Sugar-Binding Profiles of Chitin-Binding Lectins from the Hevein Family: A Comprehensive Study
Abstract
:1. Introduction
2. Results and Discussion
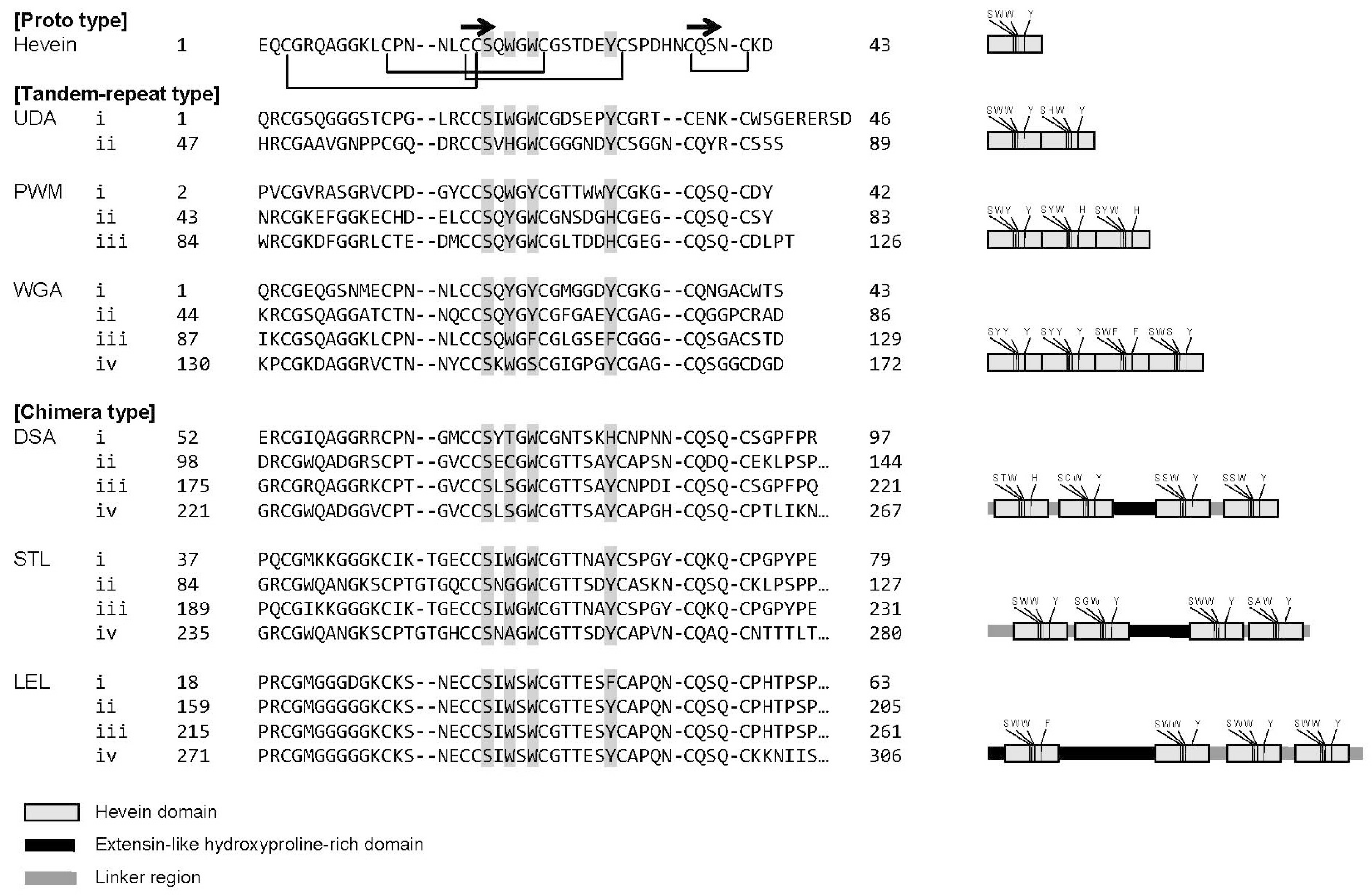
2.1. A Strategy for Comprehensive Analysis of