Biochemical and Computational Insights on a Novel Acid-Resistant and Thermal-Stable Glucose 1-Dehydrogenase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sequence Analysis
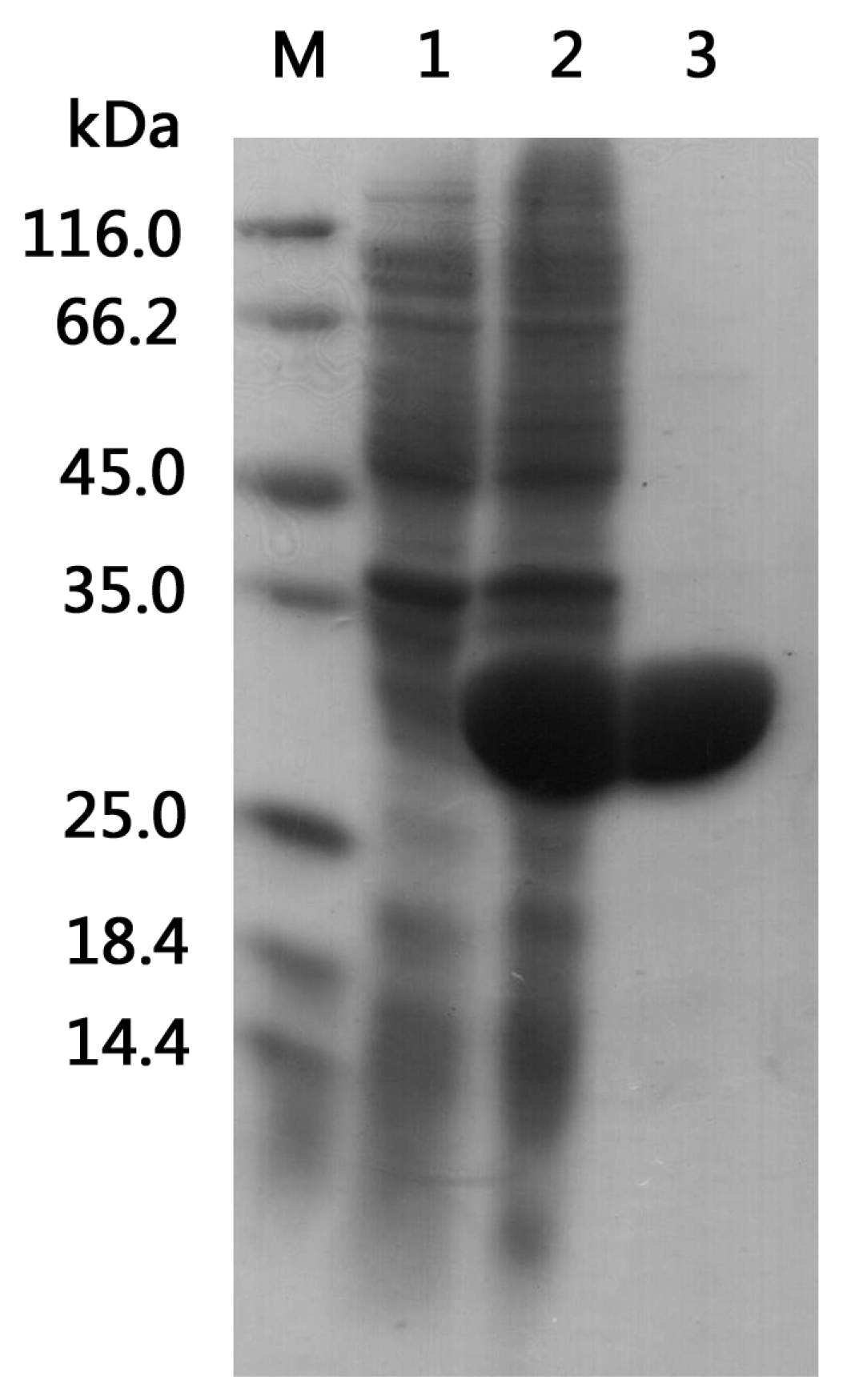
2.2. Heterologous Expression and Purification
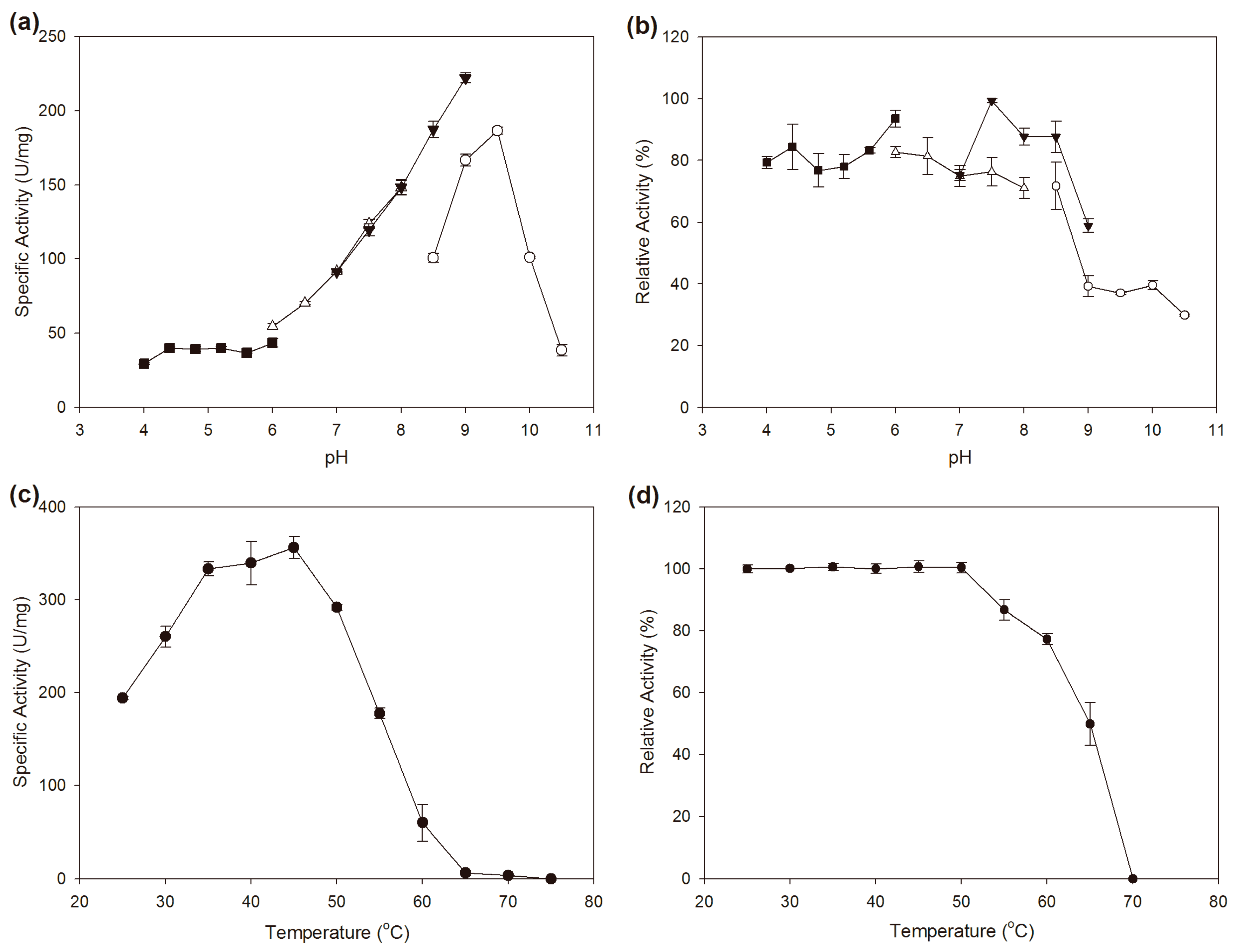
2.3. Effects of pH and Temperature on the Activity and Stability
2.4. Substrate Specificity and Steady-State Kinetics
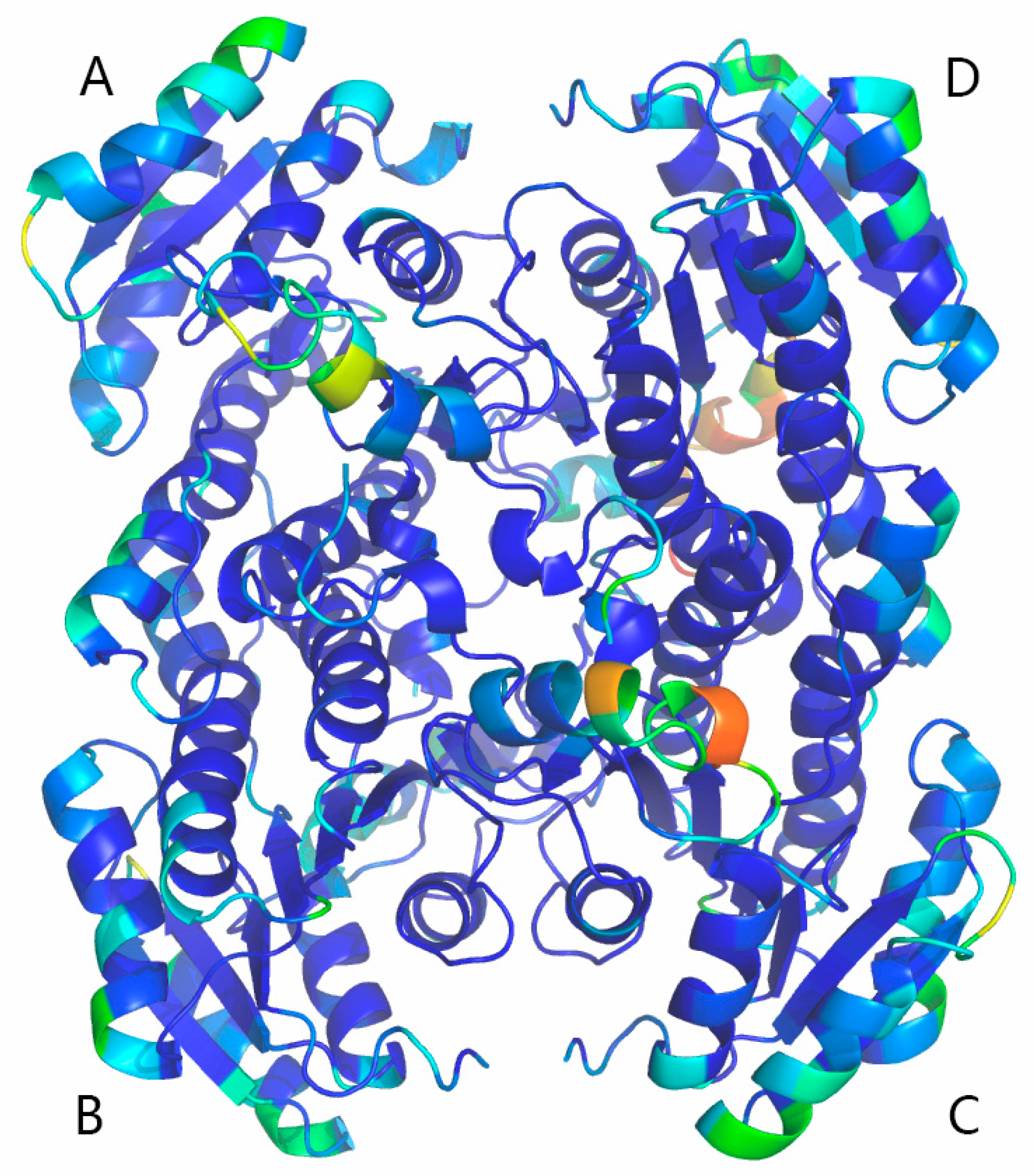
2.5. Homology Modeling and Electrostatic Potential Analysis
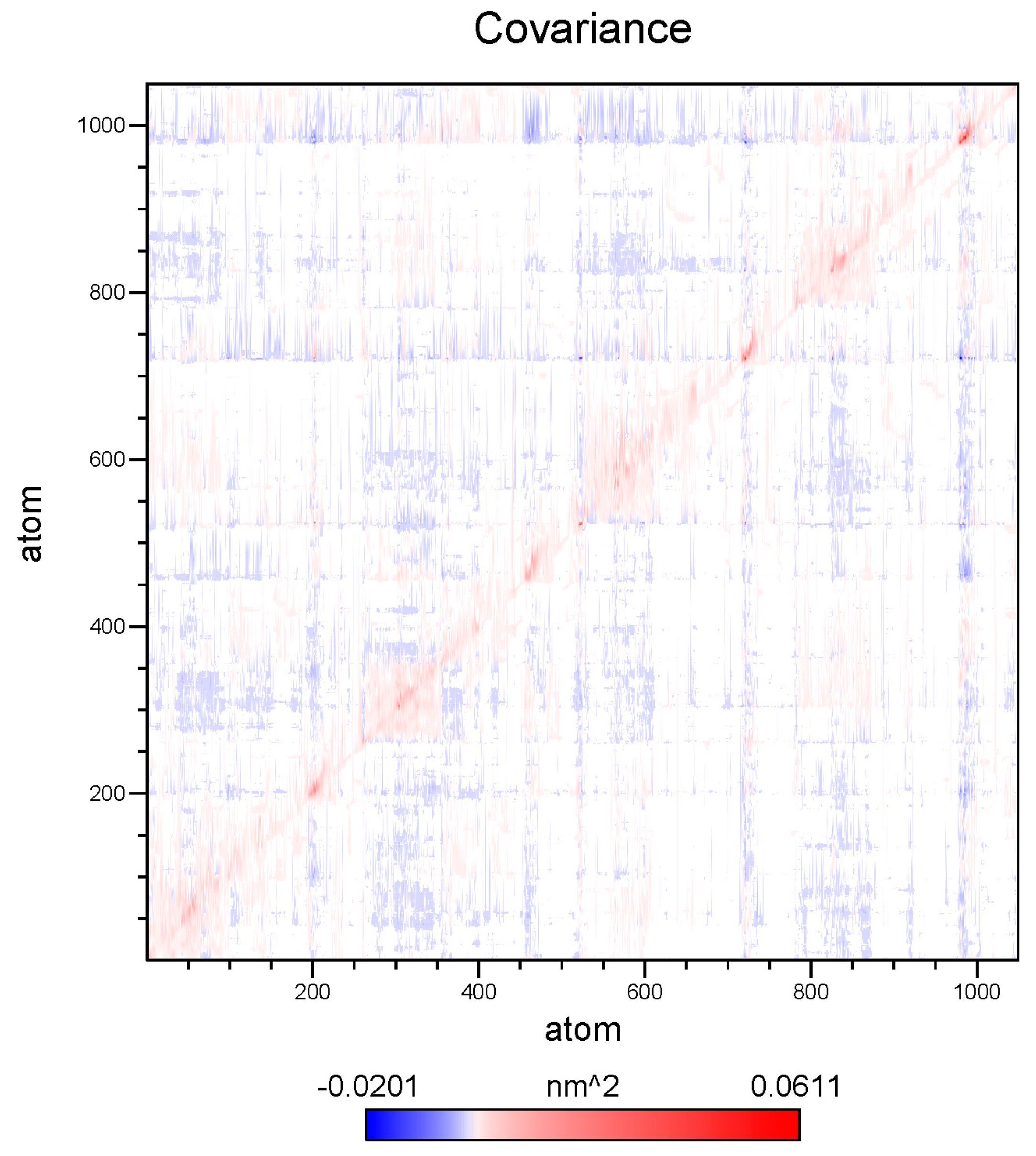
2.6. Global Structure Stability
2.7. Structure Flexibility
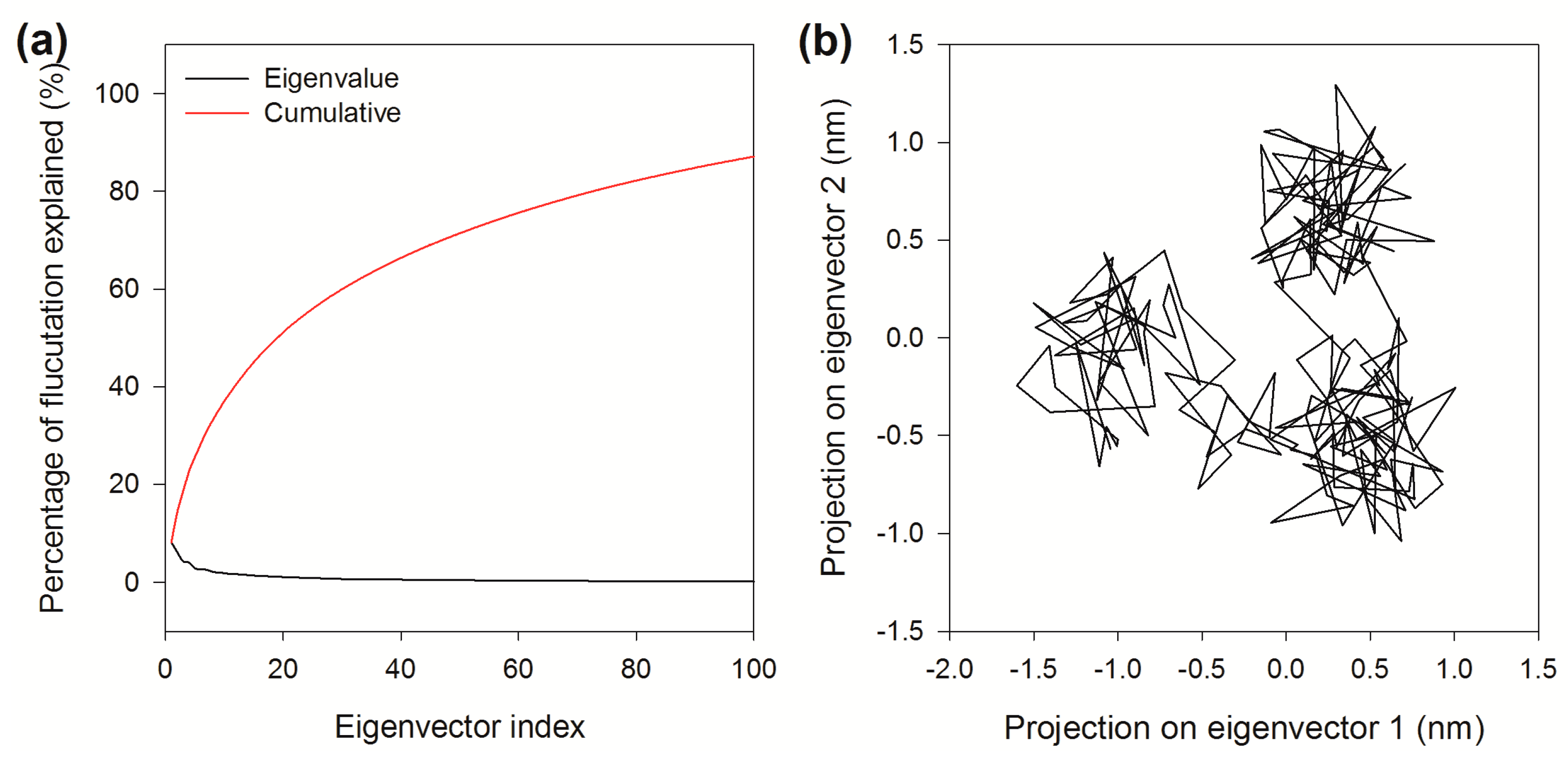
2.8. Essential Dynamics
3. Materials and Methods
3.1. Strains, Plasmids, and Chemicals
3.2. Cloning of the Bzgdh Gene and Sequence Analysis
3.3. Expression and Purification of Recombinant BzGDH
3.4. Enzyme Activity Assay
3.5. Effects of pH and Temperature on the Activity and Stability of BzGDH
3.6. Substrate Specificity of BzGDH
3.7. Steady-State Kinetics of BzGDH
3.8. Homology Modeling and Electrostatic Potential of BzGDH
3.9. Molecular Dynamic Simulations of BzGDH
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pongtharangkul, T.; Chuekitkumchorn, P.; Suwanampa, N.; Payongsri, P.; Honda, K.; Panbangred, W. Kinetic properties and stability of glucose dehydrogenase from Bacillus