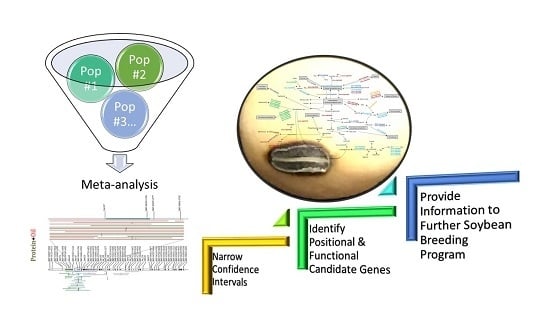
Meta-Analyses of QTLs Associated with Protein and Oil Contents and Compositions in Soybean [Glycine max (L.) Merr.] Seed
Abstract
:1. Introduction
2. Results
2.1. Collection of QTLs for Soybean Seed Protein and Oil Contents and Compositions
2.2. QTL Projection on a Soybean Consensus Map and Meta-Analysis of Seed Compositions QTL
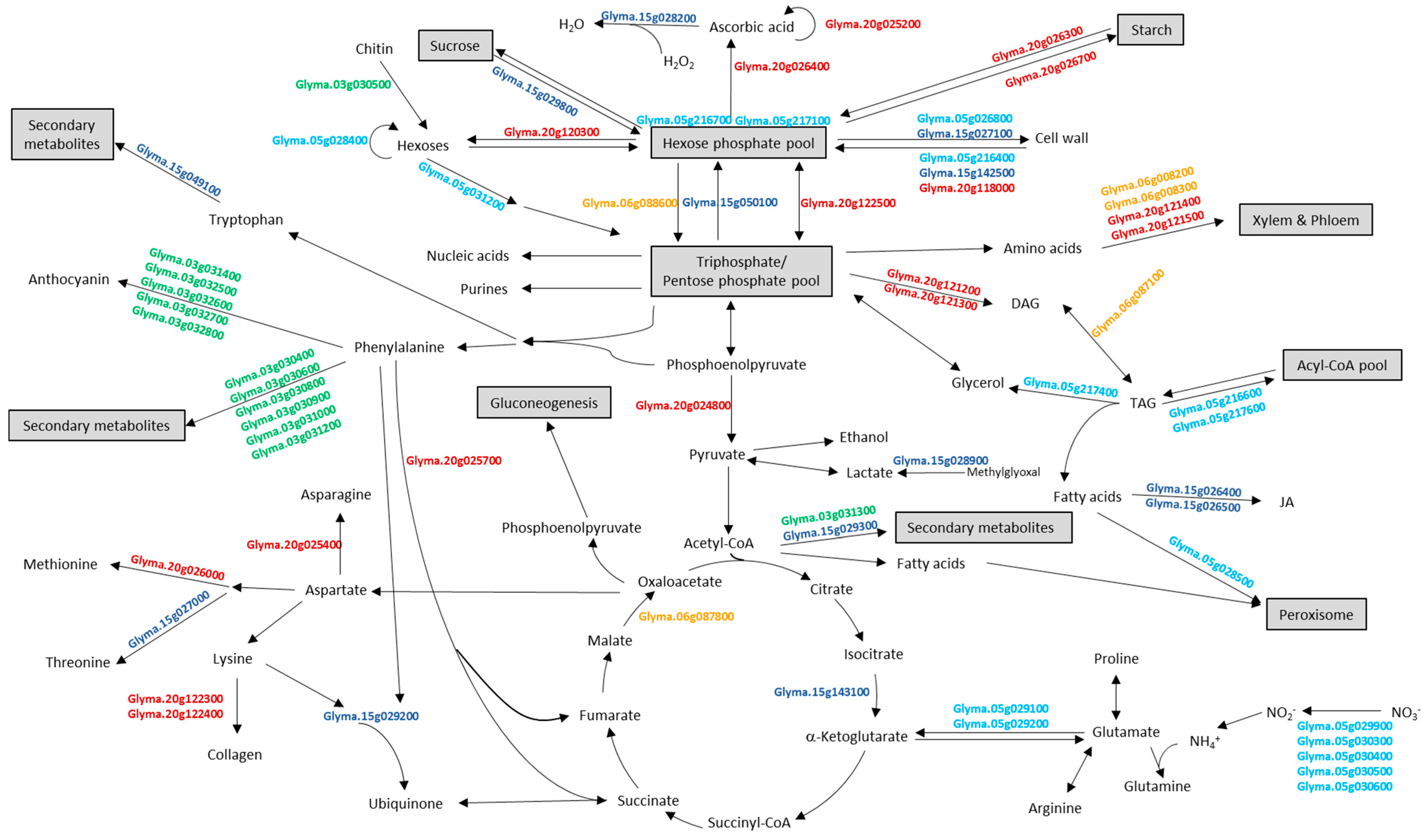
2.3. Identification of Candidate Genes for Each Meta-QTL
3. Discussion
3.1. Meta-Analysis Aids in the Identification of Robust QTLs and Narrowing of Confidence Intervals
3.2. Meta-QTLs Can Be Further Defined and Refined Through the Combined Analysis of Correlated Traits
3.3. Putative Functional Candidate Genes Were Identified from the Positional Candidates Encompassed by Meta-QTLs
4. Materials and Methods
4.1. Collection of Mapping and QTL Information for Soybean Protein, Oil, Amino Acids and Fatty Acids
4.2. Meta-QTL Analysis
4.3. Identification of Candidate Genes
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AIC | Akaike information criterion |
| AICc | Corrected Akaike information criterion |
| AIC3 | Corrected Akaike information criterion |
| Asp | Aspartate |
| AWE | Approximate weight of evidence |
| BIC | Bayesian information criterion |
| Chr | Chromosome |
| CI | Confidence interval |
| cM | Centimorgan |
| Cys | Cysteine |
| FAD2 | Microsomal oleate desaturase |
| FAD3 | ω-3 fatty-acid desaturase |
| Gmax2.0 | Glyma.Wm82.a2.v1 |
| KAS II | 3-keto-acyl-ACP synthase II |
| kb | Kilobase |
| LOD | Logarithm (base 10) of odds |
| LG | Linkage group |
| Lys | Lysine |
| Met | Methionine |
| mO | Meta-QTL for oil |
| mP | Meta-QTL for protein |
| mPO | Meta-QTL for protein and oil in combination |
| mPCM | Meta-QTL for protein, cysteine, and methionine in combination |
| PTHR | Protein analysis through evolutionary relationships |
| QTL | Quantitative trait loci |
| SACPD-C | Δ9-stearoyl-acyl-carrier-protein-desaturase |
| SoyBase | SoyBase and the Soybean Breeder’s Toolbox |
| SoyCys7.0 | Soybean metabolic pathway reference database |
| TAG | Triacylglycerol |
| TCA | Tricarboxylic acid |
| Thr | Threonine |
References
- Foyer, C.H.; Lam, H.-M.; Nguyen, H.T.; Siddique, K.H.M.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.J.; Vodkin, L.O. Using RNA-Seq to profile soybean seed development from fertilization to maturity. PLoS ONE 2013, 8, e59270. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.D.; Banerjee, A.; Acevedo, A.; Halvorsen, K.E.; Eastmond, A. Policies for the sustainable development of biofuels in the Pan American region: A review and synthesis of five countries. Environ. Manag. 2015, 56, 1276–1294. [Google Scholar] [CrossRef] [PubMed]
- Soystats. Available online: http://www.soystats.com/ (accessed on 7 June 2015).
