Novel Structurally Related Flavones Augment Cell Death Induced by rhsTRAIL
Abstract
:1. Introduction
2. Results
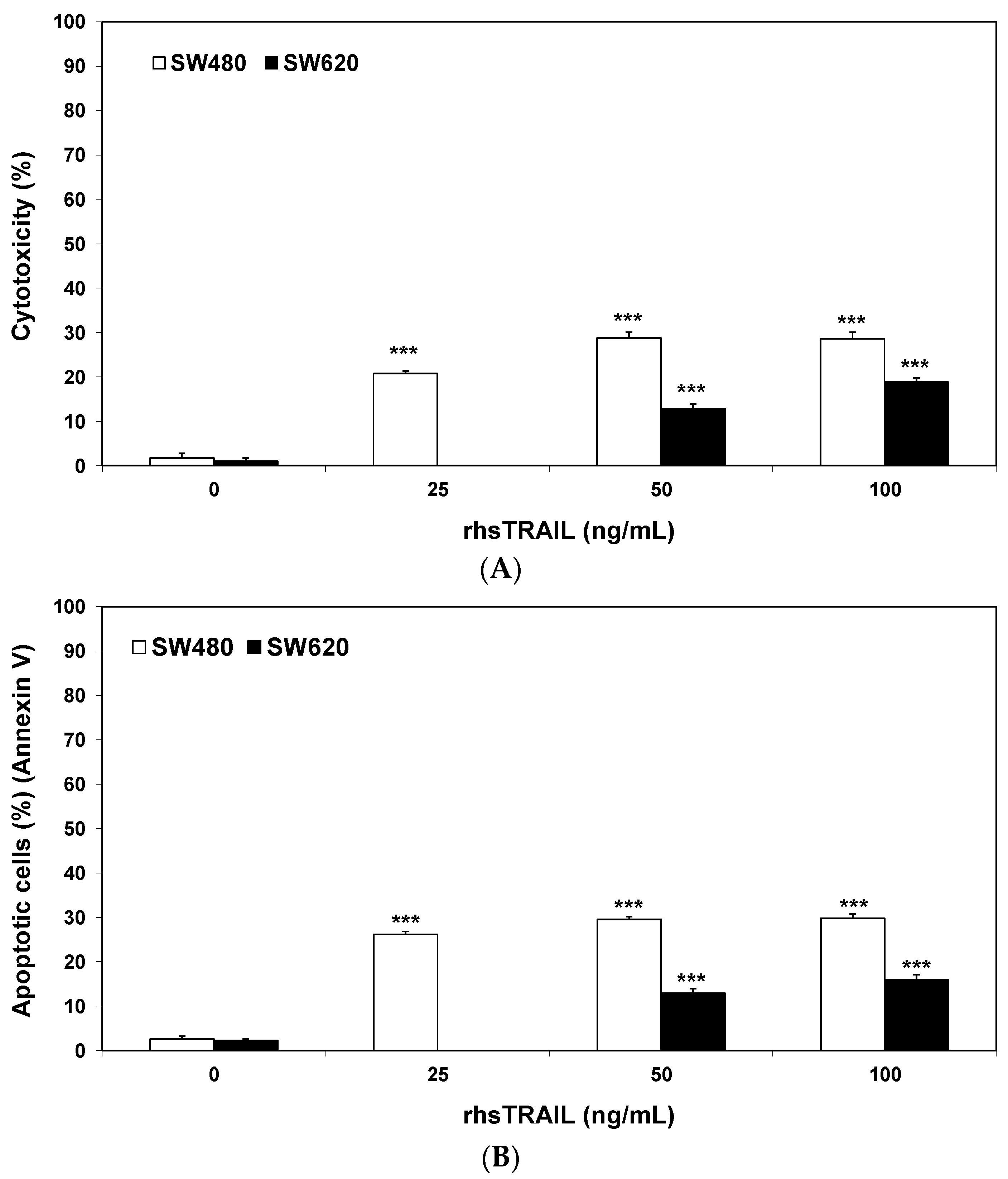
2.1. Cytotoxic and Apoptotic Effects of Flavones in Colon Cancer Cells
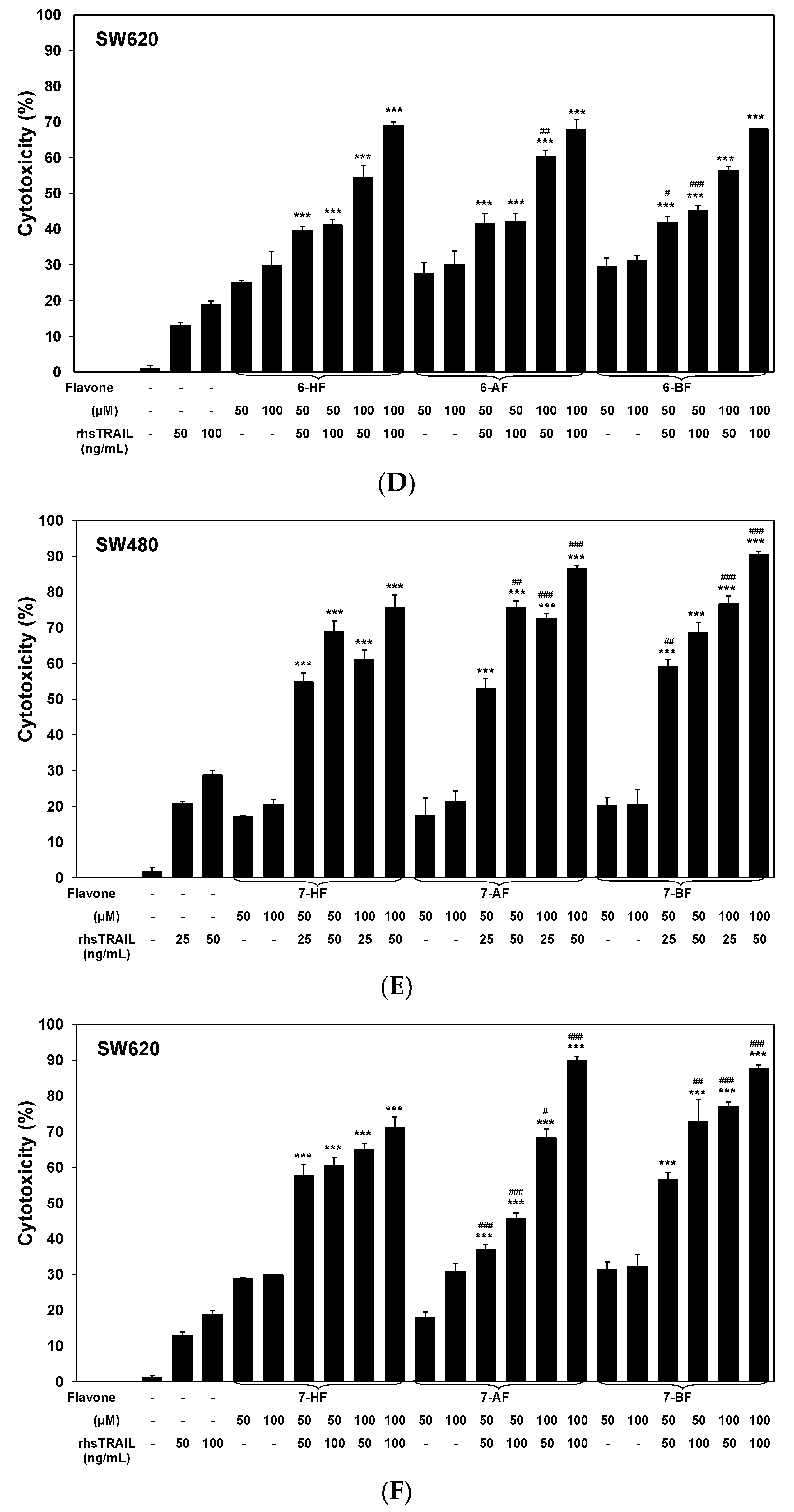
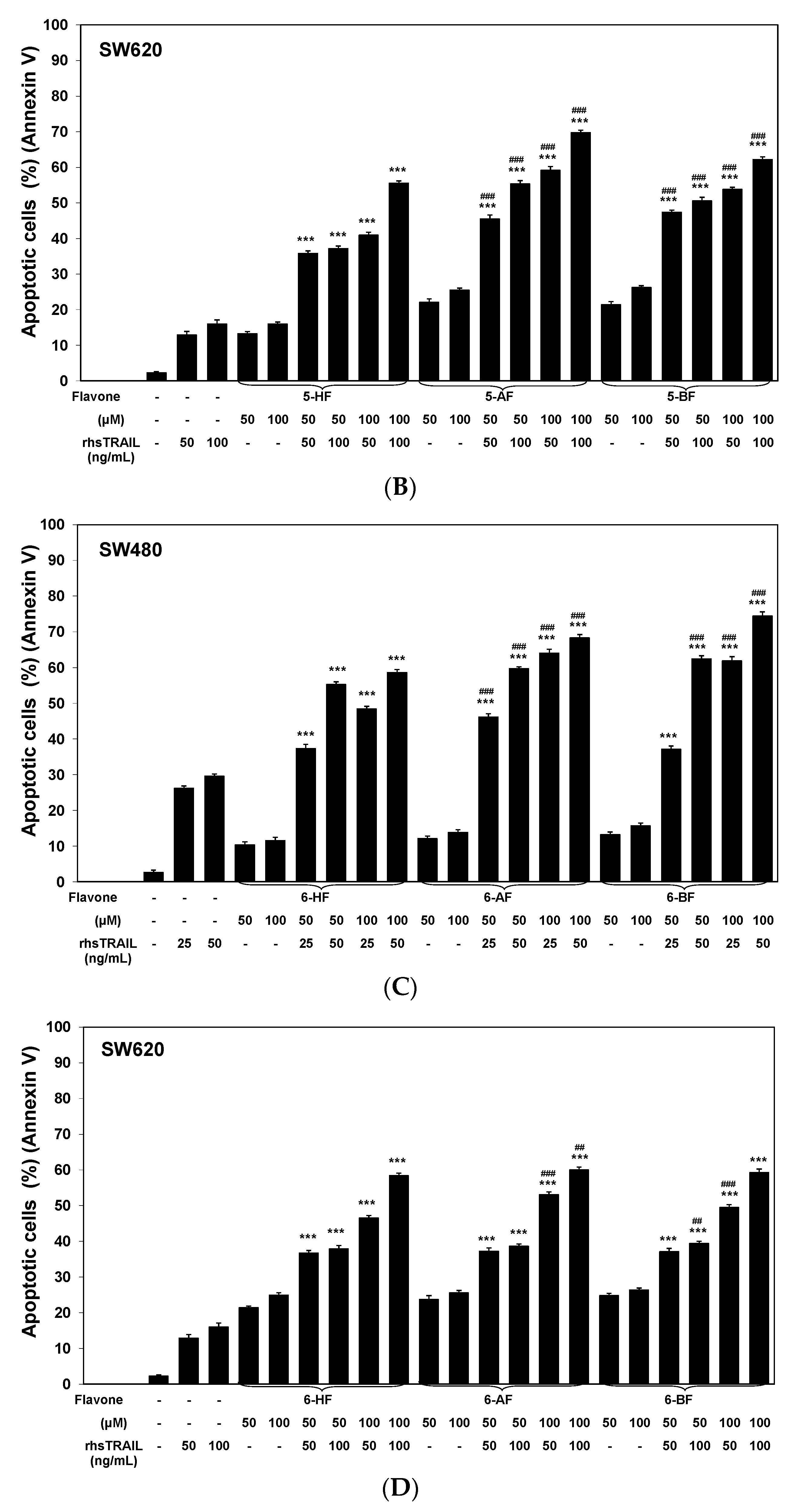
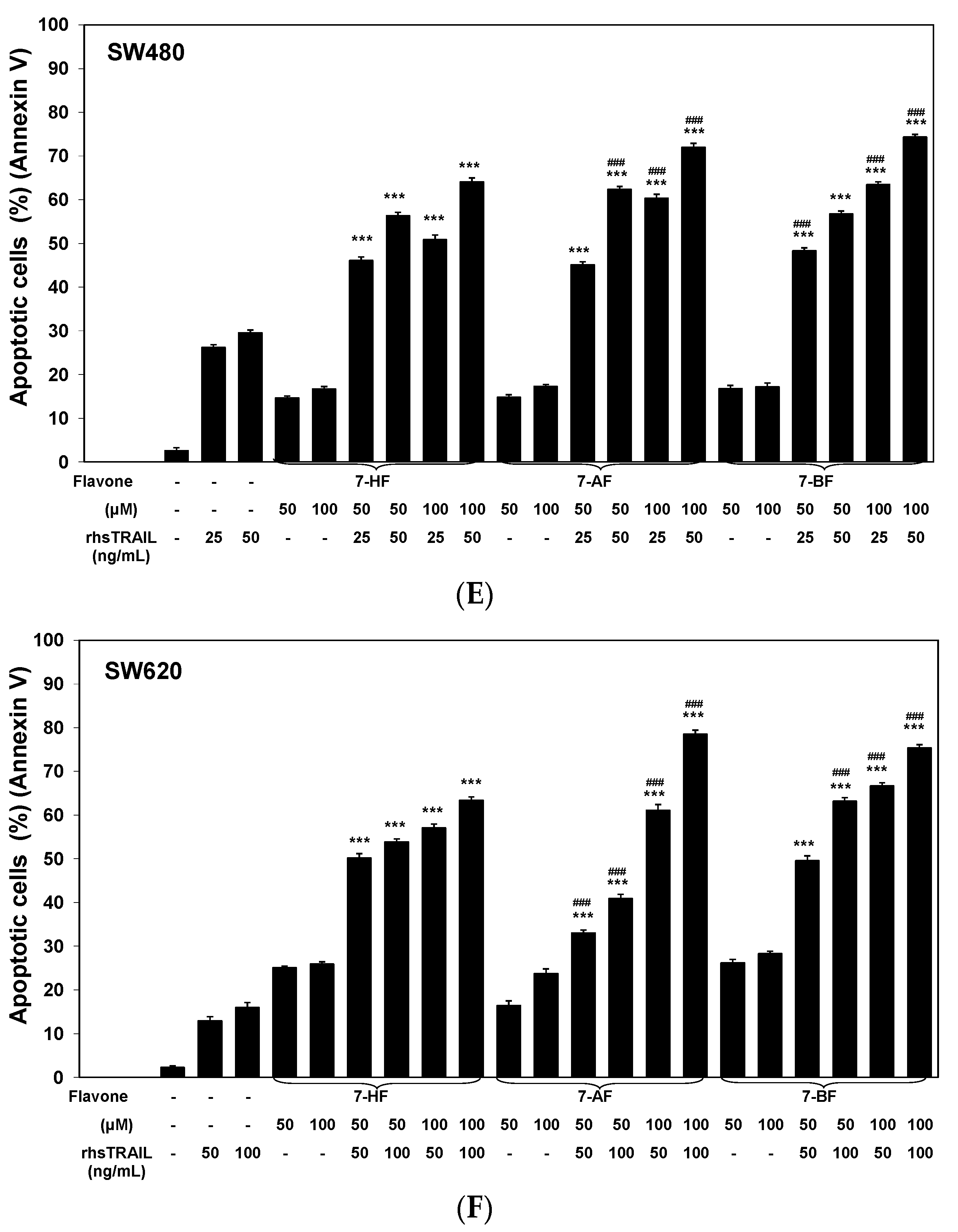
2.2. Cytotoxic and Apoptotic Effects of TRAIL in Combination with Flavones in Colon Cancer Cells
3. Discussion
4. Materials and Methods
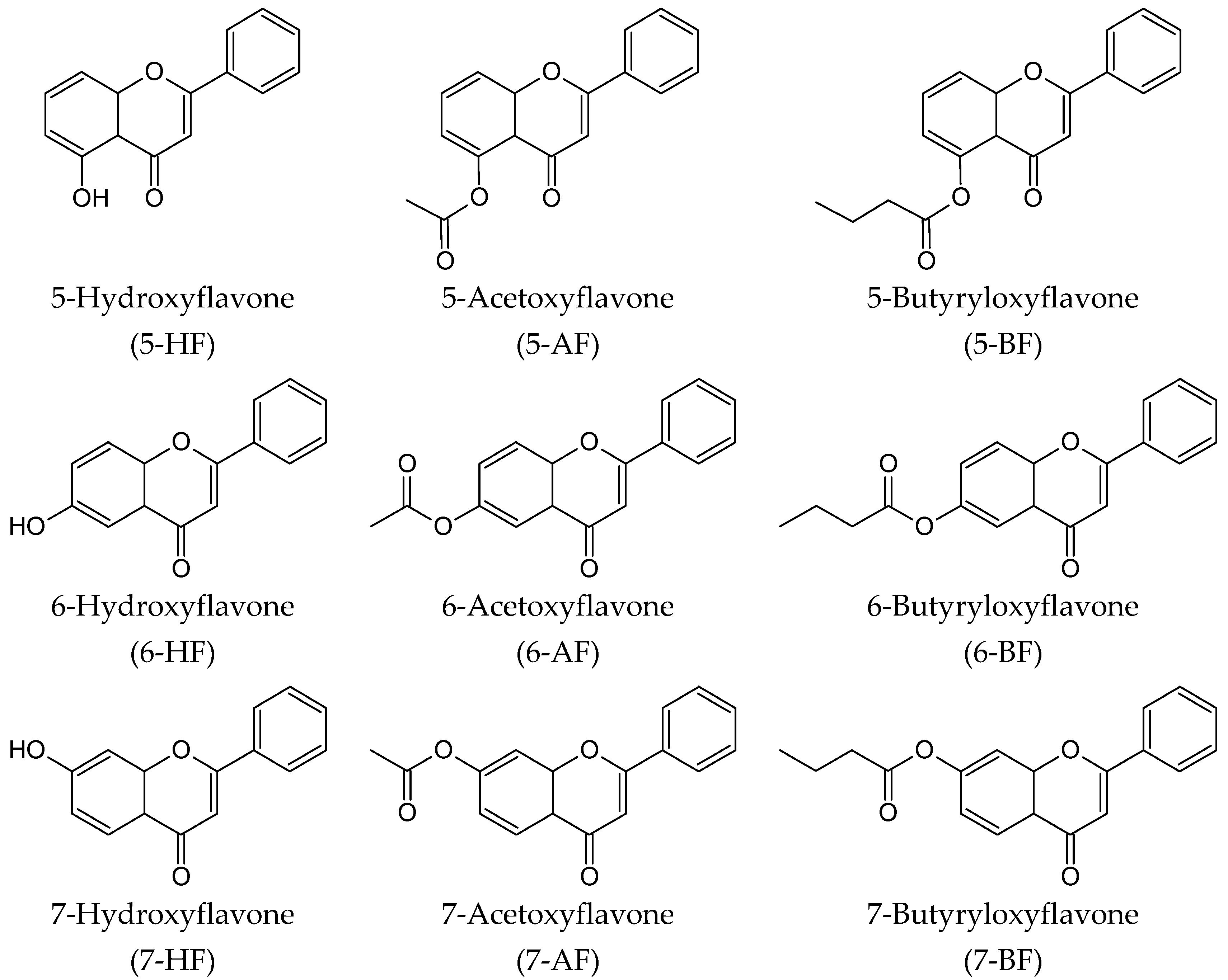
4.1. Chemistry
General
4.2. General Procedure for the Esterification of 5-HF, 6-HF and 7-HF
4.3. Biological Methods
4.3.1. Reagents
4.3.2. Cell Culture
4.3.3. Detection of Cell Death Using the MTT Colorimetric Assay
4.3.4. Lactate Dehydrogenase Release Assay
4.3.5. Determination of Apoptosis by Flow Cytometry with Annexin V-FITC Staining
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TRAIL | tumor necrosis factor-related apoptosis-inducing |
| V-FITC | annexin fluorescence staining ligand |
| TNF | tumor necrosis factor |
| LDH | lactate dehydrogenase assay |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMSO | dimethylsulfoxide |
| DMF | dimethylformamide |
| PBS | phosphate-buffered saline |
References
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones and flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007, 18, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Chen, C.; Wu, C.; Motonishi, K.; Kung, H.; Lam, K. Novel flavonoids with antiproliferative activities against breast cancer cells. J. Med. Chem. 2011, 54, 4339–4349. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef] [PubMed]
- Ghiringhelli, F.; Rebe, C.; Hichami, A.; Delmas, D. Immunomodulation and anti-inflammatory roles of polyphenols as anticancer agents. Anticancer Agents Med. Chem. 2012, 12, 852–873. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Krol, W. The role of dietary polyphenols in tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-induced apoptosis for cancer chemoprevention. Eur. J. Cancer Prev. 2011, 20, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Schauss, A.G. Mitigation of inflammation with foods. J. Agric. Food Chem. 2012, 60, 6703–6717. [Google Scholar] [CrossRef] [PubMed]