amyloliquefaciens SB5 and its potential for cofactor regeneration. AMB Express 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Du, Y.; Liu, D.; Li, Z.; Chen, X.; Zhao, Y. Cloning and expression in E. coli of an organic solvent-tolerant and alkali-resistant glucose 1-dehydrogenase from Lysinibacillus sphaericus G10. Bioresour. Technol. 2011, 102, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Boontim, N.; Yoshimune, K.; Lumyong, S.; Moriguchi, M. Purification and characterization of d-glucose dehydrogenase from Bacillus thuringiensis M15. Ann. Microbiol. 2004, 54, 481–492. [Google Scholar]
- Nagao, T.; Mitamura, T.; Wang, X.H.; Negoro, S.; Yomo, T.; Urabe, I.; Okada, H. Cloning, nucleotide sequences, and enzymatic properties of glucose dehydrogenase isozymes from Bacillus megaterium IAM1030. J. Bacteriol. 1992, 174, 5013–5020. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, T.; Urabe, I.; Okada, H. Enzymatic properties of isozymes and variants of glucose dehydrogenase from Bacillus megaterium. Eur. J. Biochem. 1989, 186, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, H.J.; Magert, H.J.; Gassen, H.G. Identification and isolation of glucose dehydrogenase genes of Bacillus megaterium M1286 and their expression in Escherichia coli. Eur. J. Biochem. 