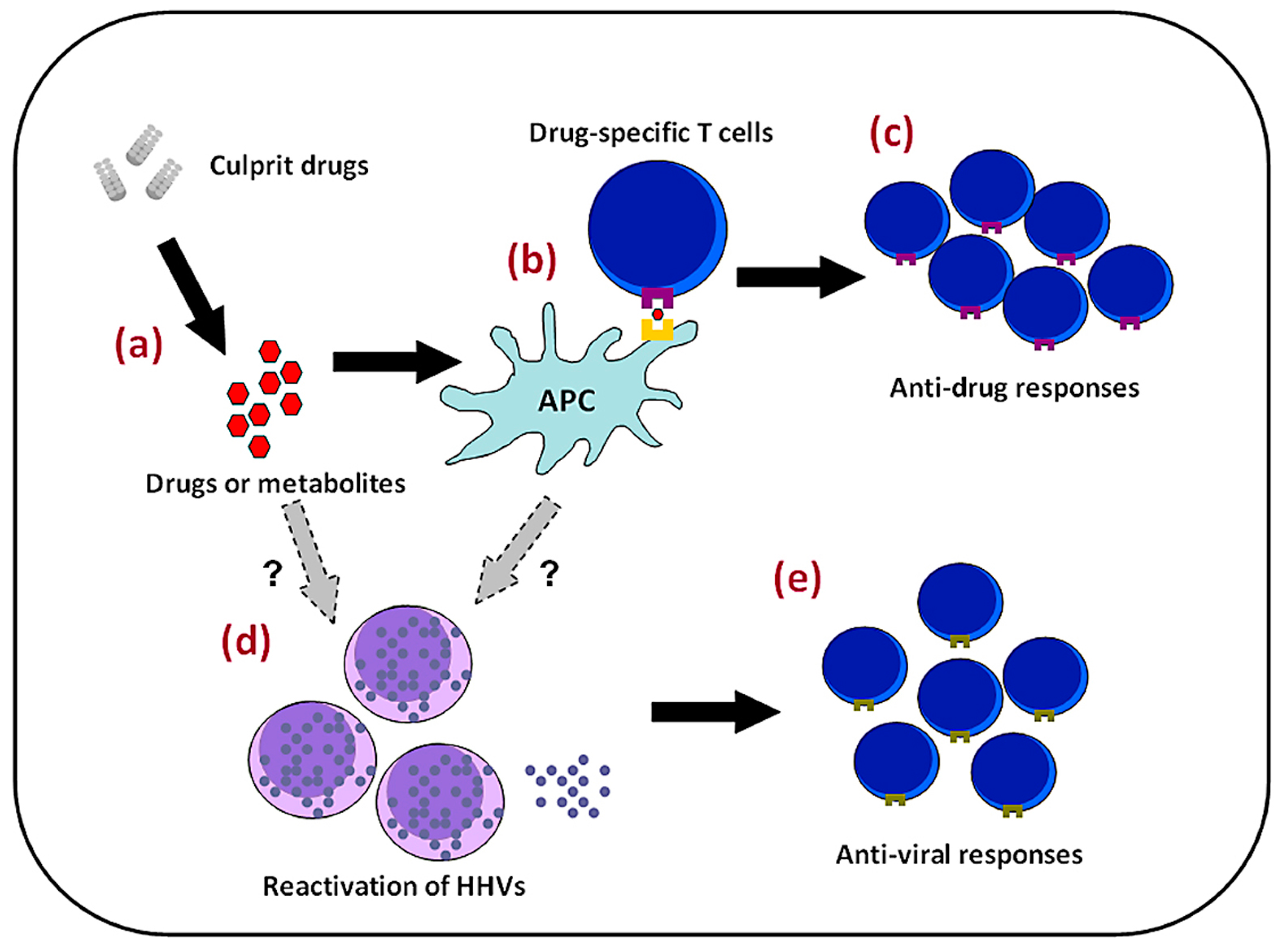
4.2. Viral Reactivation
5. Treatments
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| ALP | Alkaline phosphatase |
| CCR | C-C motif chemokine receptor |
| CMV | Cytomegalovirus |
| CXCL | C-X-C motif chemokine |
| CXCR | C-X-C motif chemokine receptor |
| CYP | Cytochrome P |
| DiHS | Drug-induced hypersensitivity syndrome |
| DM | Diabetes mellitus |
| DRESS | Drug reaction with eosinophilia and systemic symptoms |
| EBV | Epstein-Barr Virus |
| FasL | Fatty acid synthase ligand |
| HHV | Human herpes virus |
| HLA | Human leukocyte antigen |
| HMGB-1 | High mobility group box protein 1 |
| IFN | Interferon |
| IL | Interleukin |
| IP-10 | Interferon γ-induced protein-10 |
| IVIG | Intravenous immunoglobulin |
| MHC | Major histocompatibility complex |
| MPE | Maculopapular exanthema |
| NSAID | Non-steroid anti-inflammatory drug |
| pDC | Plasmacytoid dendritic cell |
| SCAR | Severe cutaneous adverse reaction |
| SJS | Stevens-Johnson syndrome |
| TARC | Thymus activation-related chemokine |
| TCR | T cell receptor |
| TEN | Toxic epidermal necrolysis |
| TEM | Effector memory T cell |
| Th | Helper T cell |
| TNF | Tumor necrosis factor |
| Treg | Regulatory T cell |
| ULN | Upper limit of normal range |
References
- Phillips, E.J.; Chung, W.H.; Mockenhaupt, M.; Roujeau, J.C.; Mallal, S.A. Drug hypersensitivity: Pharmacogenetics and clinical syndromes. J. Allergy Clin. Immunol. 2011, 127, S60–S66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chiu, H.C.; Chu, C.Y. Drug reaction with eosinophilia and systemic symptoms. A retrospective study of 60 cases. Arch. Dermatol. 2010, 146, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.C.; Yang, L.C.; Hung, S.I.; Chang, Y.C.; Kuo, T.T.; Ho, H.C.; Su, S.; Hong, H.S.; Chung, W.H. Clinicopathological features and prognosis of drug rash with eosinophilia and systemic symptoms: A study of 30 cases in Taiwan. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, T.; Kano, Y. Review of drug-induced hypersensitivity: Special emphasis on drug-induced hypersensitivity syndrome. Expert Dermatol. 2012, 7, 539–547. [Google Scholar] [CrossRef]
- Meriritt, H.H.; Putnam, T.J. Sodium diphenyl hydantoinate in the treatment of convulsive disorders. JAMA 1938, 111, 1068–1073. [Google Scholar] [CrossRef]
- Chaiken, B.H.; Goldberg, B.I.; Segal, J.P. Dilantin hypersensitivity: Report of a case of hepatitis with jaundice, pyrexia, exfoliative dermatitis. N. Engl. J. Med. 1950, 242, 897–898. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, H.; Bagot, M.; Roujeau, J.C. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (drug rash with eosinophilia and systemic symptoms: DRESS). Semin. Cutan. Med. Surg. 1996, 15, 250–257. [Google Scholar] [CrossRef]
- Descamps, V.; Bouscarat, F.; Laglenne, S.; Aslangul, E.; Veber, B.; Descamps, D.; Saraux, J.L.; Grange, M.J.; Grossin, M.; Navratil, E.; et al. Human herpesvirus 6 infection associated with anticonvulsant hypersensitivity syndrome and reactive haemophagocytic syndrome. Br. J. Dermatol. 1997, 137, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, M.; Yahata, Y.; Yasukawa, M.; Inagi, R.; Urano, Y.; Yamanishi, K.; Hashimoto, K. Severe hypersensitivity syndrome due to sulfasalazine associated with reactivation of human herpesvirus 6. Arch. Dermatol. 1998, 134, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Inagi, R.; Aono, T.; Yamanishi, K.; Shiohara, T. Human herpesvirus 6 infection as a risk factor for the development of severe drug-induced hypersensitivity syndrome. Arch. Dermatol. 1998, 134, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Musette, P.; Descamps, V.; Meyer, O.; Speirs, C.; Finzi, L.; Roujeau, J.C. The DRESS syndrome: A literature review. Am. J. Med. 2011, 124, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Peyrière, H.; Dereure, O.; Breton, H.; Demoly, P.; Cociglio, M.; Blayac, J.P.; Hillaire-Buys, D.; The Network of Pharmacovigilance Centers. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist? Br. J. Dermatol. 2006, 155, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Kardaun, S.H.; Sidoroff, A.; Valeyrie-Allanore, L.; Halevy, S.; Davidovici, B.B.; Mockenhaupt, M.; Roujeau, J.C. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: Does a DRESS syndrome really exist? Br. J. Dermatol. 2007, 156, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Kardaun, S.H.; Sekula, P.; Valeyrie-Allanore, L.; Liss, Y.; Chu, C.Y.; Creamer, D.; Sidoroff, A.; Naldi, L.; Mockenhaupt, M.; Roujeau, J.C. Drug reaction with eosinophilia and systemic symptoms (DRESS): An original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br. J. Dermatol. 