Dendritic Cell-Airway Epithelial Cell Cross-Talk Changes with Age and Contributes to Chronic Lung Inflammatory Diseases in the Elderly
Abstract
:1. Introduction
2. Age-Associated Deterioration and/or Dysregulation of Human Dendritic Cell Functions
3. Age-Associated Changes in Airway Epithelial Cell (AEC) Functions
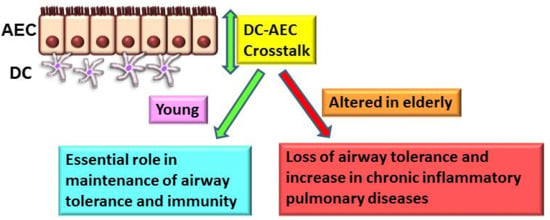
4. Immunological Cross-Talk between Airway Epithelial Cells (AECs) and Dendritic Cells (DCs) in Health and Disease
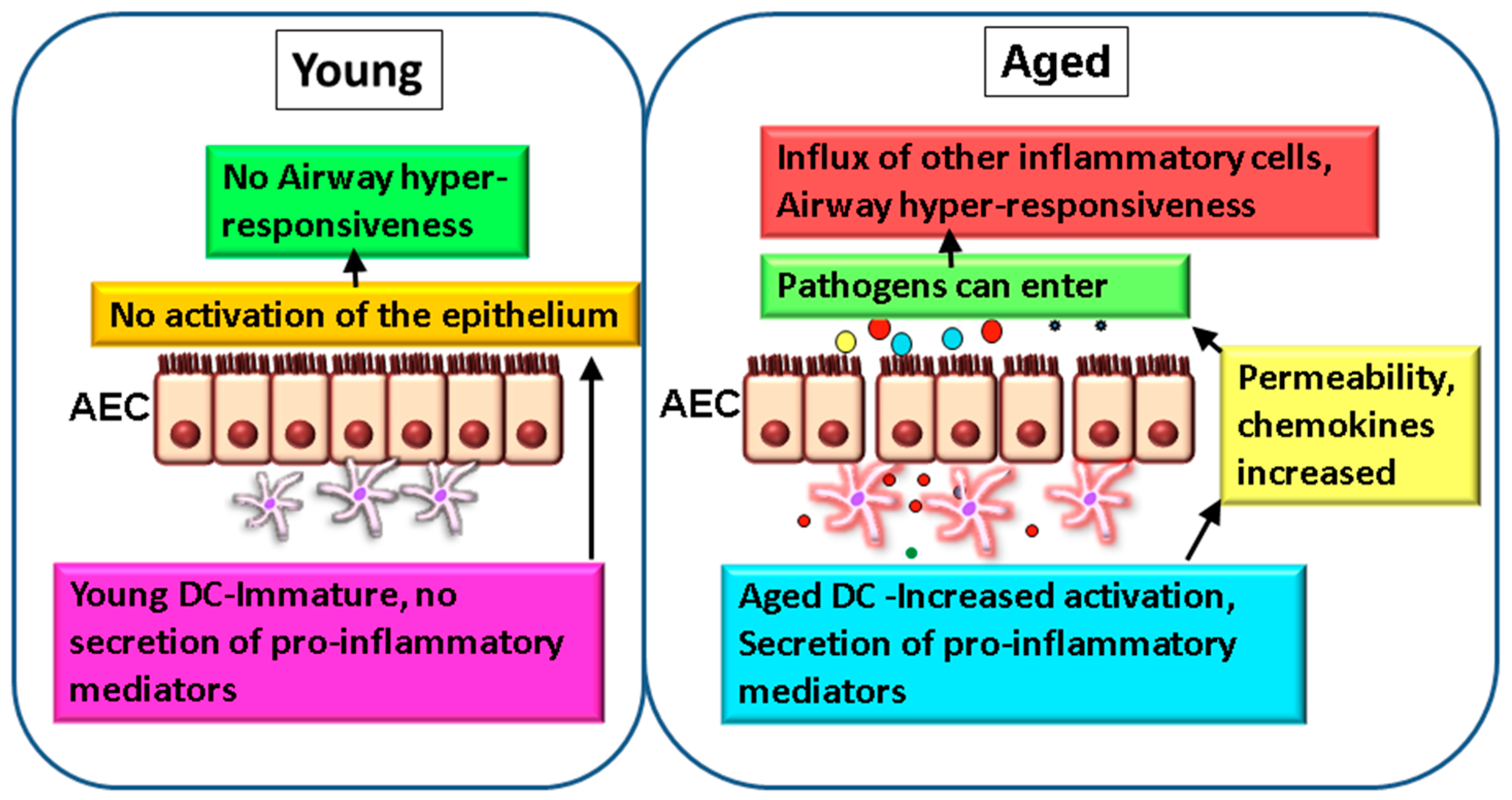
5. Age-Associated Alterations in the Crosstalk between Airway Epithelial Cells and Dendritic Cells: Effect of DCs on AECs
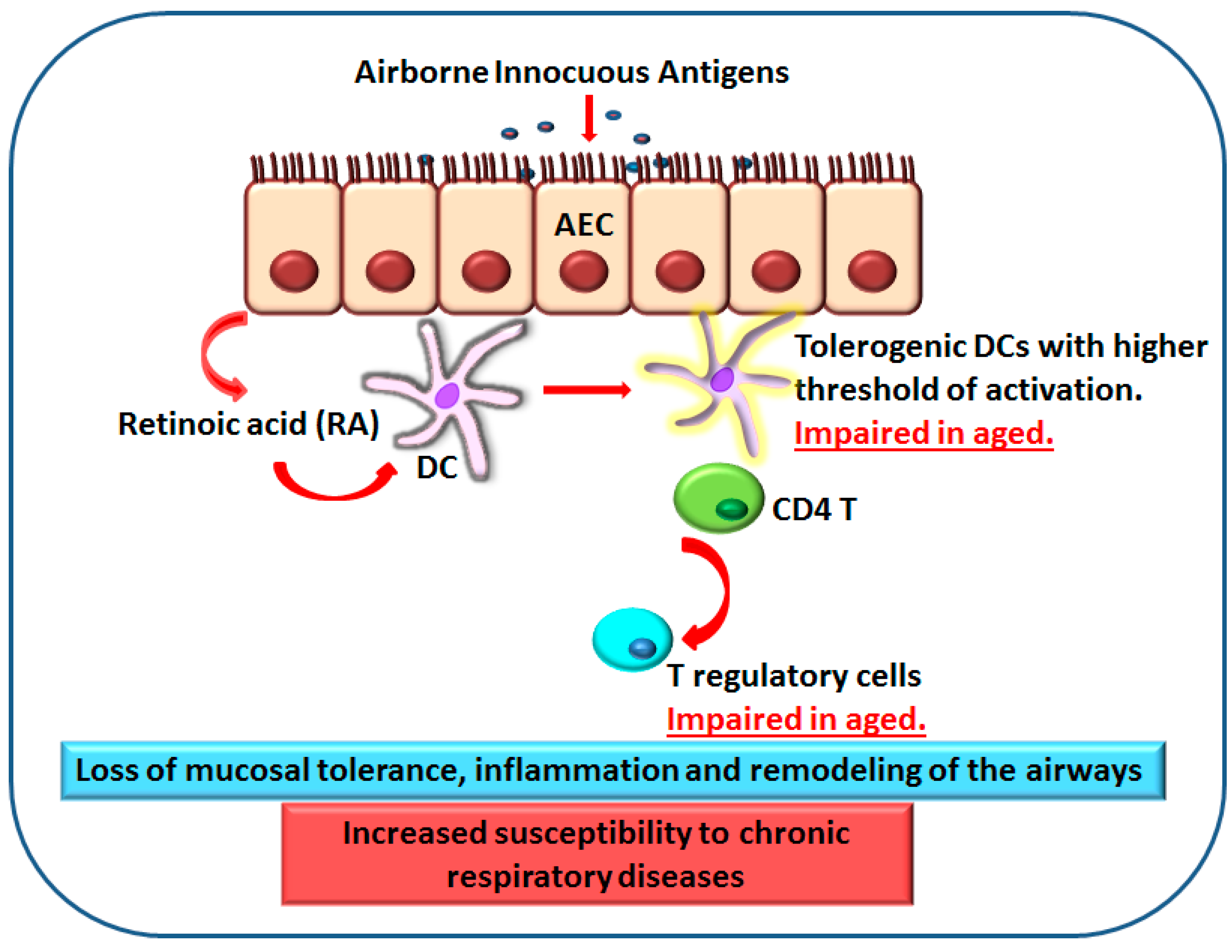
6. Age-Associated Alterations in the Crosstalk between Airway Epithelial Cells and Dendritic Cells: Effect of AECs on DCs
7. Microbial Metabolites and DC Function in the Airways
8. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Brandenberger, C.; Muhlfeld, C. Mechanisms of lung aging. Cell Tissue Res. 2017, 367, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Cellular Senescence and Lung Function during Aging. Yin and Yang. Ann. Am. Thorac. Soc. 2016, 13, S402–S406. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Selman, M. Lung Fibroblasts, Aging, and Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc. 2016, 13, S417–S421. [Google Scholar] [CrossRef] [PubMed]
- Vij, N.; Chandramani, P.; Westphal, C.V.; Hole, R.; Bodas, M. Cigarette smoke induced autophagy-impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am. J. Physiol. Cell Physiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Barnes, P.J. COPD as a disease of accelerated lung aging. Chest 2009, 135, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.; Sternberg, A.L.; Washko, G.; Make, B.J.; Han, M.K.; Martinez, F.; Criner, G.J.; National Emphysema Treatment Trial Research Group. Severe chronic bronchitis in advanced emphysema increases mortality and hospitalizations. COPD 2013, 10, 667–678. [Google Scholar]
- Lange, P.; Parner, J.; Prescott, E.; Vestbo, J. Chronic bronchitis in an elderly population. Age Ageing 2003, 32, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Gelb, A.F.; Yamamoto, A.; Mauad, T.; Kollin, J.; Schein, M.J.; Nadel, J.A. Unsuspected mild emphysema in nonsmoking patients with chronic asthma with persistent airway obstruction. J. Allergy Clin. Immunol. 2014, 133, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Chilosi, M.; Carloni, A.; Rossi, A.; Poletti, V. Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl. Res. 2013, 162, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, S.; Tsubouchi, H.; Miura, A.; Matsuo, A.; Matsumoto, N.; Nakazato, M. The Impacts of Cellular Senescence in Elderly Pneumonia and in Age-Related Lung Diseases that Increase the Risk of Respiratory Infections. Int. J. Mol. Sci. 2017, 18, 503. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.E. Two-way traffic on the bridge from innate to adaptive immunity. J. Investig. Dermatol. 2010, 130, 1773–1775. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. Lung dendritic cells in respiratory viral infection and asthma: From protection to immunopathology. Annu. Rev. Immunol. 2012, 30, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. Taking our breath away: Dendritic cells in the pathogenesis of asthma. Nat. Rev. Immunol. 2003, 3, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Chieppa, M.; Perros, F.; Willart, M.A.; Germain, R.N.; Lambrecht, B.N. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 2009, 15, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Sertl, K.; Takemura, T.; Tschachler, E.; Ferrans, V.J.; Kaliner, M.A.; Shevach, E.M. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J. Exp. Med. 1986, 163, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Agrawal, S.; Cao, J.N.; Su, H.; Osann, K.; Gupta, S. Altered innate immune functioning of dendritic cells in elderly humans: A role of phosphoinositide 3-kinase-signaling pathway. J. Immunol. 2007, 178, 6912–6922. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Tay, J.; Ton, S.; Agrawal, S.; Gupta, S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J. Immunol. 2009, 182, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, A.; Esposo, M.; Kaushal, K.; Tay, J.; Osann, K.; Agrawal, S.; Gupta, S.; Agrawal, A. Age-associated impaired plasmacytoid dendritic cell functions lead to decreased CD4 and CD8 T cell immunity. Age 2011, 33, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Agrawal, S.; Cao, J.N.; Gupta, S.; Agrawal, A. Impaired secretion of interferons by dendritic cells from aged subjects to influenza: Role of histone modifications. Age 2013, 35, 1785–1797. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Gollapudi, S.; Gupta, S.; Agrawal, A. Dendritic cells from the elderly display an intrinsic defect in the production of IL-10 in response to lithium chloride. Exp. Gerontol. 2013, 48, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: A mini-review. Gerontology 2013, 59, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Agrawal, A.; Jia, Z.; Sherman, E.; Gupta, S. Alterations in Gene Array Patterns in Dendritic Cells from Aged Humans. PLoS ONE 2014, 9, e106471. [Google Scholar] [CrossRef] [PubMed]
- Della Bella, S.; Clerici, M.; Villa, M.L. Disarming dendritic cells: A tumor strategy to escape from immune control? Expert Rev. Clin. Immunol. 2007, 3, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Shaheen, E.; Drake, R.R.; Chen, N.; Gravenstein, S.; Deng, Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 2009, 70, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Gupta, S. Impact of aging on dendritic cell functions in humans. Ageing Res. Rev. 2011, 10, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Agrawal, S.; Vahed, H.; Ngyuen, M.; Benmohamad, L.; Gupta, S.; Agrawal, A. Dendritic cells from aged subjects contribute to chronic airway inflammation by activating bronchial epithelial cells under steady state. Mucosal Immunol. 