- Warrington, C.V.; Abdel-Haleem, H.; Hyten, D.L.; Cregan, P.B.; Orf, J.H.; Killam, A.K.; Bajjalieh, N.; Li, Z.; Boerma, H.R. QTL for seed protein and amino acids in the Benning x Danbaekkong soybean population. Theor. Appl. Genet. 2015, 128, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Guschina, I.A.; Everard, J.D.; Kinney, A.J.; Quant, P.A.; Harwood, J.L. Studies on the regulation of lipid biosynthesis in plants: Application of control analysis to soybean. Biochim. Biophys. Acta 2014, 1838, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Reinprecht, Y.; Pauls, K.P. Microsomal ω-3 fatty acid desaturase genes in low linolenic acid soybean line RG10 and validation of major linolenic acid QTL. Front. Genet. 2016, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Clemente, T.E.; Cahoon, E.B. Soybean oil: Genetic approaches for modification of functionality and total content. Plant Physiol. 2009, 151, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, E.B.; Clemente, T.E.; Damude, H.G.; Kinney, A.J. Modifying vegetable oils for food and non-food purposes. In Handbook of Plant Breeding, Vol. 4, Oil Crops; Vollmann, J., Rajcan, I., Eds.; Springer: New York, NY, USA, 2010; pp. 31–56. [Google Scholar]
- Wang, X.; Jiang, G.L.; Green, M.; Scott, R.A.; Song, Q.; Hyten, D.L.; Cregan, P.B. Identification and validation of quantitative trait loci for seed yield, oil and protein contents in two recombinant inbred line populations of soybean. Mol. Genet. Genom. 2014, 289, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, P.; Wang, D.; Shannon, G.; Zeng, A.; Orazaly, M.; Wu, C. Identification and mapping of stable QTL for protein content in soybean seeds. Mol. Breed. 2015, 35, 92. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Liu, M.F.; He, J.B.; Wang, Y.F.; Xing, G.N.; Li, Y.; Yang, S.P.; Zhao, T.J.; Gai, J.Y. Molecular-assisted breeding for transgressive seed protein content in soybean [Glycine max (L.) Merr.]. Theor. Appl. Genet. 2015, 128, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Leite, D.C.; Pinheiro, J.B.; Campos, J.B.; Di Mauro, A.O.; Uneda-Trevisoli, S.H. QTL mapping of soybean oil content for marker-assisted selection in plant breeding program. Genet. Mol. Res. 2016, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Goffinet, B.; Gerber, S. Quantitative trait loci: A meta-analysis. Genet 2000, 155, 463–473. [Google Scholar]
- Khowaja, F.; Norton, G.; Courtois, B.; Price, A. Improved resolution in the position of drought-related QTLs in a single mapping population of rice by meta-analysis. BMC Genom. 2009, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Liu, D.C.; Guo, X.L.; Yang, W.L.; Sun, J.Z.; Wang, D.W.; Zhang, A. Genomic distribution of quantitative trait loci for yield and yield-related traits in common wheat. J. Integr. Plant Biol. 2010, 52, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Said, J.; Song, M.; Wang, H.; Lin, Z.; Zhang, X.; Fang, D.; Zhang, J. A comparative meta-analysis of QTL between intraspecific Gossypium hirsutum and interspecific G. hirsutum x G. barbadense populations. Mol. Genet. Genom. 2015, 290, 1003–1025. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, J.; Deng, D.; Ding, H.; Bian, Y.; Yin, Z.; Wu, Y.; Zhou, B.; Zhao, Y. A comprehensive meta-analysis of plant morphology, yield, stay-green, and virus disease resistance QTL in maize (Zea mays L.). Planta 2016, 243, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shabala, S.; Koutoulis, A.; Shabala, L.; Zhou, M. Meta-analysis of major QTL for abiotic stress tolerance in barley and implications for barley breeding. Planta 2017, 245, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sleper, D.A.; Lu, P.; Shannon, J.G. QTLs associated with resistance to soybean cyst nematode in soybean: Meta-analysis of QTL location. Crop Sci. 2006, 46, 595–602. [Google Scholar] [CrossRef]
- Sun, Y.; Luan, H.; Qi, Z.; Shan, D.; Liu, C.; Hu, G.; Chen, Q. Mapping and meta-analysis of height QTLs in soybean. Legum. Genom. Genet. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Sun, Y.N.; Pan, J.B.; Shi, X.L.; Du, X.Y.; Wu, Q.; Qi, Z.M.; Jiang, H.W.; Xin, D.W.; Liu, C.Y.; Hu, G.H.; Chen, Q.S. Multi-environment mapping and meta-analysis of 100-seed weight in soybean. Mol. Biol. Rep. 2012, 39, 9435–9443. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; King, C.A.; Chen, P.; Ray, J.D.; Cregan, P.B.; Carter, T.E., Jr.; Li, Z.; Abdel-Haleem, H.; Matson, K.W.; Schapaugh, W., Jr.; et al. Meta-analysis to refine map position and reduce confidence intervals for delayed-canopy-wilting QTLs in soybean. Mol. Breed. 2016, 36, 1–14. [Google Scholar] [CrossRef]
- Qi, Z.M.; Sun, Y.N.; Wu, Q.; Liu, C.Y.; Hu, G.H.; Chen, Q.S. A meta-analysis of seed protein concentration QTL in soybean. Can. J. Plant Sci. 2011, 91, 221–230. [Google Scholar] [CrossRef]
- Qi, Z.M.; Wu, Q.; Han, X.; Sun, Y.N.; Du, X.Y.; Liu, C.Y.; Jiang, H.W.; Hu, G.H.; Chen, Q.S. Soybean oil content QTL mapping and integrating with meta-analysis method for mining genes. Euphytica 2011, 179, 499–514. [Google Scholar] [CrossRef]
- Eskandari, M.; Cober, E.R.; Rajcan, I. Genetic control of soybean seed oil: II. QTL and genes that increase oil concentration without decreasing protein or with increased seed yield. Theor. Appl. Genet. 2013, 126, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wang, P.W.; Zhang, J.; Liu, Z.Z.; Guan, S.Y.; Liu, S.Y.; Qu, J. Inheritance analysis and mapping QTL on fat content trait in soybean. J. S. China Agric. Univ. 2012, 33, 438–443. [Google Scholar] [CrossRef]
