Identification of Novel Placentally Expressed Aspartic Proteinase in Humans
Abstract
:1. Introduction
2. Results
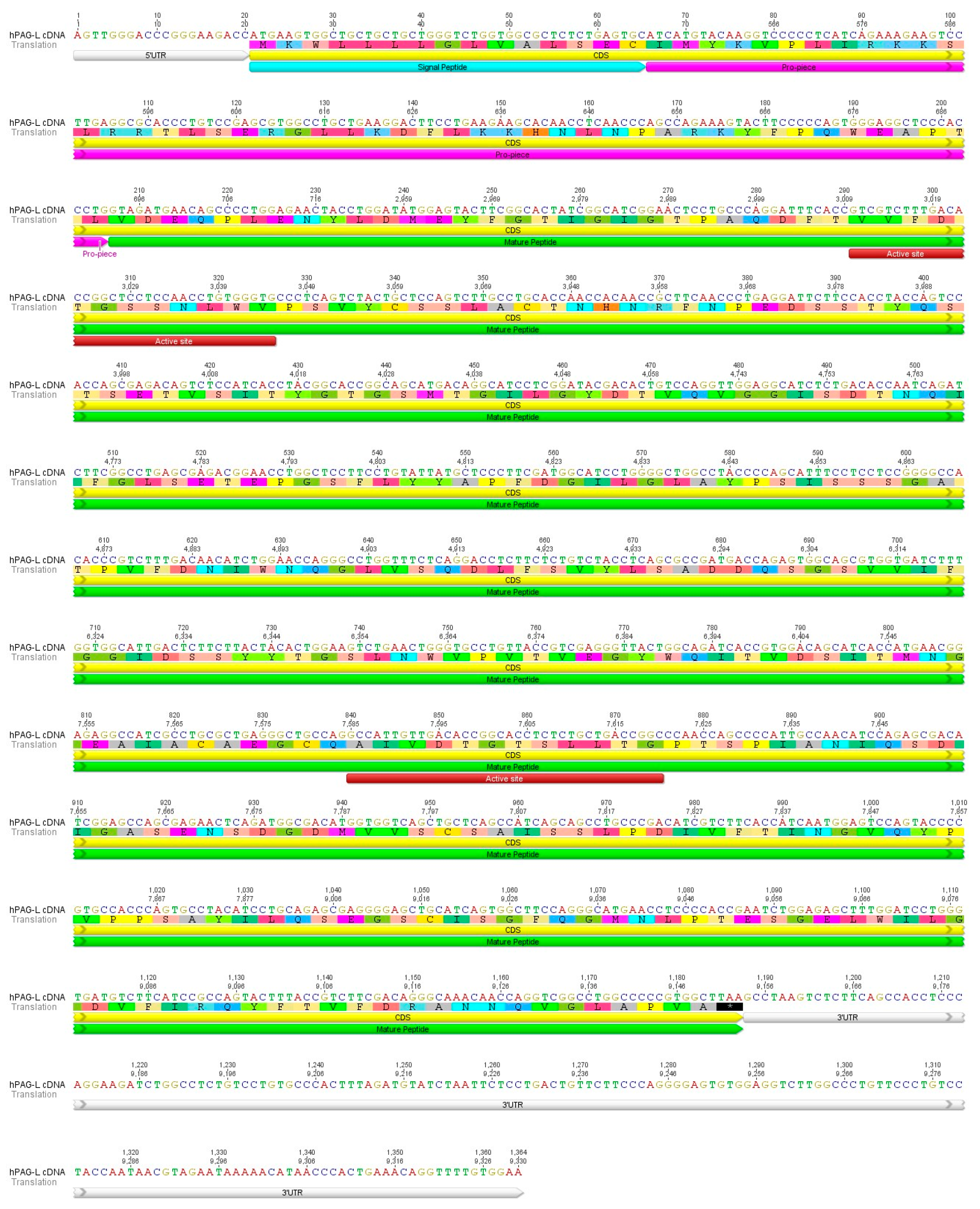
2.1. Identification of cDNA Sequence Originating from Term Placental Transcriptome
2.2. Identification of cDNA Sequence Originating from Term Placental Transcriptome
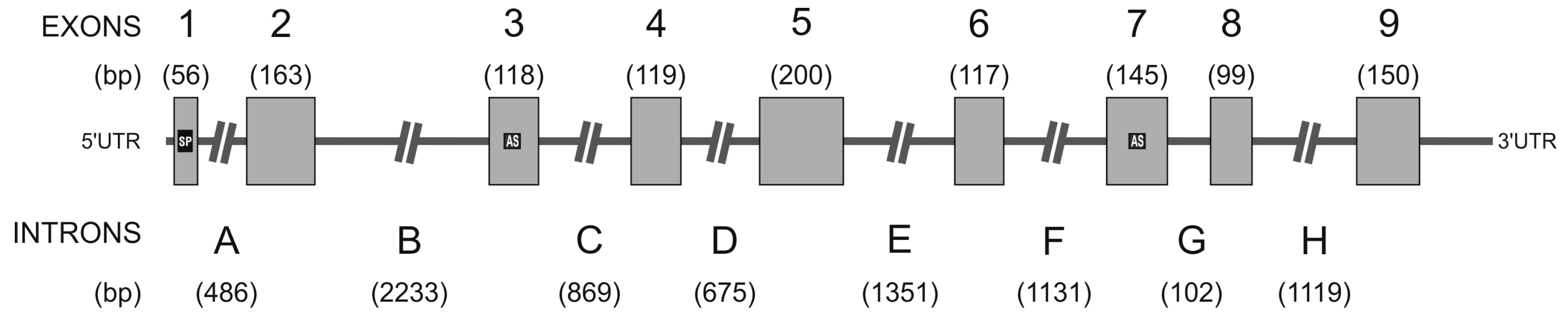
2.3. Identification of the hPAG-L/pep Sequence within the Human Genome
2.4. Identification of Placental hPAG-L/pep Protein
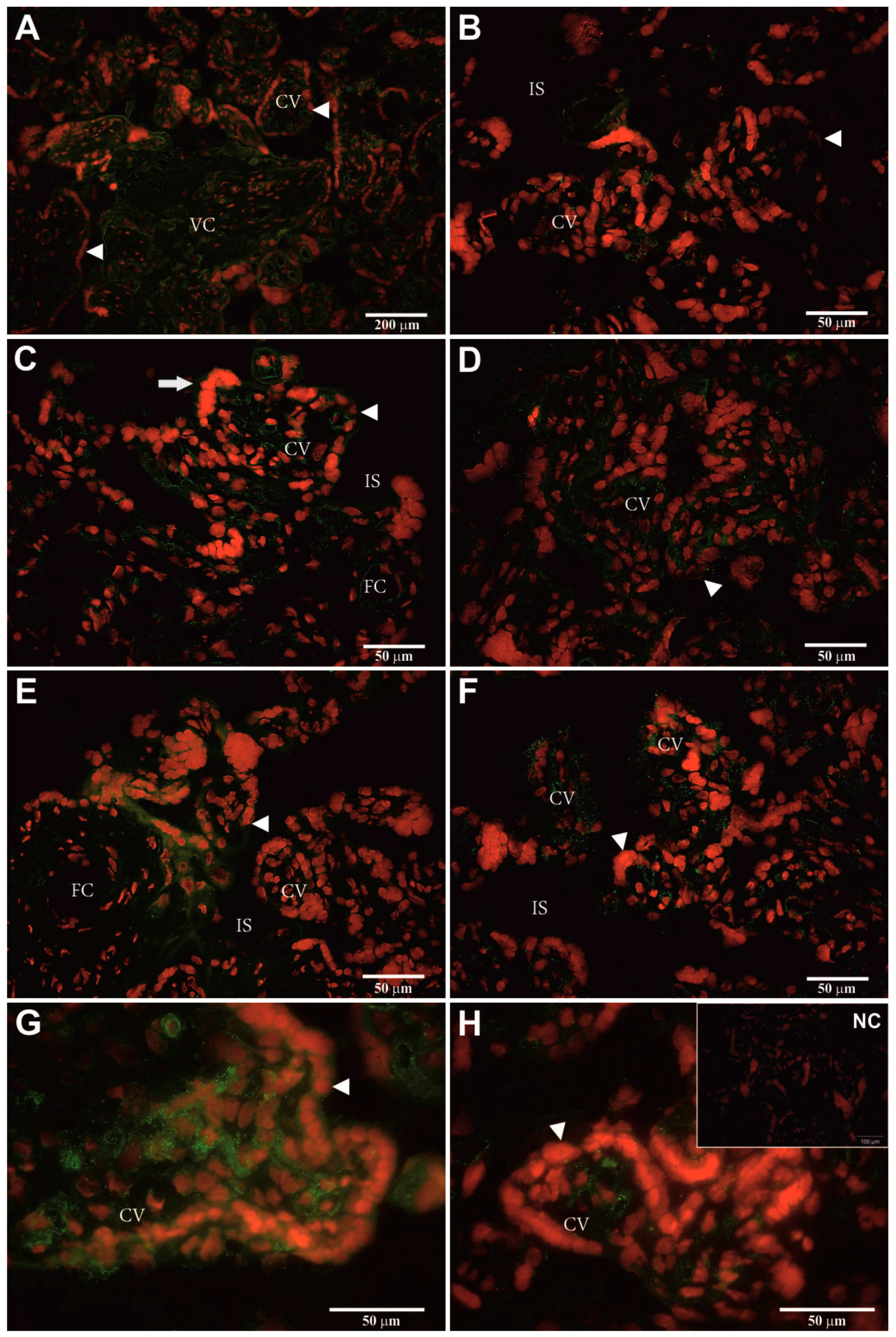
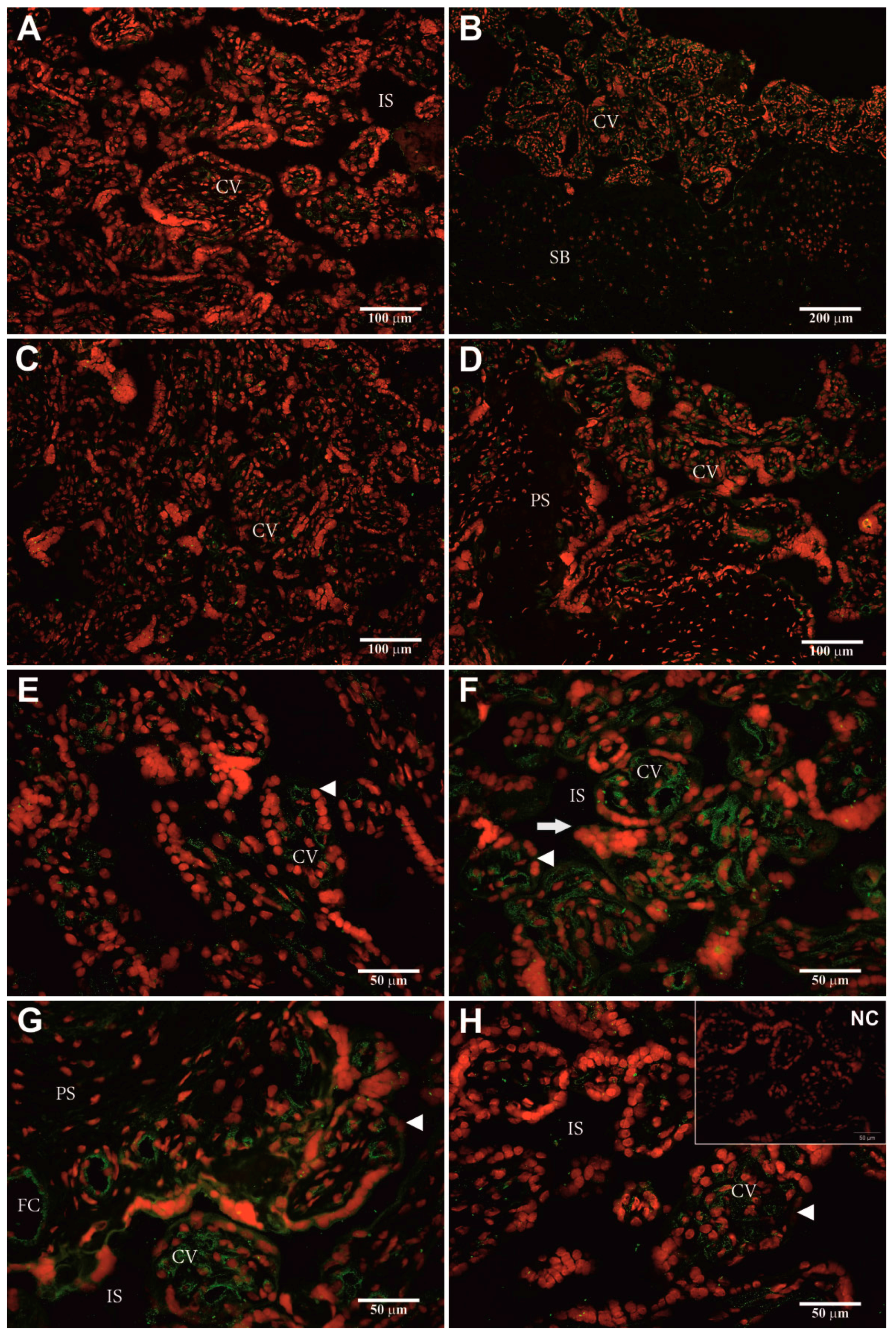
2.5. Identification of Cellular hPAG-L/pep Localization
3. Discussion
3.1. Identification of hPAG-L Transcript
3.2. Identification of hPAG-L Exonic-Intronic Structure
3.3. Identification of hPAG-L Proteins
3.4. Identification of Cellular hPAG-L Localization
4. Materials and Methods
4.1. Ethics Statement and Collection of Samples
4.2. Total RNA Extraction
4.3. High Throughput mRNA and Bioinformatics
4.4. Capillary Sequencing
4.5. Genomic Identification of the hAP/PAG-L Sequence
4.6. Identification of the Exon-Intron Organization of the hPAG-L
4.7. Cellular Placental Protein Extraction
4.8. Western Blotting
4.9. Heterologous Double Fluorescent Immunohistochemistry (htdF-IHC)
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PAG | Pregnancy-associated glycoprotein family |
| PAG-L | PAG-like |
| hPAG-L | Human PAG-L |
| Pep | Pepsinogen |
| RNA-seq | RNA sequencing |
| aa | Amino acids |
| D | Asparagine (Asp) |
| cDNA | Complementary DNA |
| gDNA | Genomic DNA |
| bPAG | Bovine PAG |
| pPAG | Porcine PAG |
| CfPAG-L | Beaver PAG-L |
| EbPAG | European bison PAG |
| LpPAG | Lama pacos PAG |
| CdPAG | Camelus dromedarius PAG |
| CfPAG | Camelus ferrus PAG |
| AaPAG-L | Alces alces PAG |
| RIA | Radioimmunological test |
| ELISA | Immunoenzymatic test |
| RT-PCR | Reverse transcriptase PCR |
| dNTP | Deoxynucleotide |
| MWCO | Molecular weight cut-off |
| SDS-PAGE | Denaturing polyacrylamide electrophoresis |
| CBB | Coomassie Brilliant Blue |
| NBT | Nitro blue tetrazolium |
| BCIP | 5-Bromo-4-chloro-3-indolyl-phosphate |
| ht | Heterologous (cross-species) |
| anti-pPAG-P | Polyclonals raised against various porcine antigens |
| anti-Rec pPAG2 | Polyclonals raised against recombinant pPAG2 antigen |
| htdF-IHC | Heterologous double fluorescent immunohistochemistry |
| A488 | Alexa 488 fluorophore |
| PI | Propidium iodide |
| HQ | High quality |
| CDS | Coding sequence |
| UTR | Untranslated region |
| QC | Query Cover |
| SP | Signal peptide |
| ID | Sequence identity |
| CV | Chorionic villi |
| VC | Villous core |
| FC | Fetal capillaries |
| IS | Intervillous space |
| PS | Placental septa |
| SB | Stratum basale |
| NC | Negative control |
| dpc | Day post coitum |
References
- Szafranska, B.; Panasiewicz, G.; Majewska, M. Biodiversity of multiple pregnancy-associated glycoprotein (PAG) family: Gene cloning and chorionic protein purification in domestic and wild eutherians (Placentalia)—A review. Reprod. Nutr. Dev. 2006, 46, 481–502. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.M.; Pohler, K.G.; Smith, M.F.; Green, J.A. Placental PAGs: Gene origins, expression patterns, and use as markers of pregnancy. Reproduction 2015, 149, R115–R126. [Google Scholar] [CrossRef] [PubMed]