2014, 7, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Qian, F.; Mohanty, S.; van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Khokha, R.; Murthy, A.; Weiss, A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Manicone, A.M.; McGuire, J.K. Matrix metalloproteinases as modulators of inflammation. Semin. Cell Dev. Biol. 2008, 19, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Agrawal, S.; Ma, D.; Gupta, S.; Peterson, E.M.; Agrawal, A. Dendritic cells from aged subjects display enhanced inflammatory responses to Chlamydophila pneumoniae. Mediat. Inflamm. 2014, 2014, 436438. [Google Scholar] [CrossRef] [PubMed]
- Garbe, K.; Bratke, K.; Wagner, S.; Virchow, J.C.; Lommatzsch, M. Plasmacytoid dendritic cells and their Toll-like receptor 9 expression selectively decrease with age. Hum. Immunol. 2012, 73, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Stout-Delgado, H.W.; Yang, X.; Walker, W.E.; Tesar, B.M.; Goldstein, D.R. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J. Immunol. 2008, 181, 6747–6756. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Wang, X.; Zhang, L.; Lin, A.; Zhao, H.; Fikrig, E.; Montgomery, R.R. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile virus. J. Infect. Dis. 2011, 203, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Hermant, P.; Michiels, T. Interferon-lambda in the context of viral infections: Production, response and therapeutic implications. J. Innate Immun. 2014, 6, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Mordstein, M.; Neugebauer, E.; Ditt, V.; Jessen, B.; Rieger, T.; Falcone, V.; Sorgeloos, F.; Ehl, S.; Mayer, D.; Kochs, G.; et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. J. Virol. 2010, 84, 5670–5677. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Hernandez, J.Z.; Wimmenauer, V.; Shepherd, B.E.; Hijano, D.; Libster, R.; Serra, M.E.; Bhat, N.; Batalle, J.P.; Mohamed, Y.; et al. A mechanistic role for type III IFN-lambda1 in asthma exacerbations mediated by human rhinoviruses. Am. J. Respir. Crit. Care Med. 2012, 185, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Chan, K.N.; Hu, W.H.; Lam, W.K.; Zheng, L.; Tipoe, G.L.; Sun, J.; Leung, R.; Tsang, K.W. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am. J. Respir. Crit. Care Med. 2001, 163, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Svartengren, M.; Falk, R.; Philipson, K. Long-term clearance from small airways decreases with age. Eur. Respir. J. 2005, 26, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Busse, P.J.; Zhang, T.F.; Srivastava, K.; Schofield, B.; Li, X.M. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin. Exp. Allergy 2007, 37, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Busse, P.J.; Mathur, S.K. Age-related changes in immune function: Effect on airway inflammation. J. Allergy Clin. Immunol. 2010, 126, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Godin, L.M.; Sandri, B.J.; Wagner, D.E.; Meyer, C.M.; Price, A.P.; Akinnola, I.; Weiss, D.J.; Panoskaltsis-Mortari, A. Decreased Laminin Expression by Human Lung Epithelial Cells and Fibroblasts Cultured in Acellular Lung Scaffolds from Aged Mice. PLoS ONE 2016, 11, e0150966. [Google Scholar] [CrossRef] [PubMed]