2013, 169, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, M.P.; Gameiro, A.; Coutinho, I.; Pereira, N.; Cardoso, J.C.; Goncalo, M. Overlap between maculopapular exanthema and drug reaction with eosinophilia and systemic symptoms among cutaneous adverse drug reactions in a dermatology ward. Br. J. Dermatol. 2016, 175, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, S.; Okazaki, H.; Sayama, K.; Tohyama, M.; Hashimoto, K. Possible association of vascular endothelial growth factor with the development of edema in drug-induced hypersensitivity syndrome. J. Dermatol. 2011, 38, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Cho, Y.T.; Chang, C.Y.; Chu, C.Y. Drug reaction with eosinophilia and systemic symptoms: A drug-induced hypersensitivity syndrome with variable clinical features. Dermatol. Sin. 2013, 31, 196–204. [Google Scholar] [CrossRef]
- Husain, Z.; Reddy, B.Y.; Schwartz, R.A. DRESS syndrome part 1. Clinical perspectives. J. Am. Acad. Dermatol. 2013, 68, 693.e1–639.e14. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Inaoaka, M.; Shiohara, T. Association between anticonvulsant hypersensitivity syndrome and human herpesvirus 6 and hypogammaglobulinemia. Arch. Dermatol. 2004, 140, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Seibima, M.; Shiohara, T. Hypogammaglobulinemia as an early sign of drug-induced hypersensitivity syndrome. J. Am. Acad. Dermatol. 2006, 55, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Yazicioglu, M.; Elmas, R.; Turgut, B.; Genchallac, T. The association between DRESS and the diminished numbers of peripheral B lymphocytes and natural killer cells. Pediatr. Allergy. Immunol. 2012, 23, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.C.; Yang, H.C.; Strong, C.; Yang, C.W.; Cho, Y.T.; Chen, K.L.; Chu, C.Y. Liver injury in patients with DRESS: A clinical study of 72 cases. J. Am. Acad. Dermatol. 2015, 72, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Lee, Y.S.; Yoon, S.Y.; Kim, S.; Bae, Y.J.; Kwon, H.S.; Cho, Y.S.; Moon, H.B.; Kim, T.B. Characteristics of liver injury in drug-induced systemic hypersensitivity reactions. J. Am. Acad. Dermatol. 2013, 69, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Danan, G.; Benichou, C. Causality assessment of adverse reactions to drugs-1. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J. Clin. Epidemiol. 1993, 46, 1323–1330. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chen, C.H.; Deng, S.T.; Huang, C.S.; Lin, Y.J.; Chen, Y.J.; Wu, C.Y.; Hung, S.I.; Chung, W.H. Allopurinol use and risk of fatal hypersensitivity reactions. A nationwide population-based study in Taiwan. JAMA Int. Med. 2015, 175, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Shiohara, T. The variable clinical picture of drug-induced hypersensitivity syndrome/drug rash with eosinophilia and systemic symptoms in relation to the eliciting drug. Immunol. Allergy Clin. N. Am. 2009, 29, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.I.; Chung, W.H.; Liou, L.B.; Chu, C.C.; Lin, M.; Huang, H.P.; Lin, Y.L.; Lan, J.L.; Yang, L.C.; Hong, H.S.; et al. HLA-B*58:01 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc. Natl. Acad. Sci. USA 2005, 102, 4134–4139. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Yeh, Y.T.; Wang, C.W.; Hung, S.I.; Yang, C.H.; Chang, Y.C.; Chang, W.C.; Lin, Y.J.; Chang, C.J.; Su, S.C.; et al. Impact of HLA-B*58:01 allele and renal impairment on allopurinol-induced cutaneous adverse reaction. J. Investig. Dermatol. 2016, 136, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Chang, W.H.; Stocker, S.L.; Juo, C.G.; Graham, G.G.; Lee, M.H.H.; Williams, K.M.; Tian, Y.C.; Juan, K.C.; Wu, Y.J.J.; et al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: The impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann. Rheum. Dis. 2015, 74, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Mattsson, J.; Schnyder, K.; Fontana, S.; Largiader, C.R.; Pichler, W.J.; Yearly, D. Allopurinol hypersensitivity is primarily mediated by dose-dependent oxypurinol-specific T cell response. Clin. Exp. Allergy 2013, 43, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Ishida, T.; Hirahara, K.; Shiohara, T. Visceral involvements and long-term sequelae in drug-induced hypersensitivity syndrome. Med. Clin. N. Am. 2010, 94, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.C.; Wang, Y.S.; Yoosuff, E.L.; Tay, Y.K. Retrospective analysis of drug-induced hypersensitivity syndrome: A study of 27 patients. J. Am. Acad. Dermatol. 2010, 63, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Thongsri, T.; Chularojanamontri, L.; Pichler, W.J. Cardiac involvement in DRESS syndrome. Asian Pac. J. Allergy Immunol. 2017, 35, 3–10. [Google Scholar] [PubMed]