- Guruprasad, K.; Blundell, T.L.; Xie, S.; Green, J.; Szafranska, B.; Nagel, R.J.; McDowell, K.; Baker, C.B.; Roberts, R.M. Comparative modelling and analysis of amino acid substitutions suggests that the family of pregnancy-associated glycoproteins includes both active and inactive aspartic proteinases. Protein Eng. 1996, 9, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K. Structure and function studies on enzymes with a catalytic carboxyl group(s): From ribonuclease T1 to carboxyl peptidases. Proc. Jpn. Acad. Ser. B 2013, 89, 201–225. [Google Scholar] [CrossRef]
- Kageyama, T. Pepsinogens progastricsins and prochymosins: Structure function evolution and development. Cell. Mol. Life Sci. 2002, 59, 288–306. [Google Scholar] [CrossRef] [PubMed]
- Carginale, V.; Trinchella, F.; Capasso, C.; Scudiero, R.; Riggio, M.; Parisi, E. Adaptive evolution and functional divergence of pepsin gene family. Gene 2004, 333, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Panasiewicz, G.; Majewska, M.; Romanowska, A.; Dajnowiec, J.; Szafranska, B. Radiocompetition of secretory pregnancy-associated glycoproteins as chorionic ligands with luteal and uterine gonadotrophin receptors of pregnant pigs. Anim. Reprod. Sci. 2007, 99, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, B.; Panasiewicz, G.; Majewska, M.; Romanowska, A.; Dajnowiec, J. Pregnancy-associated glycoprotein family (PAG)—As chorionic signaling ligands for gonadotropin receptors of cyclic animals. Anim. Reprod. Sci. 2007, 99, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.T.; Hamada, Y.; Kimura, T.; Kiso, Y. Design of potent aspartic protease inhibitors to treat various diseases. Arch. Pharm. 2008, 341, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Tsukuba, T.; Okamoto, K.; Okamoto, Y.; Yanagawa, M.; Kohmura, K.; Yasuda, Y.; Uchi, H.; Nakahara, T.; Furue, M.; Nakayama, K.; et al. Association of cathepsin E deficiency with development of atopic dermatitis. J. Biochem. 2003, 134, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Kawakubo, T.; Okamoto, K.; Iwata, J.; Shin, M.; Okamoto, Y.; Yasukochi, A.; Nakayama, K.; Kadowaki, T.; Tsukuba, T.; Yamamoto, K. Cathepsin E prevents tumor growth and metastasis by catalyzing the proteolytic release of soluble TRAIL from tumor cell surface. Cancer Res. 2007, 67, 10869–10878. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Kadowaki, T.; Iwata, J.; Kawakubo, T.; Yamaguchi, N.; Takii, R.; Tsukuba, T.; Yamamoto, K. Association of cathepsin E with tumor growth arrest through angiogenesis inhibition and enhanced immune responses. Biol. Chem. 2007, 388, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.M.; Chen, S.L.; Xing, F.Q. Expression of cathepsins B and L in early gestational decidua and chorionic villi. Di Yi Jun Yi Da Xue Xue Bao 2005, 25, 1365–1368. (In Chinese) [Google Scholar] [PubMed]
- Nakanishi, T.; Ozaki, Y.; Blomgren, K.; Tateyama, H.; Sugiura-Ogasawara, M.; Suzumori, K. Role of cathepsins and cystatins in patients with recurrent miscarriage. Mol. Hum. Reprod. 2005, 11, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Ozaki, Y.; Suzumori, N.; Yasukochi, A.; Kawakubo, T.; Furuno, T.; Nakanishi, M.; Yamamoto, K.; Sugiura-Ogasawara, M. Role of cathepsin E in decidual macrophage of patients with recurrent miscarriage. Mol. Hum. Reprod. 2014, 20, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Green, J.A.; Piontkivska, H.; Roberts, R.M. Aspartic proteinase phylogeny and the origin of pregnancy-associated glycoproteins. Mol. Biol. Evol. 2003, 20, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Green, J.; Beckers, J.F.; Roberts, R.M. The gene encoding bovine pregnancy-associated glycoprotein-1, an inactive member of the aspartic proteinase family. Gene 1995, 159, 193–197. [Google Scholar] [CrossRef]
