Depigmenting Effect of Resveratrol Is Dependent on FOXO3a Activation without SIRT1 Activation
Abstract
:1. Introduction
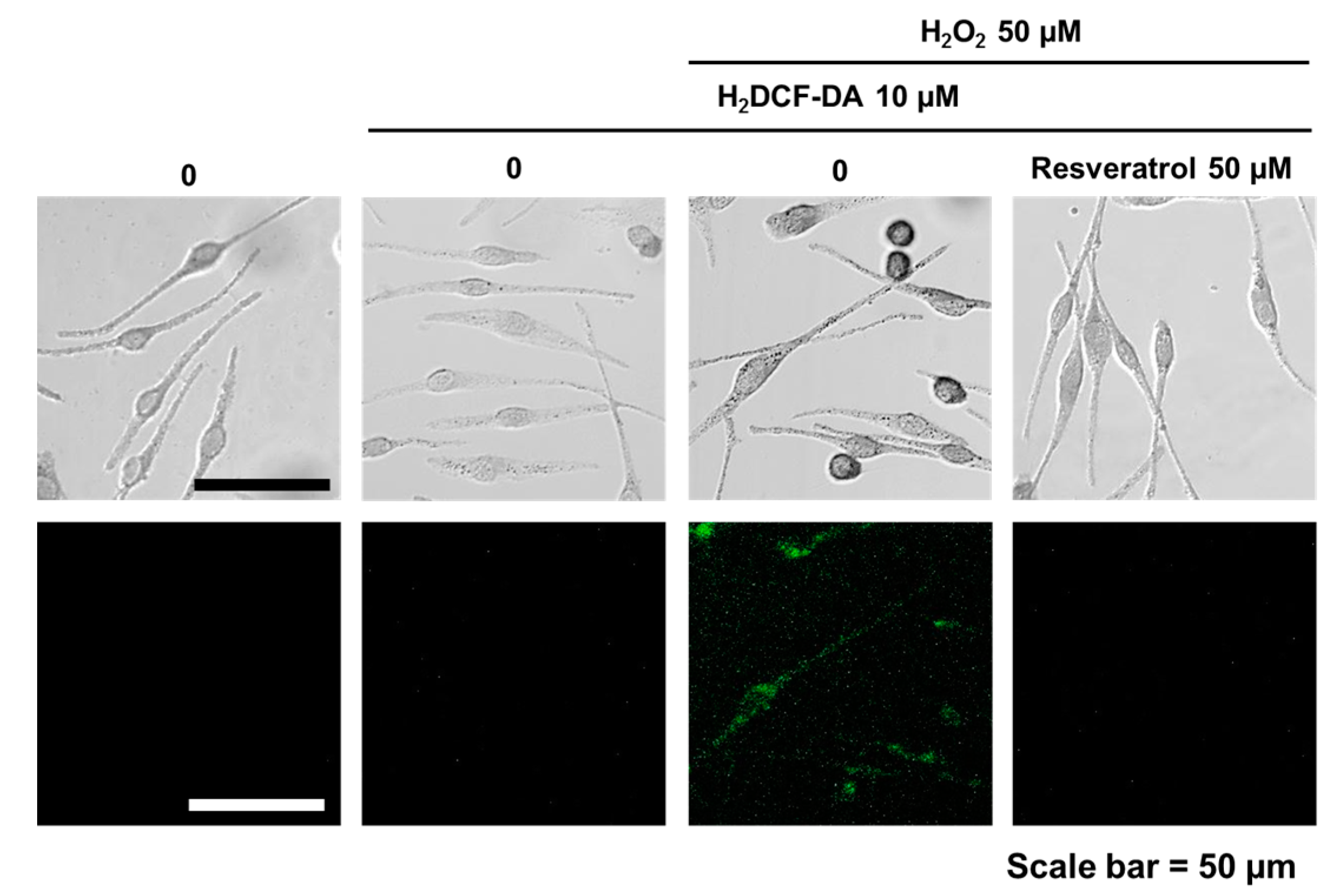
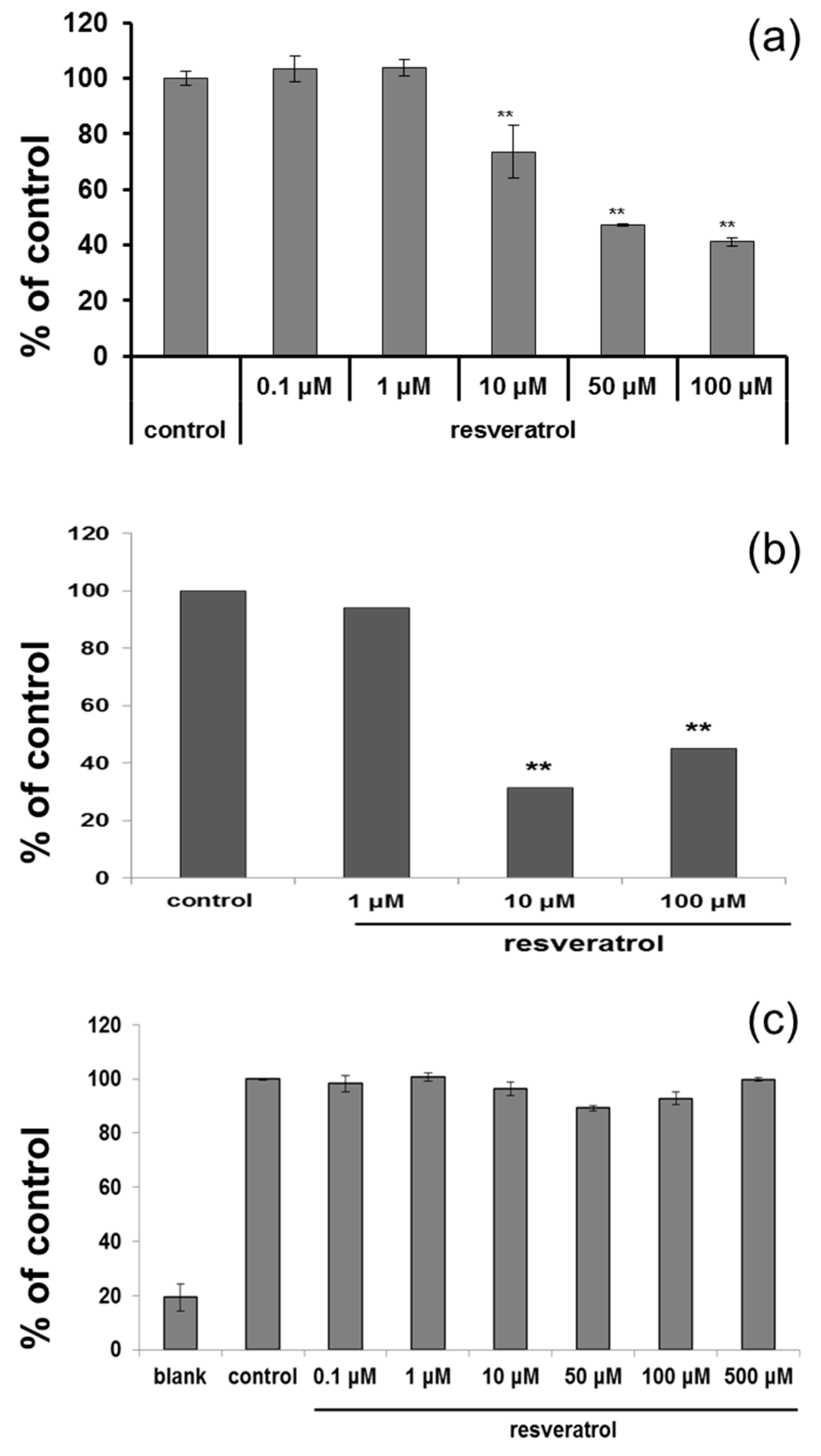
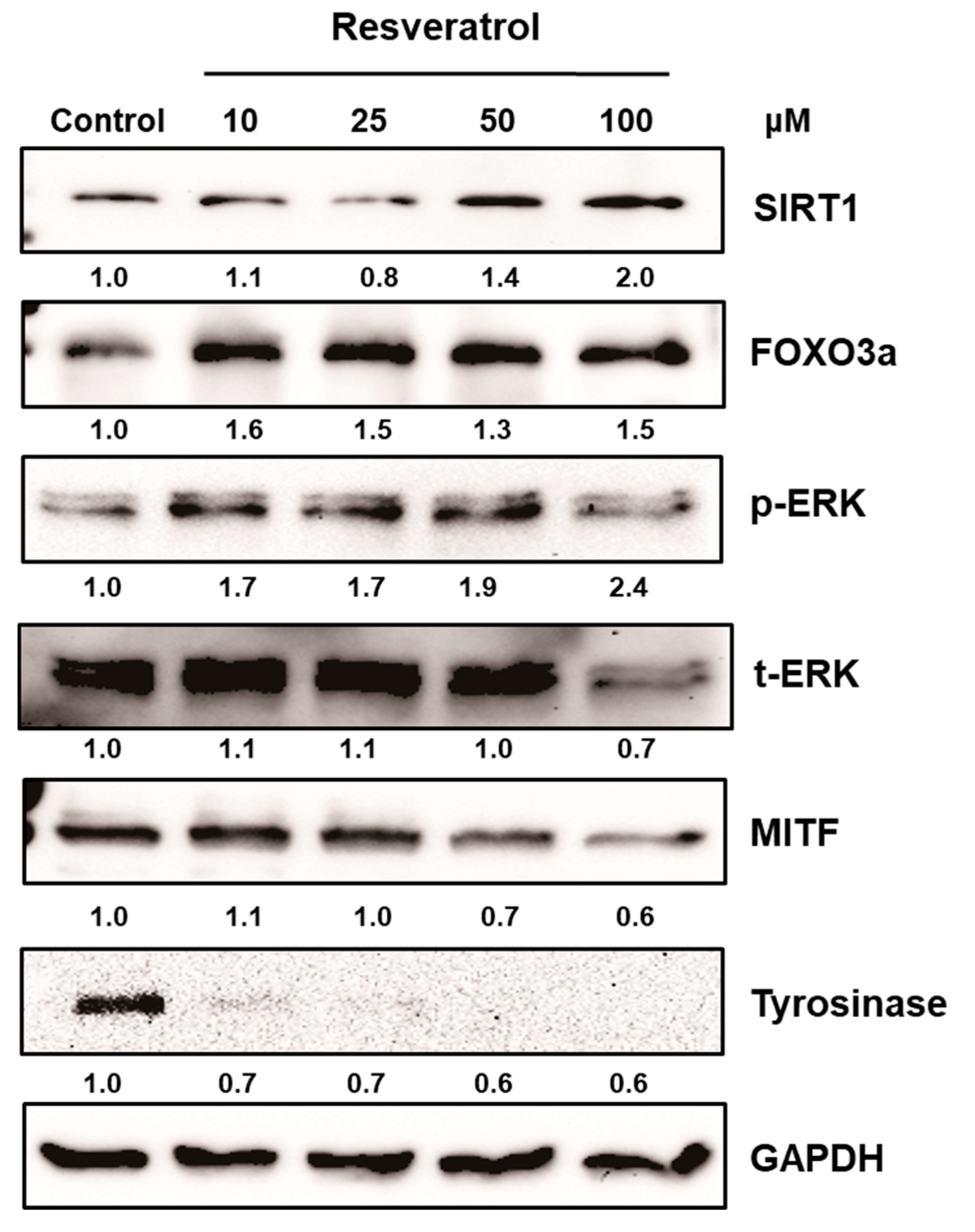
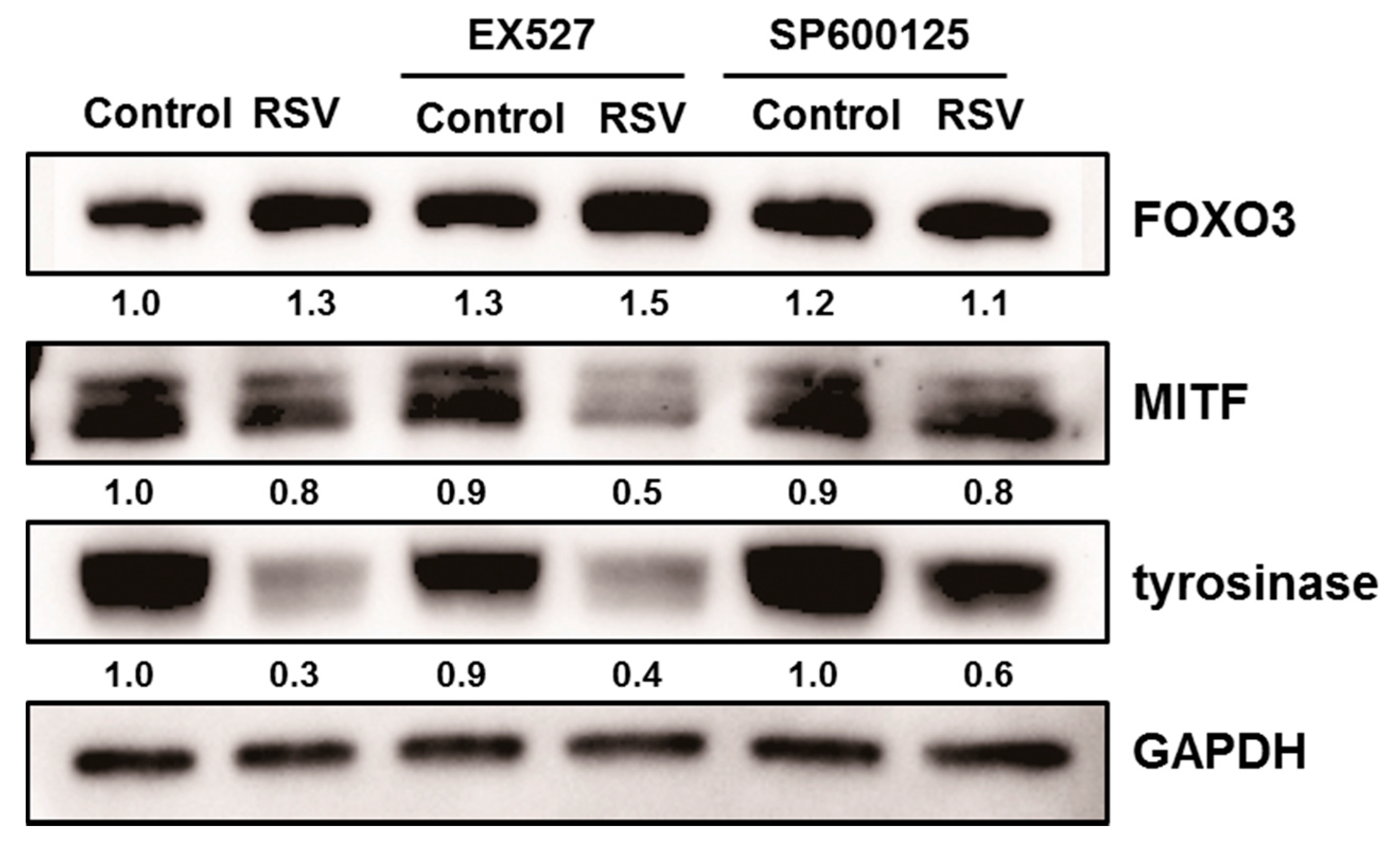
2. Results
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. DPPH Assay of Resveratrol
4.3. ROS Scavenging Effects
4.4. Cell Culture and Viability
4.5. Tyrosinase Activity and Melanin Amount
4.6. Western Blot Analysis
4.7. Fluorescence Microscopic Examination
4.8. Statistics
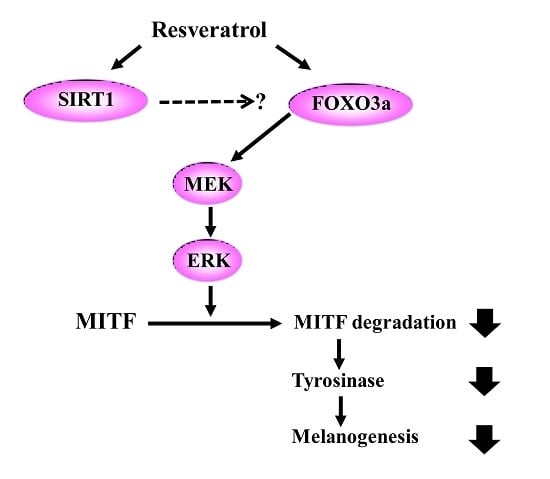
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MITF | Microphthalmia-associated transcription factor |
| SIRT1 | Sirtuin-1 |
| FOXO | Forkhead box O |
| UV | Ultraviolet |
| IL | Interleukin |
| HGF | Hepatocyte growth factor |
| ET | Endothelin |
| KGF | Keratinocyte growth factor |
| SCF | Stem cell factor |
| bFGF | Basic fibroblast growth factor |
| α-MSH | α-melanocyte-stimulating hormone |
| ROS | Reactive oxygen species |
| AMPK | AMP-activated protein kinase |
| IGF | Insulin growth factor |
| l-DOPA | l-3,4-dihydroxyphenylalanine |
| ERK | Extracellular signal-regulate kinase |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
References