1988, 174, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, T.; Yasutake, Y.; Nishiya, Y.; Tamura, T. Structure-guided mutagenesis for the improvement of substrate specificity of Bacillus megaterium glucose 1-dehydrogenase IV. FEBS J. 2012, 279, 3264–3275. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kurisu, G.; Kusunoki, M.; Tabata, S.; Urabe, I.; Osaki, S. Crystal structure of glucose dehydrogenase from Bacillus megaterium IWG3 at 1.7 A resolution. J. Biochem. 2001, 129, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Ye, J.J.; Shen, Z.Y.; Hong, H.B.; Yan, J.B.; Lin, Y.; Chen, Z.X.; Zheng, Y.G.; Shen, Y.C. Upscale production of ethyl (S)-4-chloro-3-hydroxybutanoate by using carbonyl reductase coupled with glucose dehydrogenase in aqueous-organic solvent system. Appl. Microbiol. Biotechnol. 2015, 99, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, B.; Xu, Y.; Li, Y.; Li, M.; Liang, H.; Xiao, R. Efficicent (R)-phenylethanol production with enantioselectivity-alerted (S)-carbonyl reductase II and NADPH regeneration. PLoS ONE 2013, 8, e83586. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, L.; Xie, Y.; Hu, C.; Zhang, Y.; Li, L.; Wang, Y.; Ma, C.; Xu, P. Production of (3S)-acetoin from diacetyl by using stereoselective NADPH-dependent carbonyl reductase and glucose dehydrogenase. Bioresour. Technol. 