- Lu, W.; Wen, Z.; Li, H.; Yuan, D.; Li, J.; Zhang, H.; Huang, Z.; Cui, S.; Du, W. Identification of the quantitative trait loci (QTL) underlying water soluble protein content in soybean. Theor. Appl. Genet. 2013, 126, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Jiang, Z.; Han, Y.; Teng, W.; Zhao, X.; Li, W. Identification of quantitative trait loci underlying seed protein and oil contents of soybean across multi-genetic backgrounds and environments. Plant Breed. 2013, 132, 630–641. [Google Scholar] [CrossRef]
- Pathan, S.M.; Vuong, T.; Clark, K.; Lee, J.D.; Shannon, J.G.; Roberts, C.A.; Ellersieck, M.R.; Burton, J.W.; Cregan, P.B.; Hyten, D.L.; et al. Genetic mapping and confirmation of quantitative trait loci for seed protein and oil contents and seed weight in soybean. Crop Sci. 2013, 53, 765–774. [Google Scholar] [CrossRef]
- Rossi, M.E.; Orf, J.H.; Liu, L.J.; Dong, Z.; Rajcan, I. Genetic basis of soybean adaptation to North American vs. Asian mega-environments in two independent populations from Canadian x Chinese crosses. Theor. Appl. Genet. 2013, 126, 1809–1823. [Google Scholar] [CrossRef] [PubMed]
- Yesudas, C.R.; Bashir, R.; Geisler, M.B.; Lightfoot, D.A. Identification of germplasm with stacked QTL underlying seed traits in an inbred soybean population from cultivars Essex and Forrest. Mol. Breed. 2013, 31, 693–703. [Google Scholar] [CrossRef]
- Akond, M.; Liu, S.; Boney, M.; Kantartzi, S.K.; Meksem, K.; Bellaloui, N.; Lightfoot, D.A.; Kassem, M.A. Identification of quantitative trait loci (QTL) underlying protein, oil, and five major fatty acids' contents in soybean. Am. J. Plant Sci. 2014, 5, 158–167. [Google Scholar] [CrossRef]
- Wang, J.; Chen, P.; Wang, D.; Shannon, G.; Shi, A.; Zeng, A.; Orazaly, M. Identification of quantitative trait loci for oil content in soybean seed. Crop Sci. 2015, 55, 23–34. [Google Scholar] [CrossRef]
- Kim, M.; Schultz, S.; Nelson, R.L.; Diers, B.W. Identification and fine mapping a soybean seed protein QTL from PI 407788A on chromosome 15. Crop Sci. 2016, 56, 219–225. [Google Scholar] [CrossRef]
- Qi, Z.; Pan, J.; Han, X.; Qi, H.; Xin, D.; Li, W.; Mao, X.; Wang, Z.; Jiang, H.; Liu, C.; et al. Identification of major QTLs and epistatic interactions for seed protein concentration in soybean under multiple environments based on a high-density map. Mol. Breed. 2016, 36, 1–16. [Google Scholar] [CrossRef]
- Xie, D.; Han, Y.; Zeng, Y.; Chang, W.; Teng, W.; Li, W. SSR- and SNP-related QTL underlying linolenic acid and other fatty acid contents in soybean seeds across multiple environments. Mol. Breed. 2012, 30, 169–179. [Google Scholar] [CrossRef]
- Fallen, B.D.; Hatcher, C.N.; Allen, F.L.; Kopsell, D.A.; Saxton, A.M.; Chen, P.; Kantartzi, S.K.; Cregan, P.B.; Hyten, D.L.; Pantalone, V.R. Soybean seed amino acid content QTL detected using universal soy linkage panel 1.0 with 1,536 SNPs. J. Plant Genome Ser. 2013, 1, 68–79. [Google Scholar] [CrossRef]
- Ha, B.K.; Kim, H.J.; Velusamy, V.; Vuong, T.D.; Nguyen, H.T.; Shannon, J.G.; Lee, J.D. Identification of quantitative trait loci controlling linolenic acid concentration in PI83463 (Glycine soja). Theor. Appl. Genet. 2014, 127, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, R.K.; Jedlicka, J.; Graef, G.L.; Waters, B.M. Identification of new QTLs for seed mineral, cysteine, and methionine concentrations in soybean [Glycine max (L.) Merr.]. Mol. Breed. 2014, 34, 431–445. [Google Scholar] [CrossRef]
- Wang, X.Z.; Jiang, G.L.; Green, M.; Scott, R.A.; Hyten, D.L.; Cregan, P.B. Quantitative trait locus analysis of unsaturated fatty acids in a recombinant inbred population of soybean. Mol. Breed. 2014, 33, 281–296. [Google Scholar] [CrossRef]
- Khandaker, L.; Akond, M.; Liu, S.; Kantartzi, S.K.; Meksem, K.; Bellaloui, N.; Lightfoot, D.A.; Kassem, M.A. Mapping of QTL associated with seed amino acids content in “MD96-5722” by “Spencer” RIL population of soybean using SNP markers. Food Nut. Sci. 2015, 6, 974–984. [Google Scholar] [CrossRef]
- Yan, L.; Deng, Y.; Song, Q.; Cregan, P.B.; Chen, P.; Lei, Y.; Yang, C.; Chen, Q.; Di, R.; Liu, B.; et al. Identifying and validating a quantitative trait locus on chromosome 14 underlying stearic acid in a soybean landrace. J. Crop Improv. 2016, 30, 152–164. [Google Scholar] [CrossRef]
- Hyten, D.L.; Choi, I.Y.; Song, Q.; Specht, J.E.; Carter, T.E., Jr.; Shoemaker, R.C.; Hwang, E.Y.; Matukumalli, L.K.; Cregan, P.B. A high density integrated genetic linkage map of soybean and the development of 1536 universal soy linkage panel for quantitative trait locus mapping. Crop Sci. 2010, 50, 1–9. [Google Scholar] [CrossRef]
- Burton, J.W. Quantitative genetics: Results relevant to soybean breeding. In Soybeans: Improvement, Production and Uses, 2nd ed.; Wilcox, J.R., Ed.; Agron. Monogr. 16; ASA, CSSA, and SSSA: Madison, WI, USA, 1987; pp. 211–247. [Google Scholar]
- Chung, J.; Babka, H.L.; Graef, G.L.; Staswick, P.E.; Lee, D.J.; Cregan, P.B.; Shoemaker, R.C.; Specht, J.E. The seed protein, oil, and yield QTL on soybean linkage group I. Crop Sci. 2003, 43, 1053–1067. [Google Scholar] [CrossRef]
- Panthee, D.R.; Pantalone, V.R.; Sams, C.E.; Saxton, A.M.; West, D.R.; Orf, J.H.; Killam, A.S. Quantitative trait loci controlling sulfur containing amino acids methionine and cysteine in soybean seeds. Theor. Appl. Genet. 2006, 112, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Panthee, D.R.; Pantalone, V.R.; Saxton, A.M.; West, D.R.; Sams, C.E. Genomic regions associated with amino acid composition in soybean. Mol. Breed. 2006, 17, 79–89. [Google Scholar] [CrossRef]
- Veyrieras, J.; Goffinet, B.; Charcosset, A. MetaQTL: A package of new computational methods for the meta-analysis of QTL mapping experiments. BMC Bioinform. 2007, 8, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Aghoram, K.; Wilson, R.F.; Burton, J.W.; Dewey, R.E. A mutation in a 3-keto-acyl-ACP synthase II gene is associated with elevated palmitic acid levels in soybean seeds. Crop Sci. 2006, 46, 2453–2459. [Google Scholar] [CrossRef]