- Almasan, A.; Ashkenazi, A. Apo2L/TRAIL: Apoptosis signaling, biology and potential for cancer therapy. Cytokine Growth Factor Rev. 2003, 14, 337–348. [Google Scholar] [CrossRef]
- Mellier, G.; Huang, S.; Shenoy, K.; Pervaiz, S. TRAILing death in cancer. Mol. Aspects Med. 2010, 31, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Huerta-Yepez, S.; Vega, M.; Baritaki, S.; Spandidos, D.A.; Bonavida, B. The NO TRAIL to YES TRAIL in cancer therapy. Int. J. Oncol. 2007, 685–691. [Google Scholar] [CrossRef]
- Jakóbisiak, M.; Lasek, W.; Gołąb, J. Natural mechanisms protecting against cancer. Immunol. Lett. 2003, 90, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Holland, P.M. Targeting Apo2L/TRAIL receptors by soluble Apo2L/TRAIL. Cancer Lett. 2013, 332, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Secchiero, P.; Zauli, G. State of art and recent developments of anticancer strategies based on TRAIL. Recent Pat. Anticancer Drug Discov. 2012, 7, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.; Behbakht, K.; Ford, H. TRAIL receptor-targeted therapeutics: Resistance mechanisms and strategies to avoid them. Drug Resist. Updat. 2008, 11, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Mazur, B.; Zydowicz, G.; Czuba, Z.P.; Krol, W. TRAIL-induced apoptosis and expression of death receptor TRAIL-R1 and TRAIL-R2 in bladder cancer cells. Folia Histochem. Cytobiol. 2009, 47, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Pallone, F.; Monteleone, G. Molecular targets of TRAIL-sensitizing agents in colorectal cancer. Int. J. Mol. Sci. 2012, 13, 7886–7901. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Mertas, A.; Paradysz, A.; Krol, W. The dietary isoflavone biochanin-A sensitizes prostate cancer cells to TRAIL-induced apoptosis. Urol. Oncol. 