- Telugu, B.P.; Walker, A.M.; Green, J.A. Characterization of the bovine pregnancy-associated glycoprotein gene family—Analysis of gene sequences, regulatory regions within the promoter and expression of selected genes. BMC Genom. 2009, 24, 185. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, B.; Miura, R.; Ghosh, D.; Ezashi, T.; Xie, S.; Roberts, R.M.; Green, J.A. Gene for porcine Pregnancy-Associated Glycoprotein 2 (poPAG2): Its structural organization and analysis of its promoter. Mol. Reprod. Dev. 2001, 60, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Lipka, A.; Majewska, M.; Panasiewicz, G.; Bieniek-Kobuszewska, M.; Szafranska, B. Gene structure of the pregnancy associated glycoprotein-like (PAG-L) in the Eurasian beaver (Castor fiber L.). Funct. Integr. Genom. 2017. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Xie, S.; Szafranska, B.; Gan, X.; Newman, A.G.; McDowell, K.; Roberts, R.M. Identification of a new aspartic proteinase expressed by the outer chorionic cell layer of the equine placenta. Biol. Reprod. 1999, 60, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M.; Enders, A.C.; Pijnenborg, R. The role of invasive trophoblast in implantation and placentation of primates. Philos. Trans. R. Soc. Lond. B 2015, 370, 20140070. [Google Scholar] [CrossRef] [PubMed]
- Monk, D. Genomic imprinting in the human placenta. Am. J. Obstet. Gynecol. 2015, 213, S152–162. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B.; Ghosh, D.; Sengupta, J. An integrative view on the physiology of human early placental villi. Prog. Biophys. Mol. Biol. 2014, 114, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M.; Enders, A.C. Placentation in mammals: Definitive placenta, yolk sac, and paraplacenta. Theriogenology 2016, 86, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.M.; Xie, S.; Mathialagan, N. Maternal recognition of pregnancy. Biol. Reprod. 1996, 54, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.M.; Ezashi, T.; Das, P. Trophoblast gene expression: Transcription factors in the specification of early trophoblast. Reprod. Biol. Endocrinol. 2004, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Lopes-da-Costa, L.; Chagas e Silva, J.; Deloche, M.C.; Jeanguyot, N.; Humblot, P.; Horta, A.E. Effects of embryo size at transfer (whole versus demi) and early pregnancy progesterone supplementation on embryo growth and pregnancy-specific protein bovine concentrations in recipient dairy heifers. Theriogenology 2011, 76, 522–531. [Google Scholar] [CrossRef] [PubMed]
- García-Ispierto, I.; Almería, S.; Serrano, B.; de Sousa, N.; Beckers, J.; López-Gatius, F. Plasma concentrations of pregnancy-associated glycoproteins measured using anti-bovine PAG-2 antibodies on day 120 of gestation predict abortion in dairy cows naturally infected with Neospora caninum. Reprod. Domest. Anim. 2013, 48, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Breukelman, S.P.; Perényi, Z.; Taverne, M.A.; Jonker, H.; van der Weijden, G.C.; Vos, P.L.; de Ruigh, L.; Dieleman, S.J.; Beckers, J.F.; Szenci, O. Characterisation of pregnancy losses after embryo transfer by measuring plasma progesterone and bovine pregnancy-associated glycoprotein-1concentrations. Vet. J. 2012, 194, 71–76. [Google Scholar] [CrossRef] [PubMed]
- García-Ispierto, I.; Rosselló-Visa, M.A.; Serrano-Pérez, B.; Mur-Novales, R.; deSousa, N.M.; Beckers, J.F.; López-Gatius, F. Plasma concentrations of pregnancy-associated glycoproteins I and II and progesterone on day 28 post-AI as markers of twin pregnancy in dairy cattle. Livest. Sci. 2016, 192, 44–47. [Google Scholar] [CrossRef]