- Bilyeu, K.D.; Palavalli, L.; Sleper, D.A.; Beuselinck, P.R. Three microsomal ω-3 fatty-acid desaturase genes contribute to soybean linolenic acid levels. Crop Sci. 2003, 43, 1833–1838. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Gai, J.; Yu, D. Molecular cloning and characterization of a novel microsomal oleate desaturase gene from soybean. J. Plant Physiol. 2007, 164, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Burton, J.W.; Upchurch, R.G.; Whittle, E.; Shanklin, J.; Dewey, R.E. Mutations in a Δ9-stearoyl-ACP-desaturase gene are associated with enhanced stearic acid levels in soybean seeds. Crop Sci. 2008, 48, 2305–2313. [Google Scholar] [CrossRef]
- Stasko, A.K.; Wickramasinghe, D.; Nauth, B.J.; Acharya, B.; Ellis, M.L.; Taylor, C.G.; McHale, L.K.; Dorrance, A.E. High-density mapping of resistance QTL toward Phytophthora sojae, Pythium irregulare, and Fusarium graminearum in the same soybean population. Crop Sci. 2016, 56, 2476–2492. [Google Scholar] [CrossRef]
- Salvi, S.; Tuberosa, R. To clone or not to clone plant QTLs: Present and future challenges. Trend. Plant Sci. 2005, 10, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Fasoula, V.A.; Harris, D.K.; Boerma, H.R. Validation and designation of quantitative trait loci for seed protein, seed oil, and seed weight from two soybean populations. Crop Sci. 2004, 44, 1218–1225. [Google Scholar] [CrossRef]
- Diers, B.W.; Kein, P.; Shoemaker, R.C.; Fehr, W.R. RFLP analysis of soybean seed protein and oil content. Theor. Appl. Genet. 1992, 83, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.M.; Glover, K.D.; Carlson, S.R.; Specht, J.E.; Diers, B.W. Fine mapping of a seed protein QTL on soybean linkage group I and its correlated effects on agronomic traits. Crop Sci. 2006, 46, 834–839. [Google Scholar] [CrossRef]
- Lander, E.; Kruglyak, L. Genetic dissection of complex traits: Guidelines for interpreting and reporting linkage results. Nat. Genet. 1995, 11, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Ooijen, J.W. LOD significance thresholds for QTL analysis in experimental populations of diploid species. Hered. 1999, 83, 613–624. [Google Scholar] [CrossRef]
- Brummer, E.C.; Graef, G.L.; Orf, J.H.; Wilcox, J.R.; Shoemaker, R.C. Mapping QTL for seed protein and oil content in eight soybean populations. Crop Sci. 1997, 37, 370–378. [Google Scholar] [CrossRef]
- Csanadi, G.; Vollmann, J.; Stift, G.; Lelley, T. Seed quality QTLs identified in a molecular map of early maturing soybean. Theor. Appl. Genet. 2001, 103, 912–919. [Google Scholar] [CrossRef]
- Specht, J.E.; Chase, K.; Macrander, M.; Graef, G.L.; Chung, J.; Markwell, J.P.; Germann, M.; Orf, J.H.; Lark, K.G. Soybean response to water: A QTL analysis of drought tolerance. Crop Sci. 2001, 41, 493–509. [Google Scholar] [CrossRef]
- Tajuddin, T.; Watanabe, S.; Yamanaka, N.; Harada, K. Analysis of quantitative trait loci for protein and lipid contents in soybean seeds using recombinant inbred lines. Breed. Sci. 2003, 53, 133–140. [Google Scholar] [CrossRef]
- Hyten, D.L.; Pantalone, V.R.; Sams, C.E.; Saxton, A.M.; Landau-Ellis, D.; Stefaniak, T.R.; Schmidt, M.E. Seed quality QTL in a prominent soybean population. Theor. Appl. Genet. 2004, 109, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.K.; Wang, Y.J.; Luo, G.Z.; Zhang, J.S.; He, C.Y.; Wu, X.L.; Gai, J.Y.; Chen, S.Y. QTL mapping of ten agronomic traits on the soybean (Glycine max L. Merr.) genetic map and their association with EST markers. Theor. Appl. Genet. 2004, 108, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Reinprecht, Y.; Poysa, V.; Yu, K.; Rajcan, I.; Ablett, G.; Pauls, K. Seed and agronomic QTL in low linolenic acid, lipoxygenase-free soybean (Glycine max (L.) Merrill) germplasm. Genome 2006, 49, 1510–1527. [Google Scholar] [PubMed]
- Chen, Q.S.; Zhang, Z.C.; Liu, C.Y.; Xin, D.W.; Qiu, H.M.; Shan, D.P.; Shan, C.Y.; Hu, G.H. QTL analysis of major agronomic traits in soybean. Agric. Sci. China 2007, 6, 399–405. [Google Scholar] [CrossRef]
- Li, H.; Zhao, T.; Wang, Y.; Yu, D.; Chen, S.; Zhou, R.; Gai, J. Genetic structure composed of additive QTL, epistatic QTL pairs and collective unmapped minor QTL conferring oil content and fatty acid components of soybeans. Euphytica 2011, 182, 117–132. [Google Scholar] [CrossRef]
- Akond, A.S.M.G.; Ragin, B.; Bazzelle, R.; Kantartzi, S.K.; Meksem, K.; Kassem, M.A. Quantitative trait loci associated with moisture, protein, and oil content in soybean [Glycine max (L.) Merr.]. J. Agric. Sci. 2012, 4, 16–25. [Google Scholar] [CrossRef]
- Wang, X.Z.; Jiang, G.L.; Green, M.; Scott, R.A.; Hyten, D.L.; Cregan, P.B. Quantitative trait locus analysis of saturated fatty acids in a population of recombinant inbred lines of soybean. Mol. Breed. 2012, 30, 1163–1179. [Google Scholar] [CrossRef]
- Jedlicka, J. Evaluation of Four Biparental Soybean Populations for Identification of Seed Oil QTL, Cytoplasmic Effects, and Genotype x Environment Interactions. Ph.D. Thesis, University of Nebraska, Lincoln, NE, USA, August 2014. [Google Scholar]
- Qi, Z.; Hou, M.; Han, X.; Liu, C.; Jiang, H.; Xin, D.; Hu, G.; Chen, Q. Identification of quantitative trait loci (QTLs) for seed protein concentration in soybean and analysis for additive effects and epistatic effects of QTLs under multiple environments. Plant Breed. 2014, 133, 499–507. [Google Scholar] [CrossRef]
- Fan, S.; Li, B.; Yu, F.; Han, F.; Yan, S.; Wang, L.; Sun, J. Analysis of additive and epistatic quantitative trait loci underlying fatty acid concentrations in soybean seeds across multiple environments. Euphytica 2015, 206, 689–700. [Google Scholar] [CrossRef]