- Bourgeois, G.P.; Cafardi, J.A.; Groysman, V.; Pamboukian, S.V.; Kirklin, J.K.; Andea, A.A. Fulminant myocarditis as a late sequela of DRESS: Two cases. J. Am. Acad. Dermatol. 2011, 65, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, G.P.; Cafardi, J.A.; Groysman, V.; Hughey, L.C. A review of DRESS-associated myocarditis. J. Am. Acad. Dermatol. 2012, 66, e229–e236. [Google Scholar] [CrossRef] [PubMed]
- Ozisik, L.; Tanriover, M.D.; Saka, E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern. Med. 2016, 55, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.; Duval-Modeste, A.B.; Musette, P.; Joly, P.; Massy, N.; Courville, C.; Rabehenoina, A. Drug-induced hypersensitivity syndrome due to gabapentin. Ann. Dermatol. Venereol. 2008, 135, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Sriratanaviriyakul, N.; Nquyen, L.P.; Henderson, M.C.; Albertson, T.E. Drug reaction with eosinophilia and systemic symptoms syndrome (DRESS) syndrome associated with azithromycin presenting like septic shock: A case report. J. Med. Case Rep. 2014, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Charfi, O.; Lakhoua, G.; Sahnoun, R.; Badri, T.; Daqhfous, R.; El Aidli, S.; Kastalli, S.; Zaiem, A. DRESS syndrome following levofloxacin exposure with positive patch-test. Therapie 2015, 70, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, R.; Calderon, O.; Ramirez, E.; Fiandor, A.; Prior, N.; Caballero, T.; Herranz, P.; Bobolea, I.; Lopez-Serrano, M.C.; Quirce, S.; et al. Piperacillin-induced DRESS: Distinguishing features observed in a clinical and allergy study of 8 patients. J. Investig. Allergol. Clin. Immunol. 2014, 24, 425–430. [Google Scholar] [PubMed]
- Palmero, D.; Castagnino, J.; Musella, R.M.; Mosca, C.; Gonzalez Montaner, P.; de Casado, G.C. Difficult clinical management of anti-tuberculosis DRESS syndrome. Int. J. Tuberc. Lung Dis. 2013, 17, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Biesbroeck, L.K.; Scott, J.D.; Taraska, C.; Moore, E.; Falsey, R.R.; Shinohara, M.M. Direct-acting antiviral-associated dermatitis during chronic hepatitis C virus treatment. Am. J. Clin. Dermatol. 2013, 14, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Samain, A.; Duval-Modeste, A.B.; Joly, P.; Leblanc, C.; Massy, N.; Courville, P.; Goria, O.; Riachi, G. First case of drug rash eosinophilia and systemic symptoms due to boceprevir. J. Hepatol. 2014, 60, 891–893. [Google Scholar] [CrossRef] [PubMed]
- Broccolo, F.; Ciccarese, G.; Picciotto, A.; Drago, F. A case of drug rash with eosinophilia and systemic symptoms (DRESS) induced by telaprevir associated with HHV-6 active infection. J. Hepatol. 2015, 62, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Qu, T.; Jia, L.; Shu, D.; Chen, D.; Yan, C.; Zhao, W.; Ren, R.; Sun, Q. Clinical characteristics of drug hypersensitivity syndrome in 10 patients. Zhonghua Yi Xue Za Zhi 2014, 94, 2366–2368. [Google Scholar] [PubMed]
- Kim, D.K.; Lee, S.W.; Nam, H.S.; Jeon, D.S.; Park, N.R.; Nam, Y.H.; Lee, S.K.; Baek, Y.H.; Han, S.Y.; Lee, S.W. A case of sorafenib-induced DRESS syndrome in hepatocellular carcinoma. Korean J. Gastroenterol. 2016, 67, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.L.; Arasaratnam, M.; Carlos, G.; Parasyn, A.; Baumgart, K.W.; Fernandez-Penas, P.; Marx, G. Drug reaction with eosinophilia and systemic symptoms in metastatic basal cell carcinoma treated with vismodegib. Australas. J. Dermatol. 2017, 58, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Peuvrel, L.; Quereux, G.; Saint-Jean, M.; Brocard, A.; Nguyen, J.M.; Khammari, A.; Knol, A.C.; Varey, E.; Dreno, B. Profile of vemurafenib-induced severe skin toxicities. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Radu, C.; Barnig, C.; de Blay, F. Rivaroxaban-induced drug reaction with eosinophilia and systemic symptoms. J. Investig. Allergol. Clin. Immunol. 2016, 26, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.Y.; Chen, C.B.; Cheng, C.Y.; Chen, Y.A.; Ng, C.Y.; Kuo, K.L.; Chen, W.L.; Chen, C.H. Febuxostat-associated drug reaction with eosinophilia and systemic symptoms (DRESS). J. Clin. Pharm. Ther. 2015, 40, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Descamps, V. Dysphagia, a major early manifestation in DRESS syndrome. J. Am. Acad. Dermatol. 2013, 69, 1057–1058. [Google Scholar] [CrossRef] [PubMed]