Sugar-Binding Properties of Chitin-Binding Lectins
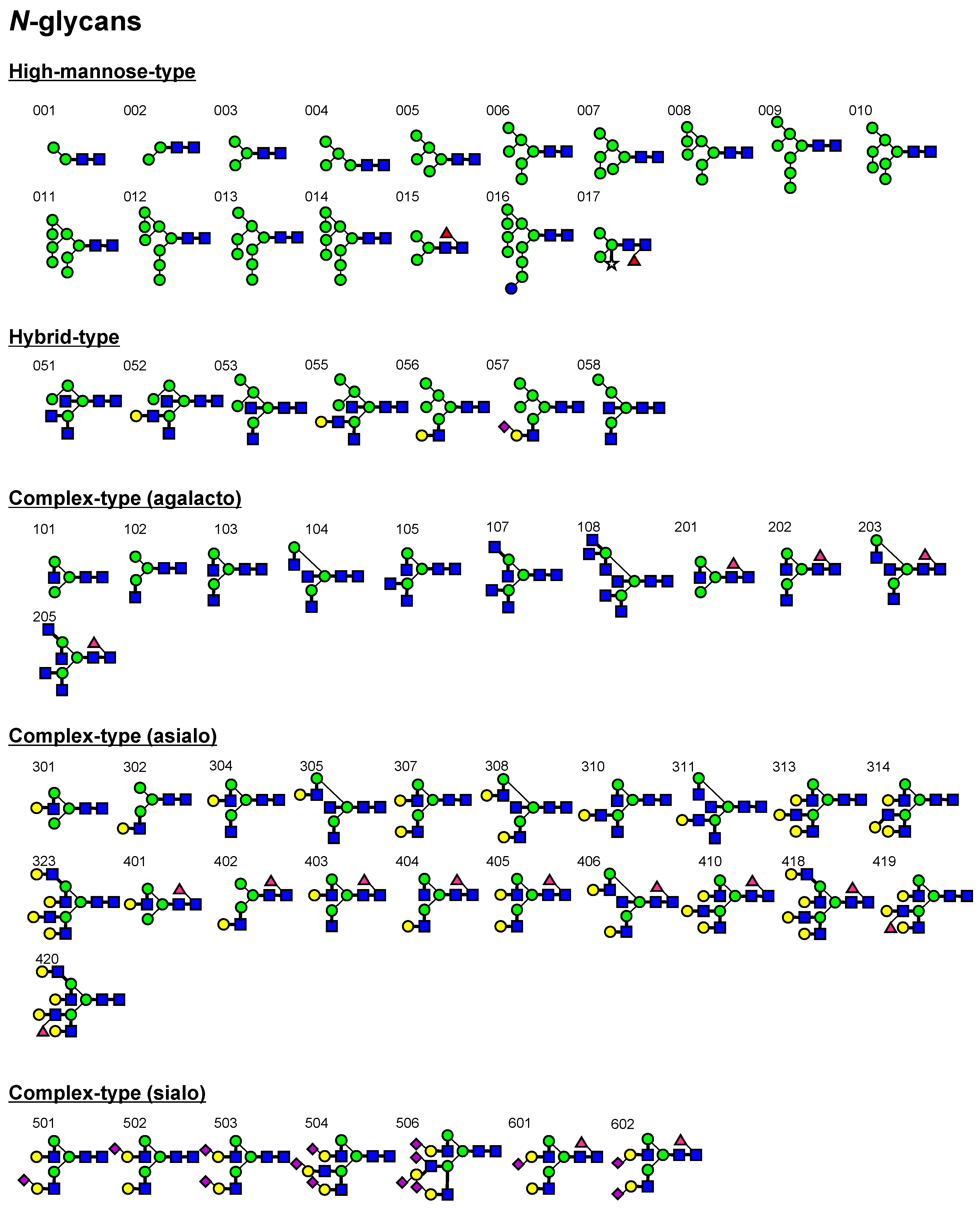
2.2. FAC for Determination of