- Tieu, D.D.; Kern, R.C.; Schleimer, R.P. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2009, 124, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ank, N.; Paludan, S.R. Type III IFNs: New layers of complexity in innate antiviral immunity. BioFactors 2009, 35, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.D.; Rochelle, L.G.; Fischer, B.M.; Krunkosky, T.M.; Adler, K.B. Airway epithelium as an effector of inflammation: Molecular regulation of secondary mediators. Eur. Respir. J. 1997, 10, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. Dendritic cells and epithelial cells: Linking innate and adaptive immunity in asthma. Nat. Rev. Immunol. 2008, 8, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, M.J.; Byers, D.E.; Alexander-Brett, J.; Wang, X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat. Rev. Immunol. 2014, 14, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.K.; Bartz, H.; Fey, F.; Schmidt, L.M.; Dalpke, A.H. Airway epithelial cells modify immune responses by inducing an anti-inflammatory microenvironment. Eur. J. Immunol. 2008, 38, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. Lung dendritic cells: Targets for therapy in allergic disease. Chem. Immunol. Allergy 2008, 94, 189–200. [Google Scholar] [PubMed]
- Lambrecht, B.N.; Hammad, H. Allergens and the airway epithelium response: Gateway to allergic sensitization. J. Allergy Clin. Immunol. 2014, 134, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Claudio, E.; Tassi, I.; Wang, H.; Tang, W.; Ha, H.L.; Siebenlist, U. Cutting Edge: IL-25 Targets Dendritic Cells To Attract IL-9-Producing T Cells in Acute Allergic Lung Inflammation. J. Immunol. 2015, 195, 3525–3529. [Google Scholar] [CrossRef] [PubMed]
- Rate, A.; Upham, J.W.; Bosco, A.; McKenna, K.L.; Holt, P.G. Airway epithelial cells regulate the functional phenotype of locally differentiating dendritic cells: Implications for the pathogenesis of infectious and allergic airway disease. J. Immunol. 2009, 182, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Croft, M. Control of regulatory T cells and airway tolerance by lung macrophages and dendritic cells. Ann. Am. Thorac. Soc. 2014, 11 (Suppl. 5), S306–S313. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Carcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Leepiyasakulchai, C.; Ignatowicz, L.; Pawlowski, A.; Kallenius, G.; Skold, M. Failure to recruit anti-inflammatory CD103+ dendritic cells and a diminished CD4+ Foxp3+ regulatory T cell pool in mice that display excessive lung inflammation and increased susceptibility to Mycobacterium tuberculosis. Infect. Immun. 2012, 80, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Manicassamy, S.; Pulendran, B. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Semin. Immunol. 2009, 21, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Baharom, F.; Thomas, S.; Rankin, G.; Lepzien, R.; Pourazar, J.; Behndig, A.F.; Ahlm, C.; Blomberg, A.; Smed-Sorensen, A. Dendritic Cells and Monocytes with Distinct Inflammatory Responses Reside in Lung Mucosa of Healthy Humans. J. Immunol. 2016, 196, 4498–4509. [Google Scholar] [CrossRef] [PubMed]