- Tetart, F.; Picard, D.; Janela, B.; Joly, P.; Musette, P. Prolonged evolution of drug reaction with eosinophilia and systemic symptoms: Clinical, virologic and biological features. JAMA Dermatol. 2014, 150, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, T.; Inaoka, M.; Kano, Y. Drug-induced hypersensitivity syndrome (DIHS): A reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol. Int. 2006, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Seishima, M.; Yamanaka, S.; Fujisawa, T.; Tohyama, M.; Hashimoto, K. Reactivation of human herpes virus (HHV) family members other than HHV-6 in drug-induced hypersensitivity syndrome. Br. J. Dermatol. 2006, 155, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, M.; Hashimoto, K.; Yasukawa, M.; Kimura, H.; Horikawa, T.; Nakajima, K.; Urano, Y.; Matsumoto, K.; Lijima, M.; Shear, N.H. Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br. J. Dermatol. 2007, 157, 934–960. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Hirahara, K.; Sakuma, K.; Shiohara, T. Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as a graft-versus-host-disease. Br. J. Dermatol. 2006, 155, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kano, Y.; Mizukawa, Y.; Shiohara, T. The dynamics of herpesvirus reactivations during and after severe drug eruptions: Their relation to the clinical phenotype and therapeutic outcome. Allergy 2014, 69, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chiang, H.H.; Cho, Y.T.; Chang, C.Y.; Chen, K.L.; Yang, C.W.; Lee, Y.H.; Chu, C.Y. Human herpes virus reactivations and dynamic cytokine profiles in patients with cutaneous adverse drug reactions—A prospective comparative study. Allergy 2015, 70, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Picard, D.; Vellar, M.; Janela, B.; Roussel, A.; Joly, P.; Musette, P. Recurrence of drug-induced reactions in DRESS patients. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Gaig, P.; Garcia-Ortega, P.; Baltasar, M.; Bartra, J. Drug neosensitization during anticonvulsant hypersensitivity syndrome. J. Investig. Allergol. Clin. Immunol. 2006, 16, 321–326. [Google Scholar] [PubMed]
- Mardivirin, L.; Valeyrie-Allanore, L.; Branlant-Redon, E.; Beneton, N.; Jidar, K.; Barbaud, A.; Crickx, B.; Ranger-Rogez, S.; Descamps, V. Amoxicillin-induced flare in patients with DRESS (drug reaction with eosinophilia and systemic symptoms): Report of seven cases and demonstration of a direct effect of amoxicillin on human herpesvirus 6 replication in vitro. Eur. J. Dermatol. 2010, 20, 68–73. [Google Scholar] [PubMed]
- Shiohara, T.; Kano, Y. Drug reaction with eosinophilia and systemic symptoms (DRESS): Incidence, pathogenesis, and management. Expert Opin. Drug Saf. 2017, 16, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, T.; Iijima, M.; Ikezawa, Z.; Hashimoto, K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br. J. Dermatol. 2007, 156, 1083–1084. [Google Scholar] [CrossRef] [PubMed]
- Ushigome, Y.; Kano, Y.; Hirahara, K.; Shiohara, T. Human herpesvirus 6 reactivation in drug-induced hypersensitivity syndrome and DRESS validation score. Am. J. Med. 2012, 125, e9–e10. [Google Scholar] [CrossRef] [PubMed]
- Ushigome, Y.; Kano, Y.; Ishida, T.; Hirahara, K.; Shiohara, T. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J. Am. Acad. Dermatol. 2013, 68, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Tohyama, M.; Aihara, M.; Matsukura, S.; Watanabe, H.; Sueki, H.; Iijima, M.; Morita, E.; Nohara, H.; Asada, H.; et al. Sequelae of 145 patients with drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: Survey conducted by the Asian Research Committee on severe cutaneous adverse reactions (ASCAR). J. Dermatol. 2015, 42, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Onuma, H.; Tohyama, M.; Imagawa, A.; Hanafusa, T.; Kobayashi, T.; Kano, Y.; Ohashi, J.; Hashimoto, K.; Osawa, H.; Makino, H. High frequency of HLA B62 in fulminant type 1 diabetes with the drug-induced hypersensitivity syndrome. J. Clin. Endocrinol. Metab. 2012, 97, e2277–e2281. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chang, C.Y.; Cho, Y.T.; Chiu, C.H.; Chu, C.Y. Long-term sequelae of drug reaction with eosinophilia and systemic symptoms: A retrospective cohort study from Taiwan. J. Am. Acad. Dermatol. 2013, 68, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Descamps, V. Drug reaction with eosinophilia and systemic symptoms and thyroiditis: Human herpesvirus-6, a possible common link. Br. J. Dermatol. 2013, 169, 952. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Zatelli, M.C.; Rizzo, R.; Benedetti, S.; Martorelli, D.; Trasforini, G.; Cassai, E.; Uberti, E.C.D.; Luca, D.D.; Dolcetti, R. Virologic and immunologic evidence supporting an association between HHV-6 and Hashimoto’s thyroiditis. PLoS Pathog. 2012, 8, e1002951. [Google Scholar] [CrossRef] [PubMed]
- Aota, N.; Hirahara, K.; Kano, Y.; Fukuoka, T.; Yamada, A.; Shiohara, T. Systemic lupus erythematosus presenting with Kikuchi-Fujimoto’s disease as a long-term sequela of drug-induced hypersensitivity syndrome: A possible role of Epstein-Barr virus reactivation. Dermatology 2009, 218, 275–577. [Google Scholar] [CrossRef] [PubMed]