2013, 137, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ding, H.; Shao, L.; Xu, X.; Zhao, Y. Molecular characterization of a novel thermal stable reductase capable of decoloration of both azo and triphenylmethane dyes. Appl. Microbiol. Biot. 2015, 99, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Lang, Q.; Tang, X.; Liu, A. Simultaneously improving stability and specificity of cell surface displayed glucose dehydrogenase mutants to construct whole-cell biocatalyst for glucose biosensor application. Bioresour. Technol. 2013, 147, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.M.; Yehezkeli, O.; Willner, I. Integrated, Electrically Contacted NAD(P)+-Dependent Enzyme–Carbon Nanotube Electrodes for Biosensors and Biofuel Cell Applications. Chem. A Eur. J. 2007, 13, 10168–10175. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.; Huisman, G.; Kazlauskas, R.; Lutz, S.; Moore, J.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, P. Cofactor regeneration for sustainable enzymatic biosynthesis. Biotechnol. Adv. 2007, 25, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; van der Donk, W.A. Regeneration of cofactors for use in biocatalysis. Curr. Opin. Biotechnol. 2003, 14, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Weckbecker, A.; Gröger, H.; Hummel, W. Regeneration of nicotinamide coenzymes: Principles and applications for the synthesis of chiral compounds. In Biosystems Engineering I; Wittmann, C., Krull, R., Eds.; Springer: Berlin, Germany, 2010; Volume 120, pp. 195–242. [Google Scholar]