- Szafranska, B.; Xie, S.; Green, J.; Roberts, R.M. Porcine pregnancy-associated glycoproteins: New members of the aspartic proteinase gene family expressed in trophectoderm. Biol. Reprod. 1995, 53, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Green, J.; Bixby, J.B.; Szafranska, B.; DeMartini, J.C.; Hecht, S.; Roberts, R.M. The diversity and evolutionary relationships of the pregnancy-associated glycoproteins an aspartic proteinase subfamily consisting of many trophoblast-expressed genes. Proc. Natl. Acad. Sci. USA 1997, 94, 12809–12816. [Google Scholar] [CrossRef] [PubMed]
- Garbayo, J.M.; Green, J.; Manikkam, M.; Beckers, J.F.; Kiesling, D.O.; Ealy, A.D.; Roberts, R.M. Caprine Pregnancy-Associated Glycoproteins (PAGs): Their cloning expression and evolutionary relationship to other PAG. Mol. Reprod. Dev. 2000, 57, 311–322. [Google Scholar] [CrossRef]
- Kageyama, T.; Ichinose, M.; Tsukada-Kato, S.; Omata, M.; Narita, Y.; Moriyama, A.; Yonezawa, S. Molecular cloning of neonate/infant-specific pepsinogens from rat stomach mucosa and their expressional change during development. Biochem. Biophys. Res. Commun. 2000, 267, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Takahashi, K. Monkey pepsinogens and pepsins III Carbohydrate moiety of Japanese monkey pepsinogens and the amino acid sequence around the site of its attachment to protein. J. Biochem. 1978, 84, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Trieu-Cuot, P.; Collin, J.C.; Ribadeau-Dumas, B. Purification and characterization of bovine gastricsin. Eur. J. Biochem. 1982, 122, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Rosenfeld, C.S.; Roberts, R.M.; Green, J.A. An aspartic proteinase expressed in the yolk sac and neonatal stomach of the mouse. Biol. Reprod. 2001, 65, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Xie, S.; Gan, X.; Roberts, R.M. An aspartic proteinase expressed in the equine placenta. Adv. Exp. Med. Biol. 1998, 436, 163–167. [Google Scholar] [PubMed]
- Panasiewicz, G.; Majewska, M.; Szafranska, B. Trophoblastic cDNA cloning of porcine pregnancy-associated glycoprotein genes (pPAG) and in silico analysis of coded polypeptide precursors. Reprod. Biol. 2004, 4, 131–141. [Google Scholar] [PubMed]
- Brandt, G.A.; Parks, T.E.; Killian, G.; Ealy, A.D.; Green, J.A. A cloning and expression analysis of pregnancy-associated glycoproteins expressed in trophoblasts of the white-tail deer placenta. Mol. Reprod. Dev. 2007, 74, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Low, B.G.; Nagel, R.J.; Kramer, K.K.; Anthony, R.V.; Zoli, A.P.; Beckers, J.F.; Roberts, R.M. Identification of the major pregnancy-specific antigens of cattle and sheep as inactive members of the aspartic proteinase family. Proc. Natl. Acad. Sci. USA 1991, 88, 10247–10251. [Google Scholar] [CrossRef] [PubMed]
- Yakabe, E.; Tanji, M.; Ichinose, M.; Goto, S.; Miki, K.; Kurokawa, K.; Ito, H.; Kageyama, T.; Takahashi, K. Purification, characterization, and amino acid sequences of pepsinogens and pepsins from the esophageal mucosa of bullfrog (Rana catesbeiana). J. Biol. Chem. 1991, 266, 22436–22443. [Google Scholar] [PubMed]
- Kurokawa, T.; Uji, S.; Suzuki, T. Identification of pepsinogen gene in the genome of stomachless fish, Takifugu rubripes. Comp. Biochem. Physiol. B 2005, 140, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Panasiewicz, G.; Louis, K.K.; Olivera, V.M.; Mamani, J.M.; Abd-Elnaeim, M.M.; Szafranska, B. Pregnancy-associated glycoprotein (PAG) family: Transcripts and gene amplicons in camelids. Reprod. Biol. 2009, 9, 127–150. [Google Scholar] [CrossRef]