- Morito, H.; Ogawa, K.; Kobayashi, N.; Fukumoto, T.; Asada, H. Drug-induced hypersensitivity syndrome followed by persistent arthritis. J. Dermatol. 2012, 39, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Takehara, A.; Aoyama, Y.; Kurosawa, M.; Shirafuji, Y.; Umemura, H.; Kamiya, K.; Ushigome, Y.; Kano, Y.; Shiohara, T.; Iwatsuki, K. Longitudinal analysis of antibody profiles against plakins in severe drug eruptions: Emphasis on correlation with tissue damage in drug-induced hypersensitivity syndrome and drug reaction with eosinophilia and systemic symptoms. Br. J. Dermatol. 2016, 175, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.T.; Liau, J.Y.; Chang, C.Y.; Yang, C.W.; Chen, K.L.; Chen, Y.C.; Song, H.L.; Chu, C.Y. Co-existence of histopathological features is characteristic in drug reaction with eosinophilia and systemic symptoms and correlates with high grades of cutaneous abnormalities. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Ortonne, N.; Valeyrie-Allanore, L.; Bastuji-Garin, S.; Wechsler, J.; de Feraudy, S.; Duong, T.A.; Delfau-Larue, M.H.; Chosidow, O.; Wolkenstein, P.; Roujeua, J.C. Histopathology of drug rash with eosinophilia and systemic symptoms: A morphological and phenotypical study. Br. J. Dermatol. 2015, 173, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.H.; Hui, R.C.Y.; Yang, C.H.; Lin, J.Y.; Lin, Y.T.; Ho, H.C.; Chung, W.H.; Kuo, T.T. Histopathologic analysis and clinical correlation of drug reaction with eosinophilia and systemic symptoms (DRESS). Br. J. Dermatol. 2014, 170, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Diaz-Cano, S.; Higgins, E.; Morris-Jones, R.; Bashir, S.; Bernal, W.; Creamer, D. Drug reaction with eosinophilia and systemic symptoms: Is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of 27 cases. Br. J. Dermatol. 2013, 168, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Skowron, F.; Bensaid, B.; Balme, B.; Depaepe, L.; Kanitakis, J.; Nosbaum, A.; Maucort-Boulch, D.; Berard, F.; D’Incan, M.; Kardaun, S.H.; et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): Clinicopathological study of 45 cases. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Goncalo, M.M.; Cardoso, J.C.; Gouveia, M.P.; Coutinho, I.; Gameiro, A.R.; Brites, M.M.; Tellechea, O.E. Histopathology of exanthema in DRESS is not specific but may indicate severity of systemic involvement. Am. J. Dermatopathol. 2016, 38, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Skowron, F.; Bensaid, B.; Balme, B.; Depaepe, L.; Kanitakis, J.; Nosbaum, A.; Maucort-Boulch, D.; Berard, F.; D’Incan, M.; Kardaun, S.H.; et al. Comparative histological analysis of drug-induced maculopapular exanthema and DRESS. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Camous, X.; Calbo, S.; Picard, D.; Musette, P. Drug reaction with eosinophilia and systemic symptoms: An update on pathogenesis. Curr. Opin. Immunol. 2012, 24, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Chang, W.C.; Lee, Y.S.; Wu, Y.Y.; Yang, C.H.; Ho, H.C.; Chen, M.J.; Lin, J.Y.; Hui, R.C.Y.; Ho, J.C.; et al. Genetic variants associated with pheytoin-related severe cutaneous adverse reactions. JAMA 2014, 312, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Shear, N.H.; Spielberg, S.P. Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk. J. Clin. Investig. 1988, 82, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Wolkenstein, P.; Charue, D.; Laurent, P.; Revuz, J.; Roujeau, J.C.; Bagot, M. Metabolic predisposition to cutaneous adverse drug reactions. Role in toxic epidermal necrolysis caused by sulfonamides and anticonvulsants. Arch. Dermatol. 1995, 131, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Shear, N.H.; Spielberg, S.P.; Grant, D.M.; Tang, B.K.; Kalow, W. Differences in metabolism of sulfonamides predisposing to idiosyncratic toxicity. Ann. Intern. Med. 1986, 105, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M.; Ostrov, D.A.; Park, B.K. New genetic findings lead a way to better understanding of fundamental mechanisms of drug hypersensitivity. J. Allergy Clin. Immunol. 2015, 136, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Lonjou, C.; Borot, N.; Sekula, P.; Ledger, N.; Thomas, L.; Halew, S.; Naldi, L.; Bouwes-Bavinck, J.N.; Sidoroff, A.; de Toma, C.; et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet. Genom. 2008, 18, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Genin, E.; Schumacher, M.; Roujeau, J.C.; Naldi, L.; Liss, Y.; Kazma, R.; Sekula, P.; Hovnanian, A.; Mockenhaupt, M. Genome-wide association study of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe. Orphanet. J. Rare Dis. 2011, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Tassaneeyakul, W.; Jantararoungtong, T.; Chen, P.; Lin, P.Y.; Tiamkao, S.; Khunarkornsiri, U.; Chucherd, P.; Konyoung, P.; Vannaprasaht, S.; Choonhakarn, C.; et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet. Genom. 