- Okazaki, M.; Yoshimura, K.; Uchida, G.; Harii, K. Correlation between age and the secretions of melanocyte-stimulating cytokines in cultured keratinocytes and fibroblasts. Br. J. Dermatol. 2005, 153 (Suppl. 2), 23–29. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Cardinali, G.; Aspite, N.; Cota, C.; Luzi, F.; Bellei, B.; Briganti, S.; Amantea, A.; Torrisi, M.R.; Picardo, M. Role of fibroblast-derived growth factors in regulating hyperpigmentation of solar lentigo. Br. J. Dermatol. 2010, 163, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.A.; Schwarz, T. Evidence for an epidermal cytokine network. J. Investig. Dermatol. 1990, 95 (Suppl. 6), 100S–104S. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Miyagishi, M.; Yada, Y. Endothelin-1 as a new melanogen: Coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J. Investig. Dermatol. 1995, 105, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Schauer, E.; Trautinger, F.; Kock, A.; Schwarz, A.; Bhardwaj, R.; Simon, M.; Ansel, J.C.; Schwarz, T.; Luger, T.A. Proopiomelanocortin-derived peptides are synthesized and released by human keratinocytes. J. Clin. Investig. 1994, 93, 2258–2262. [Google Scholar] [CrossRef] [PubMed]
- Smit, N.; Le Poole, I.; van den Wijngaard, R.; Tigges, A.; Westerhof, W.; Das, P. Expression of different immunological markers by cultured human melanocytes. Arch. Dermatol. Res. 1993, 285, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Iwamoto, M.; Masuda, T.; Tagami, H. Stimulatory effect of prostaglandin E2 on the configuration of normal human melanocytes in vitro. J. Investig. Dermatol. 1987, 89, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kim, J.H.; Kim, E.K. Repeated exposure of human fibroblasts to UVR induces secretion of stem cell factor and senescence. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1577–1580. [Google Scholar] [CrossRef] [PubMed]