- Asekova, S.; Kulkarni, K.P.; Kim, M.; Kim, J.H.; Song, J.T.; Shannon, J.G.; Lee, J.D. Novel quantitative trait loci for forage quality traits in a cross between PI 483463 and 'Hutcheson' in soybean. Crop Sci. 2016, 56, 2600–2611. [Google Scholar] [CrossRef]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, U.; Kannaste, A.; Copolovici, L. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Front. Plant Sci. 2013, 4, 262. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, C.; Li-Beisson, Y.; Philippar, K. Fatty acid and lipid transport in plant cells. Trends Plant Sci. 2016, 21, 145–158. [Google Scholar] [CrossRef] [PubMed]
- De Jong, F.; Thodey, K.; Lejay, L.V.; Bevan, M.W. Glucose elevates NITRATE TRANSPORTER2.1 protein levels and nitrate transport activity independently of its HEXOKINASE1-mediated stimulation of NITRATE TRANSPOTER2.1 expression. Plant Physiol. 2014, 164, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Liu, L.; Hu, Y.; Zhang, F.; Mergen, S.; Wang, G.; Schlappi, M.R.; Chu, C. A rice plastidial nucleotide sugar epimerase is involved in galactolipid biosynthesis and improves photosynthetic efficiency. PLoS Genet. 2011, 7, e1002196. [Google Scholar] [CrossRef] [PubMed]
- Valliyodan, B.; Nguyen, H.T. Biological mechanisms that influence soy protein concentration and composition. In Designing Soybeans for 21st Century Markets; Wilson, R.L., Ed.; Elsevier: Urbana, IL, USA, 2012; pp. 129–157. [Google Scholar]
- Dieuaide-Noubhani, M.; Alonso, A.P. Application of metabolic flux analysis to plants. In Plant Metabolic Flux Analysis, Methods and Protocols; Dieuaide-Noubhani, M., Alonso, A.P., Eds.; Humana Press: New York, NY, USA, 2014; pp. 1–17. [Google Scholar]
- Schwender, J.; Hebbelmann, J.; Heinzel, N.; Hildebrandt, T.; Rogers, A.; Naik, D.; Klapperstuck, M.; Braun, H.P.; Schreiber, F.; Denolf, P.; et al. Quantitative multilevel analysis of central metabolism in developing oilseeds of oilseed rape during in vitro culture. Plant Physiol. 2015, 168, 828–848. [Google Scholar] [CrossRef] [PubMed]
- Selinski, J.; Konlg, N.; Wellmeyer, B.; Hanke, G.T.; Linke, V.; Neuhaus, H.E.; Scheibe, R. The plastid-localized NAD-dependent malate dehydrogenase in crucial for energy homeostasis in developing Arabidopsis thaliana seeds. Mol. Plant 2014, 7, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. Oxidative environment and redox homeostasis in plants: Dissecting out significant contribution of major cellular organelles. Front. Environ. Sci. 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Hoque, T.S.; Hossain, M.A.; Mostofa, M.G.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Methylglycoxal; An emerging signaling molecule in plant abiotic stress responses and tolerance. Front. Plant Sci. 2016, 7, 1341. [Google Scholar] [CrossRef] [PubMed]
- Daie, J. Cytosolic fructose-1,6-bisphosphatase: A key enzyme in the sucrose biosynthetic pathway. Photo. Res. 1993, 38, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Preiser, A.L.; Li, Z.; Weise, S.E.; Sharkey, T.D. Triose phosphate use limitation of photosynthesis: Short-term and long-term effects. Planta 2016, 243, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Jamar, C.; du Jardin, P.; Fauconnier, M.L. Cell wall polysaccharides hydrolysis of malting barley (Hordeum vulgare L.): A review. Biotechnol. Agron. Soc. Environ. 2011, 15, 301–313. [Google Scholar]
- Troncoso-Ponce, M.A.; Nikovics, K.; Marchive, C.; Lepiniec, L.; Baud, S. New insights on the organization and regulation of the fatty acid biosynthetic network in the model higher plant Arabidopsis thaliana. Biochimie 2016, 120, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Barata-Soares, A.D.; Gomez, M.L.P.A.; de Mesquita, C.H.; Lajolo, F.M. Ascorbic acid biosynthesis: A precursor study on plants. Braz. J. Plant Physiol. 2004, 16, 147–154. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry & Molecular Biology of Plants; Wiley: Rockville, MD, USA, 2000; pp. 358–361, 498–517, 630–636. [Google Scholar]
- Darvasi, A.; Soller, M. A simple method to calculate resolving power and confidence interval of QTL map location. Behav. Genet. 1997, 27, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Nagelkerke, N.J.D. A note on a general definition of the coefficient of determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- Sosnowski, O.; Charcosset, A.; Joets, J. BioMercator V3: An upgrade of genetic map compilation and quantitative trait loci meta-analysis algorithms. Bioinformatics 2012, 28, 2082–2083. [Google Scholar] [CrossRef] [PubMed]

| Chr (LG) a | Meta-QTL | Meta-QTL Position (cM) | CI b (cM) | Weight c | Number of Projected QTL d | CI of Projected QTL (cM) | Left Markere | Right Marker e | Physical Distance (bp) | Number of Candidate Genes f | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Map Position (cM) | Physical Position f (bp) | Name | Map Position (cM) | Physical Position f (bp) | ||||||||||
| 3 | Protein+Oil | ||||||||||||||
| (N) | mPO3-1 | 20.41 | 18.33–22.49 | 0.13 | 3 | 2.41–29.89 | Satt152 | 17.36 | 3,366,405 | Satt009 | 22.59 | 3,932,012 | 565,607 | 35 | |
| mPO3-2 | 25.09 | 24.63–25.55 | 0.41 | 6 | 12.41–57.02 | BARC-064081-18547 | 23.85 | 4,802,477 | Satt530 | 25.97 | 5,664,735 | 862,258 | 56 | ||
| mPO3-3 | 31.05 | 30.22–31.88 | 0.21 | 5 | 12.41–57.02 | BARC-065251-19285 | 29.93 | 19,028,238 | BARC-011565-00290 | 32.46 | 29,807,303 | 10,779,065 | 268 | ||
| mPO3-4 | 58.97 | 58.49–59.46 | 0.24 | 3 | 34.68–69.21 | Satt549 | 57.27 | 37,342,774 | BARC-010211-00550 | 59.65 | 37,823,900 | 481,126 | 54 | ||