- Sheng, B.; Zheng, Z.; Lv, M.; Zhang, H.; Qin, T.; Gao, C.; Ma, C.; Xu, P. Efficient production of (R)-2-hydroxy-4-phenylbutyric acid by using a coupled reconstructed d-lactate dehydrogenase and formate dehydrogenase system. PLoS ONE 2014, 9, e104204. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.C.; Fish, R.H. Biomimetic NAD+ Models for Tandem Cofactor Regeneration, Horse Liver Alcohol Dehydrogenase Recognition of 1, 4-NADH Derivatives, and Chiral Synthesis. Angew. Chem. Int. Ed. 2002, 41, 478–481. [Google Scholar] [CrossRef]
- Lee, W.H.; Chin, Y.W.; Han, N.S.; Kim, M.D.; Seo, J.H. Enhanced production of GDP-l-fucose by overexpression of NADPH regenerator in recombinant Escherichia coli. Appl. Microbiol. Biot. 2011, 91, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Johannes, T.W.; Woodyer, R.D.; Zhao, H.M. Efficient regeneration of NADPH using an engineered phosphite dehydrogenase. Biotechnol. Bioeng. 2007, 96, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, H.; Du, Y.; Lin, H.; Li, Z.; Zhao, Y. Cloning, expression and characterization of a glucose dehydrogenase from Bacillus sp. G3 in Escherichia coli. Afr. J. Microbiol. Res. 2011, 5, 5882–5888. [Google Scholar]