- Torres-Alvarez, B.; Mesa-Garza, I.G.; Castanedo-Cazares, J.P.; Fuentes-Ahumada, C.; Oros-Ovalle, C.; Navarrete-Solis, J.; Moncada, B. Histochemical and immunohistochemical study in melasma: Evidence of damage in the basal membrane. Am. J. Dermatopathol. 2011, 33, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.B.; Babiarz, L.; Liebel, F.; Roydon Price, E.; Kizoulis, M.; Gendimenico, G.J.; Fisher, D.E.; Seiberg, M. Modulation of microphthalmia-associated transcription factor gene expression alters skin pigmentation. J. Investig. Dermatol. 2002, 119, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, C.; Busca, R.; Abbe, P.; Bille, K.; Aberdam, E.; Ortonne, J.P.; Ballotti, R. Different cis-acting elements are involved in the regulation of TRP1 and TRP2 promoter activities by cyclic AMP: Pivotal role of M boxes (GTCATGTGCT) and of microphthalmia. Mol. Cell. Biol. 1998, 18, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Bentley, N.J.; Eisen, T.; Goding, C.R. Melanocyte-specific expression of the human tyrosinase promoter: Activation by the microphthalmia gene product and role of the initiator. Mol. Cell. Biol. 1994, 14, 7996–8006. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.A.; Cook, A.L.; Roberts, D.W.; Leonard, J.H.; Sturm, R.A. Post-transcriptional regulation of melanin biosynthetic enzymes by cAMP and resveratrol in human melanocytes. J. Investig. Dermatol. 2007, 127, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Yun, J.; Lee, C.K.; Lee, H.; Min, K.R.; Kim, Y. Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J. Biol. Chem. 2002, 277, 16340–16344. [Google Scholar] [CrossRef] [PubMed]
- Kairisalo, M.; Bonomo, A.; Hyrskyluoto, A.; Mudo, G.; Belluardo, N.; Korhonen, L.; Lindholm, D. Resveratrol reduces oxidative stress and cell death and increases mitochondrial antioxidants and XIAP in PC6.3-cells. Neurosci. Lett. 2011, 488, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, K.C.; Kwon, S.B.; Kim, D.S. Hypopigmentary effects of 4-n-butylresorcinol and resveratrol in combination. Pharmazie 2012, 67, 542–546. [Google Scholar] [PubMed]
- Nakae, J.; Biggs, W.H., 3rd; Kitamura, T.; Cavenee, W.K.; Wright, C.V.; Arden, K.C.; Accili, D. Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat. Genet. 2002, 32, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Holzenberger, M.; Dupont, J.; Ducos, B.; Leneuve, P.; Geloen, A.; Even, P.C.; Cervera, P.; Le Bouc, Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 2003, 421, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Lee, D.F.; Xia, W.; Golfman, L.S.; Ou-Yang, F.; Yang, J.Y.; Zou, Y.; Bao, S.; Hanada, N.; Saso, H.; et al. IκB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 2004, 117, 225–237. [Google Scholar] [CrossRef]