| 5 | Protein+Oil | ||||||||||||||
| (A1) | mPO5-1 | 4.84 | 2.43–7.25 | 0.08 | 1 | 2.57–7.12 | BARC-040651-07808 | 2.45 | 2,295,488 | BARC-019485-03631 | 7.66 | 2,748,936 | 453,448 | 49 | |
| mPO5-2 | 14.61 | 10.52–18.71 | 0.08 | 2 | 10.51–27.99 | BARC-044997-08863 | 9.48 | 3,924,139 | Satt276 | 18.91 | 5,158,700 | 1,234,561 | 123 | ||
| mPO5-3 | 21.36 | 18.80–23.92 | 0.17 | 3 | 10.51–27.65 | Satt276 | 18.91 | 5,158,700 | BARC-014883-01912 | 24.07 | 5,758,793 | 600,093 | 51 | ||
| mPO5-4 | 55.73 | 54.18–57.28 | 0.50 | 6 | 47.87–62.84 | BARC-037207-06739 | 53.77 | 35,375,747 | BARC-040033-07641 | 57.47 | 35,961,573 | 585,826 | 65 | ||
| mPO5-5 | 80.34 | 80.20–80.49 | 0.17 | 2 | 76.61–82.00 | Satt200 | 80.04 | 39,622,009 | BARC-058653-17430 | 80.63 | 39,819,839 | 197,830 | 27 | ||
| 6 | Protein+Oil | ||||||||||||||
| (C2) | mPO6-1 | 5.35 | 3.38–7.33 | 0.07 | 1 | 3.37–7.33 | BARC-041825-08108 | 2.22 | 1,226,605 | BARC-035239-07157 | 11.62 | 1,673,727 | 447,122 | 59 | |
| mPO6-2 | 31.28 | 29.23–33.35 | 0.20 | 3 | 23.02–38.62 | BARC-016957-02165 | 26.08 | 3,823,755 | BARC-059985-16274 | 34.40 | 5,449,370 | 1,625,615 | 209 | ||
| mPO6-3 | 40.84 | 40.28-41.40 | 0.27 | 5 | 23.02–43.44 | BARC-027948-06704 | 40.25 | 6,712097 | BARC-056271-14211 | 41.46 | 6,919,465 | 207,368 | 23 | ||
| mPO6-4 | 48.43 | 46.56–50.31 | 0.13 | 2 | 45.45–51.30 | Satt291 | 42.94 | 7,326,519 | Satt457 | 52.51 | 8,788,659 | 1,462,140 | 161 | ||
| mPO6-5 | 68.48 | 65.44–71.52 | 0.20 | 3 | 61.33–78.84 | BARC-029937-06757 | 65.04 | 10,929,259 | BARC-018663-03235 | 71.60 | 11,925,180 | 995,921 | 133 | ||
| mPO6-6 | 122.19 | 121.81–122.58 | 0.13 | 2 | 117.73-137.09 | Sat_252 | 116.34 | 48,211,060 | BARC-016969-02170 | 126.94 | 49,267,136 | 1,057,076 | 98 | ||
| 7 | Protein+Oil | ||||||||||||||
| (M) | mPO7-1 | 13.43 | 10.83–16.03 | 0.09 | 2 | 12.88–47.24 | BARC-029703-06326 | 10.60 | 1,630781 | Satt150 | 16.86 | 2,434,308 | 755,784 | 96 | |
| mPO7-2 | 20.99 | 18.76–23.23 | 0.10 | 3 | 18.90–47.24 | Sat_316 | 18.67 | 2,722,475 | BARC-054347-12492 | 24.46 | 3,320,310 | 597,835 | 57 | ||
| mPO7-3 | 29.17 | 21.97–36.38 | 0.11 | 2 | 18.90–47.24 | Sat_316 | 18.67 | 2,722,475 | BARC-028455-05917 | 36.98 | 5,937,694 | 3,215,219 | 314 | ||
| mPO7-4 | 37.57 | 33.84–41.30 | 0.22 | 5 | 18.90–55.03 | Satt567 | 32.75 | 4,559,651 | BARC-039195-07465 | 41.37 | 6,567,400 | 2,007,749 | 199 | ||
| mPO7-5 | 44.44 | 41.84–47.04 | 0.39 | 5 | 18.90–55.03 | BARC-042815-08424 | 41.37 | 6,443,468 | BARC-048517-10647 | 47.38 | 8,461,619 | 2,018,151 | 194 | ||
| mPO7-6 | 66.69 | 57.99–75.40 | 0.09 | 1 | 58.99–74.40 | Sat_003 | 57.84 | 12,303,557 | BARC-013407-01480 | 75.42 | 31,260,765 | 18,957,208 | 590 | ||
| 9 g | Protein+Oil | ||||||||||||||
| (K) | mPO9-1 | 14.40 | 8.70–20.10 | 0.09 | 1 | 8.70–20.11 | BARC-051589-11168 | 8.77 | 1,434,250 | BARC-039923-07610 | 21.43 | 3,136,549 | 1,702,299 | 190 | |
| mPO9-2 | 30.67 | 29.23–32.11 | 0.20 | 4 | 21.47–43.20 | BARC-022201-04296 | 28.04 | 4,266,665 | BARC-014659-01609 | 34.03 | 5,901,485 | 1,634,820 | 130 | ||
| mPO9-3 | 40.46 | 39.83–41.10 | 0.61 | 8 | 21.47–52.13 | BARC-062013-17617 | 39.77 | 7,779,719 | BARC-058145-15142 | 41.31 | 21,880,468 | 14,100,749 | 370 | ||
| mPO9-4 | 51.49 | 50.49–52.49 | 0.10 | 2 | 39.00–54.41 | Satt725 | 49.08 | 31,346,707 | Sat_044 | 53.24 | 36,759,518 | 5,412,811 | 214 | ||
| 14 g | Protein+Oil | ||||||||||||||
| (B2) | mPO14-1 | 17.66 | 14.72–20.60 | 0.27 | 3 | 6.09–31.73 | BARC-051559-11161 | 14.48 | 2,598,423 | BARC-021353-04044 | 21.73 | 4,395,242 | 1,796,819 | 201 | |
| mPO14-2 | 48.70 | 46.81–50.60 | 0.62 | 7 | 38.63–61.78 | BARC-064873-18956 | 45.46 | 8,132,273 | BARC-055677-13598 | 53.92 | 9,110,441 | 978,168 | 74 | ||
| mPO14-3 | 57.12 | 55.44–58.80 | 0.11 | 6 | 38.63–61.78 | BARC-014309-01312 | 54.51 | 9,434,565 | Sat_182 | 59.99 | 31,281,020 | 21,846,455 | 482 | ||
| 15 g | Protein+Oil | ||||||||||||||
| (E) | mPO15-1 | 11.68 | 10.09–13.27 | 0.26 | 5 | 5.56–26.90 | BARC-025493-06513 | 9.97 | 2,127,717 | Satt411 | 13.66 | 2,517,428 | 389,711 | 49 | |
| mPO15-2 | 18.98 | 17.96–20.01 | 0.40 | 8 | 5.56–31.24 | BARC-008231-00112 | 17.95 | 3,964,389 | BARC-042857-08439 | 20.04 | 3,846,538 | (117,851)h | 14 | ||
| mPO15-3 | 43.58 | 40.57–46.59 | 0.13 | 2 | 38.22–49.90 | BARC-027480-06591 | 40.00 | 8,304,621 | BARC-018901-03270 | 47.29 | 9,840,775 | 1,536,154 | 179 | ||
| mPO15-4 | 53.21 | 52.75–53.68 | 0.13 | 2 | 48.84–54.45 | BARC-017283-02257 | 53.29 | 10,562,976 | BARC-052667-11557 | 53.77 | 11,139,595 | 576,619 | 57 | ||
| mPO15-5 | 58.03 | 57.93–58.13 | 0.07 | 1 | 57.17–58.89 | BARC-028607-05972 | 57.24 | 11,651,285 | BARC-017755-03124 | 59.38 | 11,818,830 | 167,545 | 12 | ||
| 19 | Protein+Oil | ||||||||||||||
| (L) | mPO19-1 | 15.29 | 12.28–18.31 | 0.27 | 4 | 7.40–35.02 | Satt446 | 10.42 | 1,678,524 | Satt388 | 21.14 | 4,244,178 | 2,565,654 | 165 | |
| mPO19-2 | 34.96 | 31.80–37.83 | 0.15 | 3 | 18.01–53.17 | Satt497 | 31.39 | 33,865,280 | BARC-013203-00448 | 39.34 | 37,425,576 | 3,560,296 | 220 | ||
| mPO19-3 | 50.85 | 48.42–53.28 | 0.24 | 4 | 36.20–62.21 | BARC-016181-02303 | 46.51 | 38,087,635 | BARC-007554-00101 | 54.93 | 39,579,279 | 1,491,644 | 121 | ||
| mPO19-4 | 58.94 | 57.31–60.58 | 0.17 | 3 | 36.20–62.21 | BARC-059657-15973 | 56.99 | 40,154,846 | Satt678 | 61.40 | 43,032,497 | 2,877,651 | 295 | ||
| mPO19-5 | 82.98 | 81.99–83.97 | 0.17 | 2 | 68.81–87.22 | Satt664 | 81.33 | 46,227,991 | BARC-014655-01607 | 84.05 | 46,596,334 | 368,343 | 54 | ||
| 20 | Protein | ||||||||||||||
| (I) | mP20-1 | 15.02 | 12.82–17.23 | 0.20 | 3 | 10.81–22.49 | BARC-055857-13795 | 11.35 | 677,408 | BARC-057033-14543 | 17.68 | 1,738,862 | 1,061,454 | 105 | |