- Bieniek-Kobuszewska, M.; Panasiewicz, G.; Lipka, A.; Majewska, M.; Szafranska, B. Novel SNPs and InDels discovered in two promoter regions of porcine pregnancy-associated glycoprotein 2-like subfamily (pPAG2-Ls) in crossbreed pigs. Funct. Integr. Genom. 2016, 16, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Tanabe, K.; Koiwai, O. Structure and development of rabbit pepsinogens stage-specific zymogens nucleotide sequences of cDNAs molecular evolution and gene expression during development. J. Biol. Chem. 1990, 265, 17031–17038. [Google Scholar] [PubMed]
- Sogawa, K.; Fujii-Kuriyama, Y.; Mizukami, Y.; Ichihara, Y.; Takahashi, K. Primary structure of human pepsinogen gene. J. Biol. Chem. 1983, 258, 5306–5311. [Google Scholar] [PubMed]
- Hayano, T.; Sogawa, K.; Ichihara, Y.; Fujii-Kuriyama, Y.; Takahashi, K. Primary structure of human pepsinogen C gene. J. Biol. Chem. 1988, 263, 1382–1385. [Google Scholar] [PubMed]
- Örd, T.; Kolmer, M.; Villems, R.; Saarma, M. Structure of the human genomic region homologous to the bovine prochymosin-encoding gene. Gene 1990, 91, 241–246. [Google Scholar] [CrossRef]
- Takahashi, K. Gene structures of pepsinogens A and C. Scand. J. Clin. Lab. Investig. 1992, 210, 97–110. [Google Scholar] [CrossRef]
- Majewska, M.; Panasiewicz, G.; Dabrowski, M.; Gizejewski, Z.; Beckers, J.F.; Szafranska, B. Multiple forms of pregnancy-associated glycoproteins released in vitro by porcine chorion or placentomal and interplacentomal explants of wild and domestic ruminants. Reprod. Biol. 2005, 5, 185–203. [Google Scholar] [PubMed]
- Szafranska, B.; Majewska, M.; Panasiewicz, G. N-glycodiversity of the pregnancy-associated glycoprotein family (PAG) produced in vitro by trophoblast and trophectoderm explants during implantation, placentation and advanced pregnancy in the pig. Reprod. Biol. 2004, 4, 67–89. [Google Scholar] [PubMed]
- Klisch, K.; Jeanrond, E.; Pang, P.C.; Pich, A.; Schuler, G.; Dantzer, V.; Kowalewski, M.P.; Dell, A. A tetraantennary glycan with bisecting N-acetylglucosamine and the Sd(a) antigen is the predominant N-glycan on bovine pregnancy-associated glycoproteins. Glycobiology 2008, 18, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kiewisz, J.; Sousa, N.M.; Beckers, J.F.; Vervaecke, H.; Panasiewicz, G.; Szafranska, B. Isolation of pregnancy-associated glycoproteins from placenta of the American bison (Bison bison) at first half of pregnancy. Gen. Comp. Endocrinol. 2008, 155, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Kiewisz, J.; Melo de Sousa, N.; Beckers, J.F.; Panasiewicz, G.; Gizejewski, Z.; Szafranska, B. Identification of multiple pregnancy-associated glycoproteins (PAGs) purified from the European bison (Eb; Bison bonasus L.) placentas. Anim. Reprod. Sci. 2009, 112, 229–250. [Google Scholar] [CrossRef] [PubMed]
- Beriot, M.; Tchimbou, A.F.; Barbato, O.; Beckers, J.F.; de Sousa, N.M. Identification of pregnancy-associated glycoproteins and alpha-fetoprotein in fallow deer (Dama dama) placenta. Acta Vet. Scand. 2014, 56, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Lipka, A.; Panasiewicz, G.; Majewska, M.; Bieniek-Kobuszewska, M.; Saveljev, A.P.; Pankratov, A.P.; Szafranska, B. Identification of the pregnancy-associated glycoprotein family (PAGs) and some aspects of placenta development in the European moose (Alces alces L.). Theriogenology 2016, 86, 2119–2135. [Google Scholar] [CrossRef] [PubMed]
- Lipka, A.; Panasiewicz, G.; Majewska, M.; Paukszto, L.; Bieniek-Kobuszewska, M.; Szafranska, B. Identification of placental aspartic proteinase in the Eurasian beaver (Castor fiber L.). Theriogenology 2017. under review. [Google Scholar]