- Paik, J.H.; Kollipara, R.; Chu, G.; Ji, H.; Xiao, Y.; Ding, Z.; Miao, L.; Tothova, Z.; Horner, J.W.; Carrasco, D.R.; et al. FOXOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 2007, 128, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Nikolaev, A.Y.; Imai, S.; Chen, D.; Su, F.; Shiloh, A.; Guarente, L.; Gu, W. Negative control of p53 by Sir2αpromotes cell survival under stress. Cell 2001, 107, 137–148. [Google Scholar] [CrossRef]
- Vaziri, H.; Dessain, S.K.; Ng Eaton, E.; Imai, S.I.; Frye, R.A.; Pandita, T.K.; Guarente, L.; Weinberg, R.A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001, 107, 149–159. [Google Scholar] [CrossRef]
- Huang, H.; Tindall, D.J. Dynamic FoxO transcription factors. J. Cell Sci. 2007, 120, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.S.; Kuno, A.; Hosoda, R.; Horio, Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS ONE 2013, 8, e73875. [Google Scholar] [CrossRef] [PubMed]
- Camins, A.; Sureda, F.X.; Junyent, F.; Verdaguer, E.; Folch, J.; Pelegri, C.; Vilaplana, J.; Beas-Zarate, C.; Pallas, M. Sirtuin activators: Designing molecules to extend life span. Biochim. Biophys. Acta 2010, 1799, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. Sirt1 and the Mitochondria. Mol. Cells 2016, 39, 87–95. [Google Scholar] [PubMed]