- Desch, A.N.; Gibbings, S.L.; Goyal, R.; Kolde, R.; Bednarek, J.; Bruno, T.; Slansky, J.E.; Jacobelli, J.; Mason, R.; Ito, Y.; et al. Flow Cytometric Analysis of Mononuclear Phagocytes in Nondiseased Human Lung and Lung-Draining Lymph Nodes. Am. J. Respir. Crit. Care Med. 2016, 193, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Granot, T.; Senda, T.; Carpenter, D.J.; Matsuoka, N.; Weiner, J.; Gordon, C.L.; Miron, M.; Kumar, B.V.; Griesemer, A.; Ho, S.H.; et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity 2017, 46, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Rate, A.; Bosco, A.; McKenna, K.L.; Holt, P.G.; Upham, J.W. Airway epithelial cells condition dendritic cells to express multiple immune surveillance genes. PLoS ONE 2012, 7, e44941. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Srivastava, R.; Rahmatpanah, F.; Madiraju, C.; BenMohamed, L.; Agrawal, A. Airway Epithelial Cells Enhance the Immunogenicity of Human Myeloid Dendritic Cells under Steady State. Clin. Exp. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A. Mucosal dendritic cells. Annu. Rev. Immunol. 2007, 25, 381–418. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Ko, H.J.; Kweon, M.N. Mucosal dendritic cells shape mucosal immunity. Exp. Mol. Med. 2014, 46, e84. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Stumbles, P.A.; McWilliam, A.S. Functional studies on dendritic cells in the respiratory tract and related mucosal tissues. J. Leukoc. Biol. 1999, 66, 272–275. [Google Scholar] [PubMed]
- Rothen-Rutishauser, B.; Mueller, L.; Blank, F.; Brandenberger, C.; Muehlfeld, C.; Gehr, P. A newly developed in vitro model of the human epithelial airway barrier to study the toxic potential of nanoparticles. Altex 2008, 25, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Blank, F.; Wehrli, M.; Lehmann, A.; Baum, O.; Gehr, P.; von Garnier, C.; Rothen-Rutishauser, B.M. Macrophages and dendritic cells express tight junction proteins and exchange particles in an in vitro model of the human airway wall. Immunobiology 2011, 216, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lewis, C.; Nadel, J.A. CCL20/CCR6 feedback exaggerates epidermal growth factor receptor-dependent MUC5AC mucin production in human airway epithelial (NCI-H292) cells. J. Immunol. 2011, 186, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Haczku, A. The dendritic cell niche in chronic obstructive pulmonary disease. Respir. Res. 2012, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Abonyo, B.O.; Alexander, M.S.; Heiman, A.S. Autoregulation of CCL26 synthesis and secretion in A549 cells: A possible mechanism by which alveolar epithelial cells modulate airway inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L478–L488. [Google Scholar] [CrossRef] [PubMed]