- Wu, X.; Ding, H.; Ke, L.; Xin, Y.; Cheng, X. Characterization of an acid-resistant glucose 1-dehydrogenase from Bacillus cereus var. mycoides. Romanian Biotechnol. Lett. 2012, 17, 7540–7548. [Google Scholar]
- Finn, R.D.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Mistry, J.; Mitchell, A.L.; Potter, S.C.; Punta, M.; Qureshi, M.; Sangrador-Vegas, A.; et al. The Pfam protein families database: Towards a more sustainable future. Nucleic. Acids Res. 2016, 44, D279–D285. [Google Scholar] [CrossRef] [PubMed]
- Boontim, N.; Yoshimune, K.; Lumyong, S.; Moriguchi, M. Cloning of d-glucose dehydrogenase with a narrow substrate specificity from Bacillus thuringiensis M15. Ann. Microbiol. 2006, 56, 237–240. [Google Scholar] [CrossRef]
- Ramaley, R.F.; Vasantha, N. Glycerol protection and purification of Bacillus subtilis glucose dehydrogenase. J. Biol. Chem. 1983, 258, 12558–12565. [Google Scholar] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Gouet, P.; Robert, X.; Courcelle, E. ESPript/ENDscript: Extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res. 2003, 31, 3320–3323. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Zuckerkandl, E.; Pauling, L. Evolutionary divergence and convergence in proteins. Evol. Genes Proteins 1965, 97, 97–166. [Google Scholar]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef] [PubMed]