2013, 31, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Newsom-Davis, T.; Prieske, S.; Walczak, H. Is TRAIL the holy grail of cancer therapy? Apoptosis 2009, 14, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Javed, K.; Ahmad, S.; Rathish, I.; Singh, S.; Chaitanya, M.; Arunasree, K.M.; Alam, M.S. Synthesis of some novel chalcones, flavanones and flavones and evaluation of their anti-inflammatory activity. Eur. J. Med. Chem. 2013, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Shukla, S.; Gupta, S. Apigenin and cancer chemoprevention: Progress, potential and promise (review). Int. J. Oncol. 2007, 30, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Esa, N.M. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: A review. Asian Pac. J. Cancer Prev. 2014, 15, 5501–5508. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, M.; Marder, M.; Blank, V.C.; Roguin, L.P. Antitumor activity of some natural flavonoids and synthetic derivatives on various human and murine cancer cell lines. Bioorg. Med. Chem. 2006, 14, 2966–2971. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Zydowicz, G.; Janoszka, B.; Dobosz, C.; Kowalczyk-Ziomek, G.; Krol, W. Ethanolic extract of Brazilian green propolis sensitizes prostate cancer cells to TRAIL-induced apoptosis. Int. J. Oncol. 2011, 38, 941–953. [Google Scholar] [PubMed]
- Jacquemin, G.; Shirley, S.; Micheau, O. Combining naturally occurring polyphenols with TNF-related apoptosis-inducing ligand: A promising approach to kill resistant cancer cells? Cell Mol. Life Sci. 2010, 67, 3115–3130. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, U.; Kuntz, S.; Brendel, M.D.; Daniel, H. Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells. Cancer Res. 2000, 60, 3823–3831. [Google Scholar] [PubMed]
- Choi, S.U.; Ryu, S.Y.; Yoon, S.K.; Jung, N.P.; Park, S.H.; Kim, K.H.; Choi, E.J.; Lee, C.O. Effects of flavonoids on the growth and cell cycle of cancer cells. Anticancer Res. 1999, 19, 5229–5233. [Google Scholar] [PubMed]