2009, 19, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.R.; Jee, Y.K.; Kim, Y.S.; Lee, C.H.; Jung, J.W.; Kim, S.H.; Park, H.W.; Chang, Y.S.; Jang, I.J.; Cho, S.H.; et al. Positive and negative associations of HLA class I alleles with allopurinol-induced SCARs in Koreans. Pharmacogenet. Genom. 2011, 21, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.I.; Chung, W.H.; Jee, S.H.; Chen, W.C.; Chang, Y.T.; Lee, W.R.; Hu, S.L.; Wu, M.T.; Chen, G.S.; Wong, T.W.; et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse reactions. Pharmacogenet. Genom. 2006, 16, 297–306. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.; Alfirevic, A.; Bourgeois, S.; Farrel, J.J.; Kasperaviciute, D.; Carrington, M.; Sillis, G.J.; Marson, T.; Jia, X.; Chinthapalli, K.; et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N. Engl. J. Med. 2011, 364, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, T.; Mushiroda, T.; Yowang, A.; Takahashi, A.; Kubo, M.; Shirakata, Y.; Ikezawa, Z.; Lijima, M.; Shiohara, T.; Hashimoto, K.; et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum. Mol. Genet. 2011, 20, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.R.; Liu, H.; Irwanto, A.; Fu, X.A.; Li, Y.; Yu, G.Q.; Yu, Y.X.; Chen, M.F.; Low, H.Q.; Li, J.H.; et al. HLA-B*13:01 and the dapsone hypersensitivity syndrome. N. Engl. J. Med. 2013, 369, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Guo, S.; Hall, D.; Cammett, A.M.; Jayadey, S.; Distel, M.; Storfer, S.; Huang, Z.; Mootsikapun, P.; Ruxrungtham, K.; et al. Toxicogenomics of nevirapine-associated cutaneous and hepatic adverse events among population of African, Asian, and European descent. AIDS 2011, 25, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Gui, X.E.; Lian, K.; Liu, Z.; Hu, J.; Dong, B. HLA-dependent hypersensitivity reaction to nevirapine in Chinese Han HIV-infected patients. AIDS Res. Hum. Retrovir. 2012, 28, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Likanonsakul, S.; Rattanatham, T.; Feangvad, S.; Uttayamakul, S.; Prasithsirikul, W.; Tunthanathip, P.; Nakayama, E.E.; Shioda, T. HLA-Cw*04 allele associated with nevirapine-induced rash in HIV-infected Thai population. AIDS Res. Ther. 2009, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Chantarangsu, S.; Mushiroda, T.; Mahasirimongkol, S.; Kiertiburanakul, S.; Sungkanupraph, S.; Manosuthi, W.; Tantisiriwat, W.; Charoenyingwattana, A.; Sura, T.; Chantratita, W.; et al. HLA-B*3505 is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai population. Pharmagenet. Genom. 2009, 19, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chen, J.K.; Ko, T.M.; Wei, C.Y.; Wu, J.Y.; Chung, W.H.; Chen, S.Y.; Liao, Y.D.; Hung, S.I.; Chen, Y.T. Immunologic basis for allopurinol-induced severe cutaneous adverse reactions: HLA-B*58:01-restricted activation of drug-specific T cells and molecular interaction. J. Allergy Clin. Immunol. 2015, 135, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- White, K.D.; Chung, W.H.; Hung, S.I.; Mallal, S.; Phillips, E.J. Evolving models of the immunopathogenesis of T cell-mediated drug allergy: The role of host, pathogens, and drug response. J. Allergy Clin. Immunol. 2015, 136, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Hansel, K.; Bellini, V.; Bianchi, L.; Brozzi, J.; Stingeni, L. Drug reaction with eosinophilia and systemic symptoms from ceftriaxone confirmed by positive patch test: An immunohistochemical study. J. Allergy Clin. Immunol. Pract. 2017, 5, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Picard, D.; Janela, B.; Descamps, V.; D’Incan, M.; Courville, P.; Jacquot, S.; Rogez, S.; Mardivirin, L.; Moins-Teisserenc, H.; Toubert, A.; et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): A multiorgan antiviral T cell response. Sci. Transl. Med. 2010, 2, 46ra62. [Google Scholar] [CrossRef] [PubMed]
- Selin, L.K.; Cornberg, M.; Brehm, M.A.; Kim, S.K.; Calcagno, C.; Ghersi, D.; Puzone, R.; Celada, F.; Welsh, R.M. CD8 memory T cells: Cross-reactivity and heterologous immunity. Semin. Immnol. 2004, 16, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Komandori, K.V.; Donahoe, S.M.; Moretto, W.J.; Schmidt, D.K.; Gillespie, G.; Ogg, G.S.; Roederer, M.; Nixon, D.F.; McCune, J.M. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology 2001, 279, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Sylwester, A.W.; Mitchell, B.L.; Edgar, J.B.; Taormina, C.; Pelte, C.; Ruchti, F.; Sleath, P.R.; Grabstein, T.H.; Hosken, N.A.; Kern, F.; et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005, 202, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Aoshima, M.; Ito, T.; Seo, N.; Takigawa, M.; Yagi, H. Emergence of circulating monomyeloid precursors predicts reactivation of human herpesvirus-6 in drug-induced hypersensitivity syndrome. Br. J. Dermatol. 