- Lipari, G.; Szabo, A. Model-free approach to the interpretation of nuclear magnetic resonance relaxation in macromolecules. 1. Theory and range of validity. J. Am. Chem. Soc. 1982, 104, 4546–4559. [Google Scholar] [CrossRef]
- Amadei, A.; Linssen, A.; Berendsen, H.J. Essential dynamics of proteins. Proteins Struct. Funct. Bioinform. 1993, 17, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Balsera, M.A.; Wriggers, W.; Oono, Y.; Schulten, K. Principal component analysis and long time protein dynamics. J. Phys. Chem. 1996, 100, 2567–2572. [Google Scholar] [CrossRef]
- Berendsen, H.J.; Hayward, S. Collective protein dynamics in relation to function. Curr. Opin. Struc. Biol. 2000, 10, 165–169. [Google Scholar] [CrossRef]
- Baik, S.H.; Michel, F.; Aghajari, N.; Haser, R.; Harayama, S. Cooperative effect of two surface amino acid mutations (Q252L and E170K) in glucose dehydrogenase from Bacillus megaterium IWG3 on stabilization of its oligomeric state. Appl. Environ. Microbiol. 2005, 71, 3285–3293. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup of Poisson–Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef] [PubMed]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Hornak, V.; Abel, R.; Okur, A.; Strockbine, B.; Roitberg, A.; Simmerling, C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins Struct. Funct. Bioinform. 2006, 65, 712–725. [Google Scholar] [CrossRef] [PubMed]





| Substrate | Relative Activity (%) 1 | ||