- Groom, J.R.; Luster, A.D. CXCR3 in T cell function. Exp. Cell Res. 2011, 317, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Wark, P.A.; Bucchieri, F.; Johnston, S.L.; Gibson, P.G.; Hamilton, L.; Mimica, J.; Zummo, G.; Holgate, S.T.; Attia, J.; Thakkinstian, A.; et al. IFN-gamma-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J. Allergy Clin. Immunol. 2007, 120, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Spurrell, J.C.; Wiehler, S.; Zaheer, R.S.; Sanders, S.P.; Proud, D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L85–L95. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, M.L.; Bernhardt, G.; Rodriguez-Barbosa, J.I.; Forster, R. Development and functional specialization of CD103+ dendritic cells. Immunol. Rev. 2010, 234, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Crozat, K.; Henri, S.; Tamoutounour, S.; Grenot, P.; Devilard, E.; de Bovis, B.; Alexopoulou, L.; Dalod, M.; Malissen, B. Skin-draining lymph nodes contain dermis-derived CD103(-) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood 2010, 115, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Napoli, J.L. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim. Biophys. Acta 2012, 1821, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Blomhoff, R.; Blomhoff, H.K. Overview of retinoid metabolism and function. J. Neurobiol. 2006, 66, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.H. Mechanisms of digestion and absorption of dietary vitamin A. Annu. Rev. Nutr. 2005, 25, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Gudas, L.J. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim. Biophys. Acta 2012, 1821, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Bchini, R.; Vasiliou, V.; Branlant, G.; Talfournier, F.; Rahuel-Clermont, S. Retinoic acid biosynthesis catalyzed by retinal dehydrogenases relies on a rate-limiting conformational transition associated with substrate recognition. Chem. Biol. Interact. 2013, 202, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Theodosiou, M.; Laudet, V.; Schubert, M. From carrot to clinic: An overview of the retinoic acid signaling pathway. Cell. Mol. Life Sci. 2010, 67, 1423–1445. [Google Scholar] [CrossRef] [PubMed]
- Pino-Lagos, K.; Benson, M.J.; Noelle, R.J. Retinoic acid in the immune system. Ann. N. Y. Acad. Sci. 2008, 1143, 170–187. [Google Scholar] [CrossRef] [PubMed]
- Soroosh, P.; Doherty, T.A.; Duan, W.; Mehta, A.K.; Choi, H.; Adams, Y.F.; Mikulski, Z.; Khorram, N.; Rosenthal, P.; Broide, D.H.; et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J. Exp. Med. 2013, 210, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lloyd, C.M.; Noble, A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol. 2013, 6, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.; Taube, C. Regulatory T cells and regulation of allergic airway disease. Am. J. Clin. Exp. Immunol. 2012, 1, 166–178. [Google Scholar] [PubMed]