2009, 161, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Fujiyama, T.; Kanebayashi, J.; Kito, Y.; Hata, M.; Yagi, H. Skin recruitment of monomyeloid precursors involves human herpesvirus-6 reactivation in drug allergy. Allergy 2013, 68, 681–699. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Matsukura, S.; Watanabe, T.; Komitsu, N.; Watanabe, Y.; Takahashi, Y.; Kambara, T.; Ikezawa, Z.L.; Aihara, M. The serum level of HMGB1 (high mobility group box 1 protein) is preferentially high in drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms. Br. J. Dermatol. 2014, 171, 1585–1588. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, F.; Nakamura, Y.; Miyashita, K.; Iioka, H.; Himuro, Y.; Ogawa, K.; Nishimura, C.; Nishikawa, M.; Mitsui, Y.; Ito, Y.; et al. Preferential expression of CD134, an HHV-6 cellular receptor, on CD4 T cells in drug-induced hypersensitivity (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS). J. Dermatol. Sci. 2016, 83, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Mardivirin, L.; Lacroix, A.D.; Descamps, V.; Ranger-Rogez, S. Augmentation de la replication in vitro de I’herpesvirus humain 6 en presence de valproate de sodium. Virologie 2007, 11, 1–3. [Google Scholar]
- Kuntz-Simon, G.; Obert, G. Sodium valproate, an anticonvulsant drug, stimulates human cytomegalovirus replication. J. Gen. Virol. 1995, 76, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Kano, Y.; Yamazaki, Y.; Kimishima, M.; Mizukawa, Y.; Shiohara, T. Defective regulatory T cells in patients with severe drug eruptions: Timing of the dysfunction is associated with the pathological phenotype and outcome. J. Immunol. 2009, 182, 8071–8079. [Google Scholar] [CrossRef] [PubMed]
- Sugita, K.; Tohyama, M.; Watanabe, H.; Otsuka, A.; Nakajima, S.; Lijima, M.; Hashimoto, K.; Tokura, Y.; Miyachi, Y.; Kabashima, K. Fluctuation of blood and skin plasmacytoid dendritic cells in drug-induced hypersensitivity syndrome. J. Allergy Clin. Immunol. 2010, 126, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Siegal, F.P.; Kadowaki, N.; Shodell, M.; Fitzgerald-Bocarsly, S.A.; Shah, K.; Ho, S.; Antonenko, S.; Liu, Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science 1999, 284, 1835–1837. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Morito, H.; Hasegawa, F.; Daikoku, N.; Miyagawa, F.; Okazaki, A.; Fukumoto, T.; Kobayashi, N.; Kasai, T.; Watanabe, H.; et al. Identification of thymus activation-regulated chemokine (TARC/CCL17) as a potential marker for early identification of disease and prediction of disease activity in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS). J. Dermatol. Sci. 2013, 69, 38–43. [Google Scholar] [PubMed]
- Ogawa, K.; Morito, H.; Hasegawa, F.; Kobayashi, N.; Watanabe, H.; Sueki, H.; Tohyama, M.; Hashimoto, K.; Kano, Y.; Shiohara, T.; et al. Elevated serum thymus and activation-regulated chemokine (TARC/CCL17) relates to reactivation of human herpesvirus 6 in drug reaction with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome (DIHS). Br. J. Dermatol. 2014, 171, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Baba, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Yoshie, O. The T cell-directed CC chemokine TARC is a highly speficic biological ligand for CC chemokine receptor 4. J. Biol. Chem. 1997, 272, 15036–15042. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lanzavecchia, A.; Mackay, C. Chemokines and chemokine receptors in T cell priming and Th1/Th2 mediated responses. Immunol. Today 1998, 19, 568–574. [Google Scholar] [CrossRef]
- Niu, J.; Jia, Q.; Ni, Q.; Yang, Y.; Chen, G.; Yang, X.; Zhai, Z.; Yu, H.; Guan, P.; Lin, R.; et al. Association of CD8+ T lymphocyte repertoire spreading with the severity of DRESS syndrome. Sci. Rep. 2015, 23, 9913. [Google Scholar] [CrossRef] [PubMed]
- Uhara, H.; Saiki, M.; Kawachi, S.; Ashida, A.; Oguchi, S.; Okuyama, R. Clinical course of drug-induced hypersensitivity syndrome treated without systemic corticosteroids. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Funck-Brentano, E.; Duong, T.A.; Bouvresse, S.; Bagot, M.; Wolkenstein, P.; Roujeau, J.C.; Chosidow, O.; Valeyrie-Allanore, L. Therapeutic management of DRESS: A retrospective study of 38 cases. J. Am. Acad. Dermatol. 2015, 72, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Descamps, V.; Ben Said, B.; Sassolas, B.; Truchetet, F.; Avenel-Audran, M.; Girardin, P.; Guinneppain, M.T.; Mathelier-Fusade, P.; Assier, H.; Milpied, B.; et al. Management of drug reaction with eosinophilia and systemic symptoms (DRESS). Ann. Dermatol. Venereol. 2010, 137, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Husain, Z.; Reddy, B.Y.; Schwartz, R.A. DRESS syndrome part II. Management and therapeutics. J. Am. Acad. Dermatol. 2013, 68, 709.e1–709.e9. [Google Scholar] [CrossRef] [PubMed]