- Hirano, T.; Oka, K.; Akiba, M. Antiproliferative effects of synthetic and naturally occurring flavonoids on tumor cells of the human breast carcinoma cell line, ZR-75–15-1. Res. Commun. Chem. Pathol. Pharmacol. 1989, 64, 69–78. [Google Scholar] [PubMed]
- Chang, H.; Mi, M.T.; Gu, Y.Y.; Yuan, J.L.; Ling, W.H.; Lin, H. Effects of flavonoids with different structures on proliferation of leukemia cell line HL-60. Ai Zheng 2007, 26, 1309–1314. [Google Scholar] [PubMed]
- Zhang, T.; Chen, X.; Qu, L.; Wu, J.; Cui, R.; Zhao, Y. Chrysin and its phosphate ester inhibit cell proliferation and induce apoptosis in Hela cells. Bioorg. Med. Chem. 2004, 12, 6097–6105. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.B.; Rahumatullah, A.; Yogarajah, T.; Ahmad, M.; Yin, K.B. Potential effects of chrysin on MDA-MB-231 cells. Int. J. Mol. Sci. 2010, 11, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Afshari, J.T.; Davoodi, S. Chrysin reduces proliferation and induces apoptosis in the human prostate cancer cell line pc-3. Clinics 2011, 66, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Turktekin, M.; Konac, E.; Onen, H.I.; Alp, E.; Yilmaz, A.; Menevse, S. Evaluation of the effects of the flavonoid apigenin on apoptotic pathway gene expression on the colon cancer cell line (HT29). J. Med. Food 2011, 14, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Kilani-Jaziri, S.; Frachet, V.; Bhouri, W.; Ghedira, K.; Chekir-Ghedira, L.; Ronot, X. Flavones inhibit the proliferation of human tumor cancer cell lines by inducing apoptosis. Drug Chem. Toxicol. 2012, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, J.; Samadder, A.; Boujedaini, N.; Khuda-Bukhsh, A.R. Apigenin-induced apoptosis in A375 and A549 cells through selective action and dysfunction of mitochondria. Exp. Biol. Med. 2012, 237, 1433–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mao, Y.; Chen, H.; Lin, Y.; Hu, Z.; Wu, J.; Xu, X.; Xu, X.; Qin, J.; Xie, L. Apigenin promotes apoptosis, inhibits invasion and induces cell cycle arrest of T24 human bladder cancer cells. Cancer Cell Int. 2013, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, H.J.; Kim, H.R.; Lee, S.H.; Cho, S.D.; Choi, C.S.; Nam, J.S.; Jung, J.Y. Antitumor actions of baicalein and wogonin in HT-29 human colorectal cancer cells. Mol. Med. Rep. 2012, 6, 1443–1449. [Google Scholar] [PubMed]