- Horio, Y.; Hayashi, T.; Kuno, A.; Kunimoto, R. Cellular and molecular effects of sirtuins in health and disease. Clin. Sci. 2011, 121, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Duranton, A.; Lan, F.; Weikel, K.A.; Breton, L.; Ruderman, N.B. Resveratrol prevents oxidative stress-induced senescence and proliferative dysfunction by activating the AMPK-FOXO3 cascade in cultured primary human keratinocytes. PLoS ONE 2015, 10, e0115341. [Google Scholar] [CrossRef] [PubMed]
- Gunschmann, C.; Stachelscheid, H.; Akyuz, M.D.; Schmitz, A.; Missero, C.; Bruning, J.C.; Niessen, C.M. Insulin/IGF-1 controls epidermal morphogenesis via regulation of FOXO-mediated p63 inhibition. Dev. Cell 2013, 26, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, H.; Cho, E.G.; Lee, T.R. FOXO3a is an antimelanogenic factor that mediates antioxidant-induced depigmentation. J. Investig. Dermatol. 2014, 134, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Ozel Turkcu, U.; Solak Tekin, N.; Gokdogan Edgunlu, T.; Karakas Celik, S.; Oner, S. The association of FOXO3a gene polymorphisms with serum FOXO3a levels and oxidative stress markers in vitiligo patients. Gene 2014, 536, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Eisinger, M.; Marko, O. Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc. Natl. Acad. Sci. USA 1982, 79, 2018–2022. [Google Scholar] [CrossRef] [PubMed]
- Medrano, E.E.; Nordlund, J.J. Successful culture of adult human melanocytes obtained from normal and vitiligo donors. J. Investig. Dermatol. 1990, 95, 441–445. [Google Scholar] [PubMed]