- Majewska, M.; Panasiewicz, G.; Majewski, M.; Szafranska, B. Localization of chorionic pregnancy-associated glycoprotein family in the pig. Reprod. Biol. 2006, 6, 205–230. [Google Scholar] [PubMed]
- Majewska, M.; Panasiewicz, G.; Szafranska, B.; Gizejewski, Z.; Majewski, M.; Borkowski, K. Cellular localization of the pregnancy-associated glycoprotein family (PAGs) in the synepitheliochorial placenta of the European bison. Gen. Comp. Endocrinol. 2008, 155, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Panasiewicz, G.; Szafranska, B. Pregnancy-associated glycoprotein (PAG) family localized in chorionic cells within the epitheliochorial/diffuse placenta of the alpaca (Lama pacos). Acta Histochem. 2011, 113, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Panasiewicz, G.; Szafranska, B. Expression of pregnancy-associated glycoprotein family in the epitheliochorial placenta of two Camelidae species (C. dromedarius and C. bactrianus). Acta Histochem. 2013, 113, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Panasiewicz, G.; Bieniek-Kobuszewska, M.; Lipka, A.; Majewska, M.; Jedryczko, R.; Szafranska, B. Novel effects of identified SNPs within the porcine pregnancy-associated glycoprotein gene family (pPAGs) on the major reproductive traits in Hirschmann hybrid-line sows. Res. Vet. Sci. 2017, 114, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Panasiewicz, G.; Zamojska, A.; Bieniek, M.; Gizejewski, Z.; Szafranska, B. Persistent Müllerian duct syndrome (PMDS) in the Polish free-ranged bull populations of the European bison (Bison bonasus L.). Anim. Reprod. Sci. 2015, 152, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, B.; Panasiewicz, G. The placental expression of the porcine pregnancy-associated glycoprotein (pPAG) gene family examined in situ and in vitro. Anim. Reprod. Sci. 2002, 72, 95–113. [Google Scholar] [CrossRef]
- Szafranska, B.; Panasiewicz, G.; Majewska, M.; Beckers, J.F. Chorionic expression of heterogeneous products of the PAG (pregnancy-associated glycoprotein) gene family secreted in vitro throughout embryonic and foetal development in the pig. Reprod. Nutr. Dev. 2003, 43, 497–516. [Google Scholar] [CrossRef] [PubMed]

| Gene Name a | SP Sequence (aa) b | Identity (%) | Positive aa (%) |
|---|---|---|---|
| hPAG-L/pep | MKWLLLLGLVALSEC | this study | this study |
| hPepsinogen A | ............... | 100 | 100 |
| bPAG2 | ....V.......... | 93.3 | 100 |
| pPAG1 | ....VI......... | 86.7 | 100 |
| mPepsinogen F | ....WV......... | 86.7 | 93.3 |
| fPAG | ....WV......... | 86.7 | 93.3 |
| pPAG2 | ....VI.......D. | 80 | 100 |
| ePAG | ...FGV....T.... | 73.3 | 80 |
| CfPAG-L | ...IVVA.LC.P.L.A | 37.5 | 62.5 |
| hCathepsin E | ..T....L..L.ELGEAQG | 60 | 60 |
| hPepsinogen C | ...MVVV-..C.QLLEA | 40 | 66.7 |
| hNapsin A | QPL....P.LNVEPSGA | 33.3 | 46.7 |
| hRenin | PR.G..--.LLWGS.TFG | 33.3 | 46.7 |
| hCathepsin D | .QPSS..P.ALCLLAAPASA | 26.7 | 33.3 |
| Gene Name a | Blocking Peptide Sequence (aa) b | Identity (%) | Positive aa (%) |
|---|---|---|---|
| hPAG-L/pep | IMYKVPLIRKKSLRRTLSERGLLKDFLKKHNLNPARKYFPQWEAPTL | this study | this study |
| hPepsinogen A | ............................................... | 100 | 100 |
| hPepsinogen C | AVV....KKF..I.E.MK.K...GE..RT.KYD..W..R.GDL | 46.5 | 65.1 |
| fPAG | -LVTI..T.V..M.EN.R.KDR.....EN.PY.L.Y.FVD | 43.6 | 59 |
| pPAG2 | -LVMI..TKV..V.ES.R.K....N...E.PY.MIQNL | 43.2 | 67.6 |
| CfPAG-L | AISRI..RKA..V.Q..K.K...EE...T.KYD..Q..LANNFGDF | 41.3 | 65.2 |