- Lim, Y.; Jeong, Y.; Tyner, A.L.; Park, J.H. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G66–G75. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, S.; Chen, J.; Zhang, Q.; Lin, S.; Chen, Z.; Jiang, J. Luteolin induces mitochondria-dependent apoptosis in human lung adenocarcinoma cell. Nat. Prod. Commun. 2012, 7, 29–32. [Google Scholar] [PubMed]
- George, V.C.; Naveen-Kumar, D.R.; Suresh, P.K.; Kumar, S.; Kumar, R.A. Comparative studies to evaluate relative in vitro potency of luteolin in inducing cell cycle arrest and apoptosis in HaCaT and A375 cells. Asian Pac. J. Cancer Prev. 2013, 14, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Dharmalingam, P.; Sadagopan, S.K.; Ramar, M.; Munusamy, A.; Ganapasam, S. Luteolin induces growth arrest in colon cancer cells through involvement of Wnt/β-catenin/GSK-3β signaling. J. Environ. Pathol. Toxicol. Oncol. 2013, 32, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Seol, D.W. TRAIL, a mighty apoptosis inducer. Mol. Cells 2003, 15, 283–293. [Google Scholar] [PubMed]
- Marsters, S.A.; Pitti, R.A.; Sheridan, J.P.; Ashkenazi, A. Control of apoptosis signaling by Apo2 ligand. Recent Prog. Horm. Res. 1999, 54, 225–234. [Google Scholar] [PubMed]
- Szliszka, E.; Zydowicz, G.; Mizgala, E.; Krol, W. Artepillin C (3,5-diprenyl-4-hydroxycinnamic acid) sensitizes prostate cancer LNCaP cells to TRAIL-induced apoptosis. Int. J. Oncol. 2012, 41, 818–828. [Google Scholar] [PubMed]
- Tanaguchi, H.; Yoshida, T.; Horinaka, M.; Yasuda, T.; Goda, A.E.; Konishi, M.; Wakada, M.; Kataoka, K.; Yoshikawa, T.; Sakai, T. Baicalein overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance via two different cell specific pathways in cancer cells but not in normal cells. Cancer. Res. 2008, 68, 8918–8927. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.K.; Lam, W.S.; Chiu, L.C.; Ooi, V.E.; Sun, S.S.; Wong, Y.S. A rice bran polyphenol, cycloartenyl ferulate, elicits apoptosis in human colorectal adenocarcinoma SW480 and sensitizes metastatic SW620 cells to TRAIL-induced apoptosis. Biochem. Pharmacol. 2009, 77, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, X.; Zha, D.; Cai, F.; Zhang, W.; He, Y.; Huang, Q.; Zhuang, H.; Hua, Z.C. Apigenin potentiates TRAIL therapy of non-small cell lung cancer via upregulating DR4/DR5 expression in a p53-dependent manner. Sci. Rep. 2016, 6, 35468. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Kim, A.K. Apigenin Sensitizes Huh-7 Human Hepatocellular Carcinoma Cells to TRAIL-induced Apoptosis. Biomol. Ther. 2012, 20, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Kostrzewa-Susłow, E.; Bronikowska, J.; Jaworska, D.; Janeczko, T.; Czuba, Z.P.; Krol, W. Synthetic flavanones augment the anticancer effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Molecules 2012, 17, 11693–11711. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic extract of propolis (EEP) enhances the apoptosis-inducing potential of TRAIL in cancer cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Polier, G.; Köhler, R.; Giaisi, M.; Krammer, P.H.; Li-Weber, M. Wogonin and related natural flavones overcome tumor necrosis factor-related apoptosis inducing ligand (TRAIL) protein resistance of tumors by down-regulation of c-FLIP protein and up-regulation of TRAIL receptor 2 expression. J. Biol. Chem. 2012, 287, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Czuba, Z.P.; Bronikowska, J.; Mertas, A.; Paradysz, A.; Krol, W. Ethanolic extract of propolis (EEP) augments TRAIL-induced apoptotic death in prostate cancer cells. Evid. Based Complement. Altern. Med. 2011, 2011, 535172. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, J.N.; Huang, J.M.; Xiong, X.K.; Chen, M.F.; Ong, C.N.; Shen, H.M.; Yang, X.F. Chrysin promotes tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced apoptosis in human cancer cell lines. Toxicol. In Vitro 2011, 25, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Lirdprapamongkol, K.; Sakurai, H.; Abdelhamed, S.; Yokoyama, S.; Athikomkulchai, S.; Viriyaroj, A.; Awale, S.; Ruchirawat, S.; Svasti, J.; Saiki, I. Chrysin overcomes TRAIL resistance of cancer cells through Mcl-1 downregulation by inhibiting STAT3 phosphorylation. Int. J. Oncol. 