- Choi, H.R.; Kang, Y.A.; Lee, H.S.; Park, K.C. Disulfanyl peptide decreases melanin synthesis via receptor-mediated ERK activation and the subsequent downregulation of MITF and tyrosinase. Int. J. Cosmet. Sci. 2016, 38, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Busca, R.; Bertolotto, C.; Ortonne, J.P.; Ballotti, R. Inhibition of the phosphatidylinositol 3-kinase/p70(S6)-kinase pathway induces B16 melanoma cell differentiation. J. Biol. Chem. 1996, 271, 31824–31830. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.J.; Kim, H.J.; Kim, S.Y.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Wan Kyun, W.; Choi, H.R.; Park, K.C.; Kim, D.S. Hypopigmentary effects of ethyl P-methoxycinnamate isolated from Kaempferia galanga. Phytother. Res. 2014, 28, 274–279. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, S.-H.; Choi, H.-R.; Kang, Y.-A.; Park, K.-C. Depigmenting Effect of Resveratrol Is Dependent on FOXO3a Activation without SIRT1 Activation. Int. J. Mol. Sci. 2017, 18, 1213. https://doi.org/10.3390/ijms18061213
Kwon S-H, Choi H-R, Kang Y-A, Park K-C. Depigmenting Effect of Resveratrol Is Dependent on FOXO3a Activation without SIRT1 Activation. International Journal of Molecular Sciences. 2017; 18(6):1213. https://doi.org/10.3390/ijms18061213
Chicago/Turabian StyleKwon, Soon-Hyo, Hye-Ryung Choi, Youn-A Kang, and Kyoung-Chan Park. 2017. "Depigmenting Effect of Resveratrol Is Dependent on FOXO3a Activation without SIRT1 Activation" International Journal of Molecular Sciences 18, no. 6: 1213. https://doi.org/10.3390/ijms18061213