| pPAG1 | -LVII..TKV..I.EN.R.KD..LN...E.PY.MIQ.F | 40.5 | 64.9 |
| ePAG | -LVTI..VKI....EN.R.KDM..EY.E.YPFRL | 36.4 | 66.7 |
| mPepsinogen F | -LV.I..MKI..M.EN.R.SQV...Y.E.YPRSR.HVLLE.RRN. | 36.4 | 59.1 |
| bPAG2 | .VIL-..KKM.T..E..R.KN..NN..EEQAYRLSKNDS | 33.3 | 56.4 |
| Gene Name a | NH2-Domain b | Identity (%) | COOH-Domain b | Identity (%) |
|---|---|---|---|---|
| hPAG-L/pep. | VVFDTGSSNLWV | this study | AIVDTGTSLLTG | this study |
| hPepsinogen A | ............. | 100 | ............ | 100 |
| hCathepsin D | ............ | 100 | .........MV. | 83.3 |
| hPepsinogen C | .L.......... | 91.7 | ...........V | 91.7 |
| hCathepsin E | .I.......... | 91.7 | .........I.. | 91.7 |
| CfPAG-L | .L.......... | 91.7 | G..........V | 83.3 |
| TrNothepsin | ........D... | 91.7 | .........IA. | 83.3 |
| hNapsin A | .A.......... | 91.7 | ..L......I.. | 83.3 |
| pPAG2, 4, 6, 10 | ........D... | 91.7 | ........MLH. | 75 |
| oPAG2 | ........D... | 91.7 | .L.......IH. | 75 |
| bPAG2 | .......A.... | 91.7 | .LL.....MIY. | 58.3 |
| hRenin A | .........V.. | 91.7 | .L....A.YIS. | 58.3 |
| mPepsinogen F | ..L.....V... | 83.3 | G.M......... | 83.3 |
| fPAG | .I......D... | 83.3 | ..I.......I. | 83.3 |
| ePAG | .I.....AD... | 75 | ..........L. | 91.7 |
| zPAG | .I.....AD... | 75 | ..........L. | 91.7 |
| pPAG1, 3, 5 | .I...A..D... | 75 | ..L.S.SAF.L. | 50 |
| Sequence Length (bp) | |||||
|---|---|---|---|---|---|
| Gene Segment | hPAG-L/pep | bPAG1 | bPAG2 | pPAG2 | CfPAG-L |
| Exon 1 | 56 | 53 | 53 | 53 | 59 |
| Intron A | 486 | 1100 | 1300 | 1093 | 1937 |
| Exon 2 | 163 | 151 | 151 | 166 | 160 |
| Intron B | 2233 | 1000 | 1000 | 1324 | 385 |
| Exon 3 | 118 | 118 | 118 | 118 | 118 |
| Intron C | 869 | 100 | 100 | 90 | 917 |
| Exon 4 | 119 | 119 | 119 | 119 | 119 |
| Intron D | 675 | 1200 | 1200 | 1124 | 451 |
| Exon 5 | 200 | 194 | 194 | 200 | 200 |
| Intron E | 1351 | 900 | 1100 | 927 | 1138 |
| Exon 6 | 117 | 117 | 117 | 117 | 123 |
| Intron F | 1131 | 1900 | 1700 | 1455 | 288 |
| Exon 7 | 145 | 142 | 142 | 136 | 148 |
| Intron G | 102 | 100 | 100 | 85 | 681 |
| Exon 8 | 99 | 99 | 99 | 99 | 99 |
| Intron H | 1119 | 1700 | 1700 | 292 | 603 |
| Exon 9 | 150 | 150 | 150 | 156 | 147 |
| Total length | 9133 | 9143 | 9343 | 8031 | 7573 |
| Donor Splice Sites | Acceptor Splice Sites | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Exon | 5′→3′ | Phase | Intron | 5′→3′ | Intron | 5′→3′ | Phase | Exon | 5′→3′ |
| 1 | TCATGTACAA | 0 | A | GTGAGTCCGG | A | CAAACCACAG | 2 | 2 | GGTCCCCCTC |
| 2 | CTACCTGGAT | 0 | B | GTGAGTGTGC | B | GCCTGGACAG | 0 | 3 | ATGGAGTACT |
| 3 | CTTGCCTGCA | 1 | C | GTAAGTGCCC | C | GTCCTTGCAG | 1 | 4 | CCAACCACAA |
| 4 | CACTGTCCAG | 0 | D | GTGGGCACCT | D | CCCCACCCAG | 0 | 5 | GTTGGAGGCA |
| 5 | ACCTCAGCGC | 2 | E | GTAAGTTGAG | E | CTTTCCACAG | 2 | 6 | CGATGACCAG |
| 6 | CCGTGGACAG | 2 | F | GTGAGACTGC | F | TTGCCCTCAG | 2 | 7 | CATCACCATG |
| 7 | AGATGGCGAC | 0 | G | GTGAGTCCAG | G | CTCTTTCCAG | 0 | 8 | ATGGTGGTCA |
| 8 | CATCCTGCAG | 0 | H | GTGAGGAGGC | H | TTTTCTCCAG | 0 | 9 | AGCGAGGGGA |
| Pairwise Identity (%) | |||
|---|---|---|---|
| hPAG-L/pep | bPAG1 | pPAG2 | CfPAG-L |
| Exon 1 | 78.6 | 75.5 | 63.3 |
| Intron A | 49.8 | 51.1 | 50.3 |
| Exon 2 | 52.1 | 58.8 | 59.6 |
| Intron B | 51.7 | 50.7 | 52.7 |
| Exon 3 | 69.5 | 74.6 | 71.2 |
| Intron C | 55.6 | 54.5 | 25.4 |
| Exon 4 | 59.7 | 58.4 | 64.7 |
| Intron D | 52.5 | 52.2 | 52.7 |
| Exon 5 | 58.5 | 65.3 | 65.5 |
| Intron E | 51.3 | 53.3 | 50.9 |
| Exon 6 | 61.9 | 69.7 | 56.9 |
| Intron F | 51.2 | 51.0 | 50.3 |
| Exon 7 | 62.0 | 63.3 | 64.7 |