2013, 43, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Sakai, T. The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor-related apoptosis-inducing ligand. Mol. Cancer Ther. 2006, 5, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Nakanishi, R.; Nishino, H.; Sakai, T. The combination of TRAIL and luteolin enhances apoptosis in human cervical cancer HeLa cells. Biochem. Biophys. Res. Commun. 2005, 333, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.X.; Ong, C.N.; Shen, H.M. Protein kinase C inhibition and x-linked inhibitor of apoptosis protein degradation contribute to the sensitization effect of luteolin on tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in cancer cells. Cancer Res. 2005, 65, 7815–7823. [Google Scholar] [PubMed]
- Yan, J.; Wang, Q.; Zheng, X.; Sun, H.; Zhou, Y.; Li, D.; Lin, Y.; Wang, X. Luteolin enhances TNF-related apoptosis-inducing ligand’s anticancer activity in a lung cancer xenograft mouse model. Biochem. Biophys. Res. Commun. 2012, 417, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.C.; Li, J.R.; Kuan, Y.H.; Raung, S.L.; Wang, C.C.; Hung, Y.Y.; Pan, P.H.; Lu, H.C.; Chen, CJ. Luteolin sensitizes human 786-O renal cell carcinoma cells to TRAIL-induced apoptosis. Life Sci. 2014, 100, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa-Susłow, E.; Janeczko, T. Microbial transformations of 5-hydroxy- and 5-methoxyflavone in Aspergillus niger and Penicillium chermesinum cultures. J. Microb. Biotech. Food Sci. 2014, 3, 448–452. [Google Scholar]