- Scheuerman, O.; Nofech-Moses, Y.; Rachmel, A.; Ashkenazi, S. Successful treatment of antiepileptic drug hypersensitivity syndrome with intravenous immune globulin. Pediatrics 2001, 107, e14. [Google Scholar] [CrossRef] [PubMed]
- Fields, K.S.; Petersen, M.J.; Chiao, E.; Tristani-Firouzi, P. Case reports: Treatmnt of nevirapine-associated dress syndrome with intravenous immune globulin (IVIG). J. Drugs Dermatol. 2005, 4, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Joly, P.; Janela, B.; Tetart, F.; Rogez, S.; Picard, D.; D’Incan, M.; Descamps, V.; Collet, E.; Roujeau, J.C.; Musette, P. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch. Dermatol. 2012, 148, 543–544. [Google Scholar] [CrossRef] [PubMed]
- Mark, G.K.; Wong, A.; Dutz, J.P. Cyclosporine treatment of drug-induced hypersensitivity syndrome. JAMA Dermatol. 2016, 152, 1254–1257. [Google Scholar]
- Laban, E.; Hainaut-Wierzbicka, E.; Pourreau, F.; Yacoub, M.; Sztermer, E.; Guillet, G. Cyclophosphamide therapy for corticosresistant drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome in a patient with severe kidney and eye involvement and Epstein-Barr virus reactivation. Am. J. Kidney Dis. 2010, 55, e11–e14. [Google Scholar] [CrossRef] [PubMed]


| Category | Drugs |
|---|---|
| Anti-convulsants | Carbamazepine [2,11,14,18], lamotrigine [2,11,14,18], phenobarbital [11,14,18], phenytoin [2,11,14,18], oxcarbazepine [11,14], gabapentin [37] |
| Anti-bacterial | Amoxicillin [11,14], ampicillin [14,18], azithromycin [38], levofloxacin [39], minocycline [11,14,18], piperacillin/tazobactam [40], vancomycin [11,14,18] |
| Anti-tuberculosis | Ethambutol [18,41], isoniazid [2,18,41], pyrazinamide [18,41], rifampin [18,41], streptomycin [11,18,41] |
| Anti-retroviral agents | Abacavir [11,18], nevirapine [11,14,18] |
| Anti-hepatitis C virus agents | Boceprevir [42,43], telaprevir [42,44] |
| Anti-pyretic/analgesics | Acetaminophen [45], diclofenac [2], celecoxib [11,18], ibuprofen [11,18] |
| Sulfonamides | Dapsone [2,11,14,18], sulfamethoxazole-trimethoprim [2,11,14,18], sulfasalazine [2,11,14,18] |
| Targeted therapeutic agents | Dorafenib [46], vismodegib [47], vemurafenib [48] |
| Others | Allopurinol [2,11,14,18], chinese herbal medicine [2], imatinib [11], mexiletine [11,18], omeprazole [11], strontium ranelate [11] |
| Items | Score | Comments | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Fever ≧ 38.5 °C | N/U | Y | ||
| Enlarged lymph nodes | N/U | Y | >1 cm and ≧ 2 different areas | |
| Eosinophilia ≧ 0.7 × 109/L or ≧ 10% if WBC < 4.0 × 109/L | N/U | Y | Score 2, when ≧ 1.5 × 109/L or ≧ 20% if WBC < 4.0 × 109/L | |
| Atypical lymphocytosis | N/U | Y | ||
| Skin rash Extent > 50% of BSA Rash suggesting DRESS | N | N/U U | Y Y | Rash suggesting DRESS: ≧ 2 symptoms: purpuric lesions (other than legs), infiltration, facial edema, psoriasiform desquamation |
| Skin biopsy suggesting DRESS | N | Y/U | ||
| Organ involvement | N | Y | Score 1 for each organ involvement, maximal score: 2 | |
| Rash resolution ≧ 15 days | N/U | Y | ||
| Excluding other causes | N/U | Y | Score 1 if 3 tests of the following tests were performed and all were negative: HAV, HBV, HCV, Mycoplasma, Chlamydia, ANA, blood culture | |
| Drug | Associated HLA alleles | Population |
|---|---|---|
| Allopurinol | B*58:01 | Han Chinese [27], European [87,88], Thai [89], Korean [90] |
| Carbamazepine | A*31:01 | Han Chinese [91], European [92], Japanese [93] |
| A*11, B*51 | Japanese [93] | |
| Dapsone | B*13:01 | Han Chinese [94] |
| Nevirapine | DRB1*01:01, DRB1*01:02 | African, Asian, European [95] |
| Cw*4 | African, Asian, European [95,96,97] | |
| B*35 | Asian [95,98] | |
| Phenytoin | B*13:01 and B*51:01 | Han Chinese [82] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.-T.; Yang, C.-W.; Chu, C.-Y. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): An Interplay among Drugs, Viruses, and Immune System. Int. J. Mol. Sci. 2017, 18, 1243. https://doi.org/10.3390/ijms18061243
Cho Y-T, Yang C-W, Chu C-Y. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): An Interplay among Drugs, Viruses, and Immune System. International Journal of Molecular Sciences. 2017; 18(6):1243. https://doi.org/10.3390/ijms18061243
Chicago/Turabian StyleCho, Yung-Tsu, Che-Wen Yang, and Chia-Yu Chu. 2017. "Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): An Interplay among Drugs, Viruses, and Immune System" International Journal of Molecular Sciences 18, no. 6: 1243. https://doi.org/10.3390/ijms18061243