|---|---|---|---|
| BzGDH | BgGDH [23] | BcGDH [24] | |
| d-glucose | 100 | 100 | 100 |
| d-galactose | 6.8 | 22.0 | 7.3 |
| d-mannose | 3.2 | 7.1 | 4.4 |
| d-fructose | 0.9 | 0.6 | 0 |
| d-xylose | 6.1 | 6.4 | 6.0 |
| d-arabinose | 0 | 0.2 | 0 |
| d-maltose | 10.0 | 13.0 | 11.0 |
| d-lactose | 3.1 | 2.6 | 5.2 |
| d-sucrose | 0.9 | 6.3 | 2.51 |
| Substrate/Cofactor | Km (mM) | kcat (s−1) 1 | kcat/Km (mM−1·s−1) |
|---|---|---|---|
| d-glucose | 17.126 ± 0.946 | 87.844 ± 1.362 | 5.129 |
| NAD | 0.072 ± 0.009 | 84.521 ± 2.175 | 1166.294 |
| NADP | 0.404 ± 0.088 | 73.960 ± 2.677 | 182.978 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Gao, F.; Yu, Y.; Chen, B. Biochemical and Computational Insights on a Novel Acid-Resistant and Thermal-Stable Glucose 1-Dehydrogenase. Int. J. Mol. Sci. 2017, 18, 1198. https://doi.org/10.3390/ijms18061198
Ding H, Gao F, Yu Y, Chen B. Biochemical and Computational Insights on a Novel Acid-Resistant and Thermal-Stable Glucose 1-Dehydrogenase. International Journal of Molecular Sciences. 2017; 18(6):1198. https://doi.org/10.3390/ijms18061198
Chicago/Turabian StyleDing, Haitao, Fen Gao, Yong Yu, and Bo Chen. 2017. "Biochemical and Computational Insights on a Novel Acid-Resistant and Thermal-Stable Glucose 1-Dehydrogenase" International Journal of Molecular Sciences 18, no. 6: 1198. https://doi.org/10.3390/ijms18061198