| mP20-2 | 25.43 | 24.37–26.50 | 0.60 | 10 | 10.81–39.24 | Satt367 | 24.01 | 2,615,668 | BARC-040841-07852 | 27.14 | 2,946,641 | 330,973 | 26 | ||
| mP20-3 | 49.47 | 49.17–49.78 | 0.13 | 3 | 47.33–67.38 | BARC-055423-13277 | 44.95 | 36,055,353 | BARC-050455-09643 | 49.92 | 36,575,544 | 520,191 | 49 | ||
| mP20-4 | 60.48 | 54.19–66.77 | 0.07 | 1 | 53.58–67.38 | BARC-025987-05207 | 53.77 | 37,350,343 | Sat_418 | 66.82 | 39,876,415 | 2,526,072 | 270 | ||
| Oil | |||||||||||||||
| mO20-1 | 15.86 | 13.56–18.16 | 0.19 | 5 | 11.13–34.78 | BARC-055857-13795 | 11.35 | 677,408 | BARC-021887-04232 | 18.51 | 1,900,702 | 1.223.294 | 111 | ||
| mO20-2 | 24.08 | 21.90–26.27 | 0.36 | 8 | 13.93–48.44 | BARC-052017-11314 | 19.96 | 2,103,067 | BARC-040841-07852 | 27.14 | 2,946,641 | 843,574 | 63 | ||
| mO20-3 | 35.26 | 29.44–41.09 | 0.14 | 3 | 14.28–48.44 | BARC-057867-14973 | 29.16 | 28,540,212 | BARC-039921-07608 | 42.13 | 35,080,674 | 6,540,462 | 307 | ||
| mO20-4 | 49.47 | 49.47–49.48 | 0.16 | 3 | 29.23–49.64 | BARC-055423-13277 | 44.95 | 36,055,353 | BARC-050455-09643 | 49.92 | 36,575,544 | 520,191 | 49 | ||
| mO20-5 | 64.99 | 62.63–67.36 | 0.15 | 2 | 54.48–75.56 | BARC-017939-02461 | 60.30 | 38,750,487 | BARC-021323-04037 | 67.42 | 39,876,415 | 1,125,928 | 115 | ||
| Protein+Oil | |||||||||||||||
| mPO20-1 | 15.40 | 13.82–16.98 | 0.19 | 9 | 10.81–29.58 | BARC-055857-13795 | 11.35 | 677,408 | BARC-057041-14548 | 17.03 | 1,666,918 | 989,510 | 100 | ||
| mPO20-2 | 24.98 | 24.07–25.90 | 0.46 | 19 | 10.81–48.44 | Satt367 | 24.01 | 2,615,668 | BARC-065829-19777 | 26.05 | 2,795,596 | 179,928 | 14 | ||
| mPO20-3 | 34.29 | 30.15–38.43 | 0.09 | 5 | 14.28–48.44 | BARC-039387-07311 | 30.14 | 27,997,262 | BARC-020713-04700 | 38.61 | 34,052,339 | 6,055,077 | 201 | ||
| mPO20-4 | 49.47 | 49.47–49.47 | 0.26 | 8 | 29.23–66.48 | BARC-055423-13277 | 44.95 | 36,055,353 | BARC-050455-09643 | 49.92 | 36,575,544 | 520,191 | 49 | ||
| Protein+Cys+Met | |||||||||||||||
| mPCM20-1 | 15.03 | 12.84–17.22 | 0.17 | 3 | 10.81–22.49 | BARC-055857-13795 | 11.35 | 677,408 | BARC-057033-14543 | 17.68 | 1,738,862 | 1,061,454 | 105 | ||
| mPCM20-2 | 26.09 | 25.28–26.90 | 0.56 | 11 | 10.81–39.24 | Satt367 | 24.01 | 2,615,668 | BARC-040841-07852 | 27.14 | 2,946,641 | 330,973 | 26 | ||
| mPCM20-3 | 49.47 | 49.40–49.54 | 0.17 | 3 | 47.33–67.38 | BARC-055423-13277 | 44.95 | 36,055,353 | BARC-050455-09643 | 49.92 | 36,575,544 | 520,191 | 49 | ||
| mPCM20-4 | 76.75 | 75.05–78.46 | 0.11 | 2 | 69.83–93.95 | Satt292 | 74.78 | 40,623,844 | Satt162 | 78.82 | 41,416,130 | 792,286 | 85 | ||
| Chr | Meta-QTL | Gene Name | Start (bp) | Stop (bp) | Annotation a | Metabolism b |
|---|---|---|---|---|---|---|
| 3 | mPO3-1 | 3,366,405 | 3,932,012 | |||
| Glyma.03g030400 | 3,372,331 | 3,374,781 | Phenylacetaldoxime monooxygenase | Glucosinolate biosynthesis from phenylalanine | ||
| Glyma.03g030500 | 3,402,824 | 3,403,934 | Chitinase | Chitinase degradation | ||
| Glyma.03g030600 | 3,427,088 | 3,429,159 | Phenylacetaldoxime monooxygenase | Glucosinolate biosynthesis from phenylalanine | ||
| Glyma.03g030800 | 3,458,451 | 3,463,303 | Phenylacetaldoxime monooxygenase | Glucosinolate biosynthesis from phenylalanine | ||
| Glyma.03g030900 | 3,462,650 | 3,465,320 | Phenylacetaldoxime monooxygenase | Glucosinolate biosynthesis from phenylalanine | ||
| Glyma.03g031000 | 3,483,334 | 3,486,055 | Phenylacetaldoxime monooxygenase | Glucosinolate biosynthesis from phenylalanine | ||
| Glyma.03g031200 | 3,503,057 | 3,504,917 | Phenylacetaldoxime monooxygenase | Glucosinolate biosynthesis from phenylalanine | ||
| Glyma.03g031300 | 3,515,990 | 3,517,595 | Costunolide synthase | Constunolide biosynthesis | ||
| Glyma.03g031400 | 3,522,165 | 3,527,021 | Anthocyanidin 3-O-glucosyltransferase | Flavonoid biosynthesis | ||
| Glyma.03g032500 | 3,716,149 | 3,718,912 | Anthocyanidin 3-O-glucosyltransferase | Flavonoid biosynthesis | ||
| Glyma.03g032600 | 3,738,632 | 3,739,843 | Anthocyanidin 3-O-glucosyltransferase | Flavonoid biosynthesis | ||
| Glyma.03g032700 | 3,740,621 | 3,743,020 | Anthocyanidin 3-O-glucosyltransferase | Flavonoid biosynthesis | ||
| Glyma.03g032800 | 3,786,702 | 3,789,334 | Anthocyanidin 3-O-glucosyltransferase | Flavonoid biosynthesis | ||
| 5 | mPO5-1 | 2,295,488 | 2,748,936 | |||
| Glyma.05g026800 | 2,301,052 | 2,303,249 | Xylogalacturonan β-1,3-xylosyltransferase | Xylogalacturonan biosynthesis | ||
| Glyma.05g028400 | 2,444,345 | 2,448,119 | Aldose 1-epimerase | Trehalose degradation II (trehalase) | ||
| Glyma.05g028500 | 2,449,777 | 2,459,772 | Lipid exporter ABCA1 and related proteins, ABC superfamily | Fatty acid transportation | ||
| Glyma.05g029100 | 2,501,803 | 2,508,656 | Aldehyde dehydrogenase family | Arginine degradation I (arginase pathway)/proline degradation | ||
| Glyma.05g029200 | 2,510,682 | 2,517,754 | Aldehyde dehydrogenase family | Arginine degradation I (arginase pathway)/proline degradation | ||
| Glyma.05g029900 | 2,565,716 | 2,569,741 | Nitrate transporter | Nitrate assimilation | ||
| Glyma.05g030300 | 2,603,033 | 2,605,522 | Nitrate transporter | Nitrate assimilation | ||
| Glyma.05g030400 | 2,609,893 | 2,613,480 | Nitrate transporter | Nitrate assimilation | ||
| Glyma.05g030500 | 2,615,166 | 2,618,849 | Nitrate transporter | Nitrate assimilation | ||
| Glyma.05g030600 | 2,626,715 | 2,629,668 | Nitrate transporter | Nitrate assimilation | ||
| Glyma.05g031200 | 2,712,680 | 2,715,373 | Glucose/Sorbosone dehydrogenase | Carbohydrate metabolic process | ||
| mPO5-5 | 39,622,009 | 39,819,839 | ||||
| Glyma.05g216400 | 39,673,239 | 39,678,762 | Cellulase/ENDO-1,4-BETA-GLUCANASE | Carbohydrate metabolic process | ||