- Strickland, D.H.; Wikstrom, M.E.; Turner, D.J.; Holt, P.G. Mucosal regulatory T cells in airway hyperresponsiveness. Chem. Immunol. Allergy 2008, 94, 40–47. [Google Scholar] [PubMed]
- Navarro, S.; Driscoll, B. Regeneration of the Aging Lung: A Mini-Review. Gerontology 2016, 63, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Wansleeben, C.; Bowie, E.; Hotten, D.F.; Yu, Y.R.; Hogan, B.L. Age-related changes in the cellular composition and epithelial organization of the mouse trachea. PLoS ONE 2014, 9, e93496. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Ganguly, S.; Tran, A.; Sundaram, P.; Agrawal, A. Retinoic acid treated human dendritic cells induce T regulatory cells via the expression of CD141 and GARP which is impaired with age. Aging 2016, 8, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.R.; Stetson, C.L.; Daugherty, C.; Shimizu, I.; Syapin, P.J.; Garrel, G.; Cohen-Tannoudji, J.; Huhtaniemi, I.; Slominski, A.T.; Pruitt, K.; et al. Up-regulation of steroid biosynthesis by retinoid signaling: Implications for aging. Mech. Ageing Dev. 2015, 150, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, M.L.; Rodriguez-Barbosa, J.I.; Kremmer, E.; Forster, R. CD103- and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J. Immunol. 2007, 178, 6861–6866. [Google Scholar] [CrossRef] [PubMed]
- Haniffa, M.; Shin, A.; Bigley, V.; McGovern, N.; Teo, P.; See, P.; Wasan, P.S.; Wang, X.N.; Malinarich, F.; Malleret, B.; et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 2012, 37, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Nastasi, C.; Candela, M.; Bonefeld, C.M.; Geisler, C.; Hansen, M.; Krejsgaard, T.; Biagi, E.; Andersen, M.H.; Brigidi, P.; Odum, N.; et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 2015, 5, 16148. [Google Scholar] [CrossRef] [PubMed]
- Whiteson, K.; Agrawal, S.; Agrawal, A. Differential responses of human dendritic cells to metabolites from the oral/airway microbiome. Clin. Exp. Immunol. 2017, 188, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Whelan, F.J.; Verschoor, C.P.; Stearns, J.C.; Rossi, L.; Luinstra, K.; Loeb, M.; Smieja, M.; Johnstone, J.; Surette, M.G.; Bowdish, D.M. The loss of topography in the microbial communities of the upper respiratory tract in the elderly. Ann. Am. Thorac. Soc. 2014, 11, 513–521. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, A. Dendritic Cell-Airway Epithelial Cell Cross-Talk Changes with Age and Contributes to Chronic Lung Inflammatory Diseases in the Elderly. Int. J. Mol. Sci. 2017, 18, 1206. https://doi.org/10.3390/ijms18061206
Agrawal A. Dendritic Cell-Airway Epithelial Cell Cross-Talk Changes with Age and Contributes to Chronic Lung Inflammatory Diseases in the Elderly. International Journal of Molecular Sciences. 2017; 18(6):1206. https://doi.org/10.3390/ijms18061206
Chicago/Turabian StyleAgrawal, Anshu. 2017. "Dendritic Cell-Airway Epithelial Cell Cross-Talk Changes with Age and Contributes to Chronic Lung Inflammatory Diseases in the Elderly" International Journal of Molecular Sciences 18, no. 6: 1206. https://doi.org/10.3390/ijms18061206