| Intron G | 58.5 | 54.2 | 54.7 |
| Exon 8 | 67.7 | 66.7 | 70.3 |
| Intron H | 52.9 | 52.3 | 51.8 |
| Exon 9 | 58.7 | 61.4 | 61.5 |
| Primers Name | Sequence (5′–3′) | Position (bp) a | Amplicon Length (bp) a | |
|---|---|---|---|---|
| 1 | MMstart | AGTTGGGACCCGGGAAGA | 1–18 | 1363 |
| MMutrR | TCCACAAAACCTGTTTCAGTG | 1343–1364 | ||
| 2 | MM2s | TCATCAGAAAGAAGTCCTTGAG | 85–106 | 496 |
| MM5as | TAGGCCAGSCCCAKGATGCCATC | 558–580 | ||
| 3 | MM3s | GCTCCTCCAACCTGTGGGT | 307–325 | 560 |
| MM7as | CAGAGAGGTGCCKGTGTCMACAA | 844–866 | ||
| 4 | MM5s | GATGGCATCMTGGGSCTGGCCTA | 558–580 | 564 |
| MM9as | GAAGACATCWCCMAGGATCCAA | 1100–1121 | ||
| 5 | MM7s | TTGTKGACACMGGCACCTCTCTG | 844–866 | 520 |
| MMutrR | TCCACAAAACCTGTTTCAGTG | 1343–1364 | ||
| Primers Name | Sequence (5′–3′) | Position (bp) a | Amplicon Length (bp) a | |
|---|---|---|---|---|
| 1 | MMstart | AGTTGGGACCCGGGAAGA | 1–18 | 725 |
| MM2as | ATCCAGGTAGTTCTCCAGGG | 706–725 | ||
| 2 | MM2s | TCATCAGAAAGAAGTCCTTGAG | 571–592 | 1789 |
| MMintronBr | ATTCTCCTGCCTCAACCTCCCAA | 2337–2359 | ||
| 3 | MMintronB | CTCCGCATAGCCTGATCCCTT | 1180–1200 | 1180 |
| MMintronBr | ATTCTCCTGCCTCAACCTCCCAA | 2337–2359 | ||
| 4 | MMintronB3 | CCTCCTGCAGATATTGTATGTCC | 1429–1451 | 1616 |
| MM3as | ACCCACAGGTTGGAGGAGCC | 3025–3044 | ||
| 5 | MMintronB2 | TGTGAGGAATGAAGGAAAAGATGG | 2840–2863 | 1533 |
| MMintronDr | GGTGCTGCATGTCGGGAGAA | 4353–4372 | ||
| 6 | MMintronC | GCTGTAGAATAGCCCACCAGG | 3381–3401 | 992 |
| MMintronDr | GGTGCTGCATGTCGGGAGAA | 4353–4372 | ||
| 7 | MMintronC | GCTGTAGAATAGCCCACCAGG | 3381–3401 | 1663 |
| MMintronEr | AAGACCCTCTCCATCGCACCCA | 5022–5043 | ||
| 8 | MMintronD(N) | AGTCCTGCATGAGATGAACCA | 4636–4656 | 1284 |
| MMintronEr3 | CTTAAGGACTTGAGGGTGGAGGTC | 5896–5919 | ||
| 9 | MMintronE3 | GCACAACTCAAATGTCATCAGCCA | 5178–5201 | 742 |
| MMintronEr3 | CTTAAGGACTTGAGGGTGGAGGTC | 5896–5919 | ||
| 10 | MMintronE3 | GCACAACTCAAATGTCATCAGCCA | 5178–5201 | 1325 |
| MMintronFr2 | CTGGGGGGATTCTGGAAAGCTGA | 6480–6502 | ||
| 14 | MM6sens | AGTGGCAGCGTGGTGATCTTTG | 6301–6322 | 1311 |
| MM7as | CAGAGAGGTGCCKGTGTCMACAA | 7589–7611 | ||
| 15 | MMintronF2 | TCAGCTTTCCAGAATCCCCCCAG | 6480–6502 | 1132 |
| MM7as | CAGAGAGGTGCCKGTGTCMACAA | 7589–7611 | ||
| 16 | MMintronF | TGGATGGGTGGGGAAGAAATGT | 7438–7459 | 1650 |
| MM9as | GAAGACATCWCCMAGGATCCAA | 9066–9087 | ||
| 17 | MM8s | GACATCGTCTTCACCATCAAT | 7822–7842 | 1266 |
| MM9as | GAAGACATCWCCMAGGATCCAA | 9066–9087 | ||
| 18 | MMintronF | TGGATGGGTGGGGAAGAAATGT | 7438–7459 | 405 |
| MM8as | ATTGATGGTGAAGACGATGTC | 7822–7842 | ||
| 19 | MM992 | GAGCTGCATCAGTGGCTTCC | 9012–9031 | 318 |
| MMutrR | TCCACAAAACCTGTTTCAGTG | 9309–9329 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewska, M.; Lipka, A.; Panasiewicz, G.; Gowkielewicz, M.; Jozwik, M.; Majewski, M.K.; Szafranska, B. Identification of Novel Placentally Expressed Aspartic Proteinase in Humans. Int. J. Mol. Sci. 2017, 18, 1227. https://doi.org/10.3390/ijms18061227
Majewska M, Lipka A, Panasiewicz G, Gowkielewicz M, Jozwik M, Majewski MK, Szafranska B. Identification of Novel Placentally Expressed Aspartic Proteinase in Humans. International Journal of Molecular Sciences. 2017; 18(6):1227. https://doi.org/10.3390/ijms18061227
Chicago/Turabian StyleMajewska, Marta, Aleksandra Lipka, Grzegorz Panasiewicz, Marek Gowkielewicz, Marcin Jozwik, Mariusz Krzysztof Majewski, and Bozena Szafranska. 2017. "Identification of Novel Placentally Expressed Aspartic Proteinase in Humans" International Journal of Molecular Sciences 18, no. 6: 1227. https://doi.org/10.3390/ijms18061227