- Kostrzewa-Susłow, E.; Dmochowska-Gładysz, J.; Janeczko, T.; Środa, K.; Michalak, K.; Palko, A. Microbial transformations of 6-methoxyflavone and 7-methoxyflavone in Aspergillus niger and Penicillium chermesinum cultures. Z. Naturforsch. C 2012, 67, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa-Susłow, E.; Dmochowska-Gładysz, J.; Białońska, A.; Ciunik, Z.; Rymowicz, W. Microbial transformations of flavanone and 6-Hydroxyflavone by Aspergillus niger strains. J. Mol. Catal. B Enzym. 2006, 39, 18–23. [Google Scholar] [CrossRef]
- Kostrzewa-Susłow, E.; Janeczko, T. Microbial transformations of 7-Hydroxyflavone. Sci. World J. 2012. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa-Susłow, E.; Dymarska, M.; Białońska, A.; Janeczko, T. Enantioselective conversion of certain derivatives of 6-Hydroxyflavone. J. Mol. Catal. B Enzym. 2014, 102, 59–65. [Google Scholar] [CrossRef]
- Bronikowska, J.; Szliszka, E.; Jaworska, D.; Czuba, Z.P.; Krol, W. The coumarin psoralidin enhances anticancer effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Molecules 2012, 17, 6449–6464. [Google Scholar] [CrossRef] [PubMed]
- Kłósek, M.; Mertas, A.; Król, W.; Jaworska, D.; Szymszal, J.; Szliszka, E. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in prostate cancer cells after treatment with xanthohumol—A natural compound present in Humulus lupulus L. Int. J. Mol. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bronikowska, J.; Szliszka, E.; Kostrzewa-Susłow, E.; Jaworska, D.; Czuba, Z.P.; Bednarski, P.; Król, W. Novel Structurally Related Flavones Augment Cell Death Induced by rhsTRAIL. Int. J. Mol. Sci. 2017, 18, 1211. https://doi.org/10.3390/ijms18061211
Bronikowska J, Szliszka E, Kostrzewa-Susłow E, Jaworska D, Czuba ZP, Bednarski P, Król W. Novel Structurally Related Flavones Augment Cell Death Induced by rhsTRAIL. International Journal of Molecular Sciences. 2017; 18(6):1211. https://doi.org/10.3390/ijms18061211
Chicago/Turabian StyleBronikowska, Joanna, Ewelina Szliszka, Edyta Kostrzewa-Susłow, Dagmara Jaworska, Zenon P. Czuba, Piotr Bednarski, and Wojciech Król. 2017. "Novel Structurally Related Flavones Augment Cell Death Induced by rhsTRAIL" International Journal of Molecular Sciences 18, no. 6: 1211. https://doi.org/10.3390/ijms18061211