| Glyma.05g216600 | 39,686,415 | 39,694,722 | Long-chain-fatty-acid-CoA ligase | D-myo-inositol (1,4,5)-trisphosphate biosynthesis/fatty acid activation | ||
| Glyma.05g216700 | 39,696,647 | 39,699,812 | Nucleoside-diphosphate kinase | UTP and CTP de novo biosynthesis | ||
| Glyma.05g217100 | 39,735,139 | 39,739,763 | UDP-glucose 4-epimerase | Galactose degradation I (Leloir pathway) | ||
| Glyma.05g217400 | 39,752,430 | 39,754,981 | Lipase (class 3) | Fatty acid biosynthesis | ||
| Glyma.05g217600 | 39,762,157 | 39,765,360 | 1-phosphatidylinositol 4-kinase | D-myo-inositol (1,4,5)-trisphosphate biosynthesis/3-phosphoinositide biosynthesis | ||
| 6 | mPO6-3 | 6,712,097 | 6,919,465 | |||
| Glyma.06g087100 | 6,750974 | 6,754,953 | Acylglycerol lipase | Triacylglycerol degradation | ||
| Glyma.06g087800 | 6,788436 | 6,793,747 | Malate dehydrogenase (oxaloacetate-decarboxylating) (NADP+) | Gluconeogenesis | ||
| Glyma.06g088200 | 6,824021 | 6,828,704 | Amino acid permease 1 | Amino acid transportation | ||
| Glyma.06g088300 | 6,838983 | 6,846,287 | Amino acid permease 8 | Amino acid transportation | ||
| Glyma.06g088600 | 6,865939 | 6,873,001 | 6-phosphofructokinase | Glycolysis | ||
| 15 | mPO15-1 c | 2,127,717 | 2,517,428 | |||
| Glyma.15g026400 | 2,130531 | 2,134,563 | Linoleate 9S-lipoxygenase | Jasmonic acid biosynthesis | ||
| Glyma.15g026500 | 2,142191 | 2,147,489 | Linoleate 9S-lipoxygenase | Jasmonic acid biosynthesis | ||
| Glyma.15g027000 | 2,171688 | 2,174,398 | Threonine synthase | Threonine biosynthesis | ||
| Glyma.15g027100 | 2,177956 | 2,179,285 | Glucuronosyl-N-acetylglucosaminyl-proteoglycan 4-α-N-acetylglucosaminyltransferase | Cell wall synthesis | ||
| Glyma.15g028200 | 2,254166 | 2,256,553 | Peroxidase | Active oxygen species-scavenging systems | ||
| Glyma.15g028900 | 2,325622 | 2,329,211 | Hydroxyacylglutathione hydrolase | Methylglyoxal degradation | ||
| Glyma.15g029200 | 2,356317 | 2,366,181 | Electron-transferring-flavoprotein dehydrogenase | Protein degradation | ||
| Glyma.15g029300 | 2,366688 | 2,369,189 | Strictosidine synthase | Alkaloid biosynthesis | ||
| Glyma.15g029800 | 2,387957 | 2,391,720 | Fructokinase | Sucrose degradation | ||
| mPO15-2 c | 3,846,538 | 3,964,389 | ||||
| Glyma.15g049100 | 3,869,436 | 3,870,986 | Vinorine synthase | Alkaloid biosynthesis | ||
| Glyma.15g050100 | 3,958,743 | 3,960,926 | Fructose-bisphosphatase | Glycolysis/Sucrose biosynthesis | ||
| mPO15-5 c | 11,651,285 | 11,818,830 | ||||
| Glyma.15g142500 | 11,655,353 | 11,656,913 | Glucan endo-1,3-β-d-glucosidase | Cell wall degradation | ||
| Glyma.15g143100 | 11,744,760 | 11,750,567 | Dihydrolipoyl dehydrogenase | Acetyl-CoA biosynthesis | ||
| 20 | mP20-2, | 2,615,668 | 2,946,641 | |||
| mPCM20-2 | Glyma.20g024800 | 2,679,571 | 2,683,022 | Pyruvate kinase | Glycolysis | |
| Glyma.20g025200 | 2,743,092 | 2,745,282 | l-ascorbate oxidase | Ascorbic acid biosynthesis/degradation | ||
| Glyma.20g025400 | 2,768,770 | 2,781,380 | Asparagine synthase (glutamine-hydrolyzing) | Asparagine biosynthesis | ||
| Glyma.20g025700 | 2,813,089 | 2,825,575 | 2-hydroxy-6-oxonona-2,4-dienedioate hydrolase | TCA cycle | ||
| Glyma.20g026000 | 2,856,949 | 2,863,351 | Sarcosine/dimethylglycine N-methyltransferase | Amino acid biosynthesis | ||
| Glyma.20g026300 | 2,894,580 | 2,905,241 | β-glucosidase | Starch degradation | ||
| Glyma.20g026400 | 2,909,731 | 2,912,529 | UDP-glucose 6-dehydrogenase | l-ascorbate biosynthesis | ||
| Glyma.20g026700 | 2,935,653 | 2,945,665 | Phosphorylase | Carbohydrate metabolism | ||
| mPO20-2 | 2,615,668 | 2,795,596 | ||||
| Glyma.20g024800 | 2,679,571 | 2,683,022 | Pyruvate kinase | Glycolysis | ||
| Glyma.20g025200 | 2,743,092 | 2,745,282 | l-ascorbate oxidase | Ascorbic acid biosynthesis/degradation | ||
| Glyma.20g025400 | 2,768,770 | 2,781,380 | Asparagine synthase (glutamine-hydrolyzing) | Asparagine biosynthesis | ||
| mP20-3, | 36,055,353 | 36,575,544 | ||||
| mO20-4, | Glyma.20g118000 | 36,083,279 | 36,091,841 | β-lactamase | Cell wall metabolism | |
| mPO20-4, | Glyma.20g120300 | 36,306,759 | 36,311,703 | Trehalose-phosphatase | Trehalose biosynthesis | |
| mPCM20-3 | Glyma.20g121200 | 36,384,081 | 36,391,030 | Diacylglycerol diphosphate phosphatase | Triacylglycerol biosynthesis | |
| Glyma.20g121300 | 36,395,415 | 36,400,718 | Diacylglycerol diphosphate phosphatase | Triacylglycerol biosynthesis | ||
| Glyma.20g121400 | 36,402,262 | 36,406,832 | Amino acid permease | Amino acid transportation | ||
| Glyma.20g121500 | 36,414,549 | 36,419,060 | Amino acid permease | Amino acid transportation | ||
| Glyma.20g122300 | 36,506,583 | 36,511,326 | Procollagen-lysine 5-dioxygenase | Collagen biosynthesis via Lysine | ||
| Glyma.20g122400 | 36,516,501 | 36,521,436 | Procollagen-lysine 5-dioxygenase | Collagen biosynthesis via Lysine | ||
| Glyma.20g122500 | 36,524,182 | 36,526,957 | Fructose-bisphosphate aldolase | Sucrose biosynthesis |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van, K.; McHale, L.K. Meta-Analyses of QTLs Associated with Protein and Oil Contents and Compositions in Soybean [Glycine max (L.) Merr.] Seed. Int. J. Mol. Sci. 2017, 18, 1180. https://doi.org/10.3390/ijms18061180
Van K, McHale LK. Meta-Analyses of QTLs Associated with Protein and Oil Contents and Compositions in Soybean [Glycine max (L.) Merr.] Seed. International Journal of Molecular Sciences. 2017; 18(6):1180. https://doi.org/10.3390/ijms18061180
Chicago/Turabian StyleVan, Kyujung, and Leah K. McHale. 2017. "Meta-Analyses of QTLs Associated with Protein and Oil Contents and Compositions in Soybean [Glycine max (L.) Merr.] Seed" International Journal of Molecular Sciences 18, no. 6: 1180. https://doi.org/10.3390/ijms18061180