Kd’s to a Panel of PA-Oligosaccharides
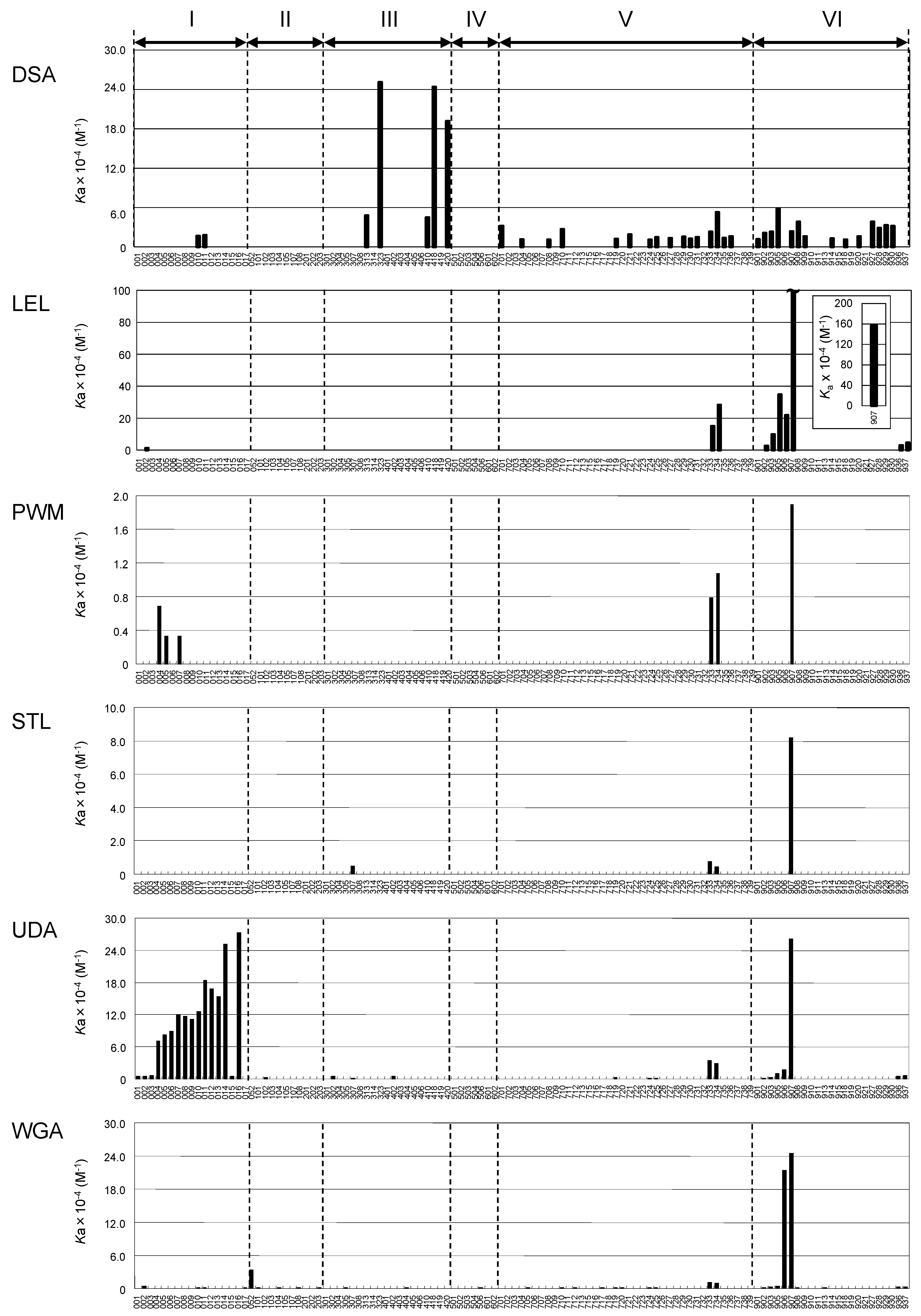
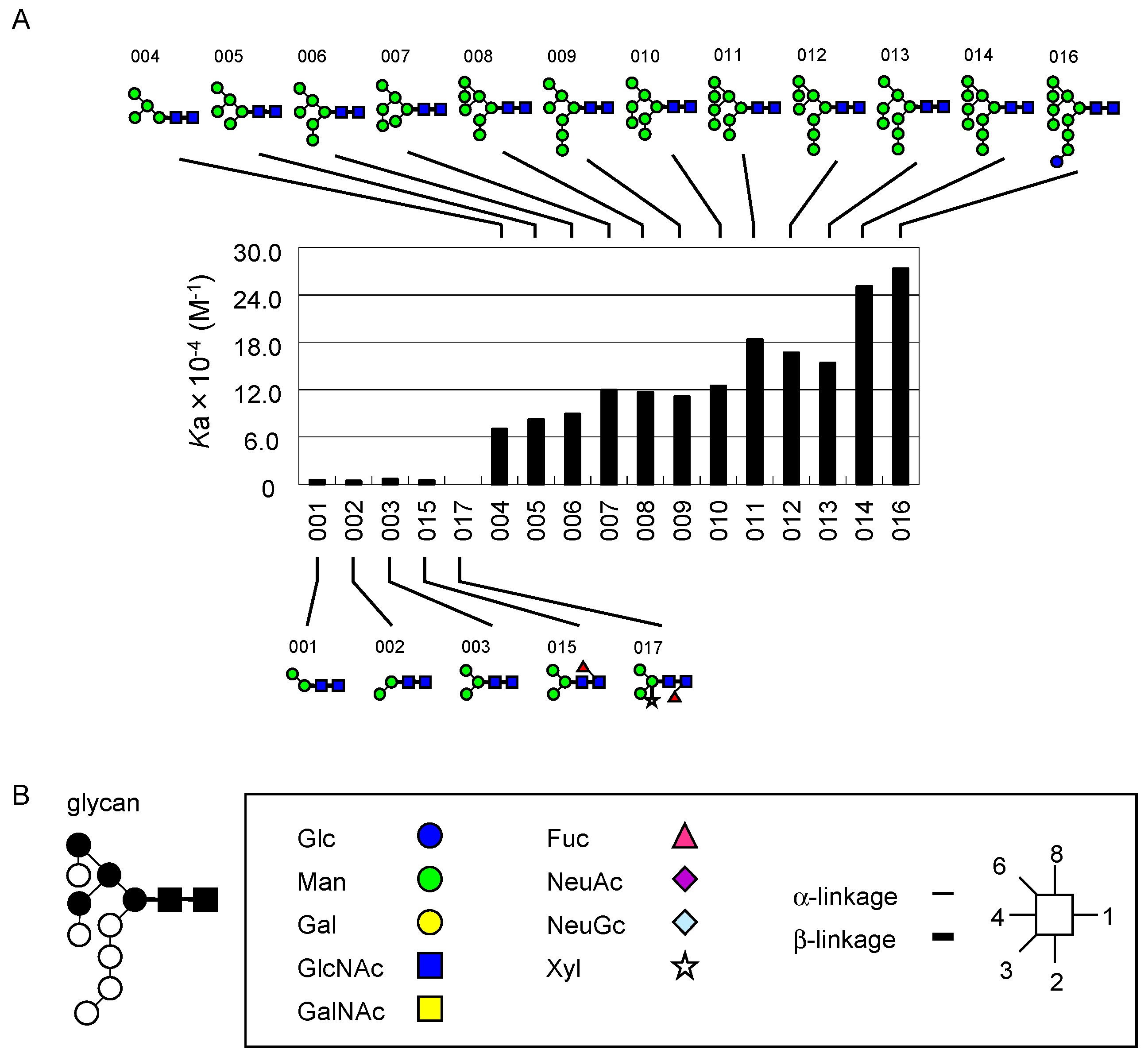
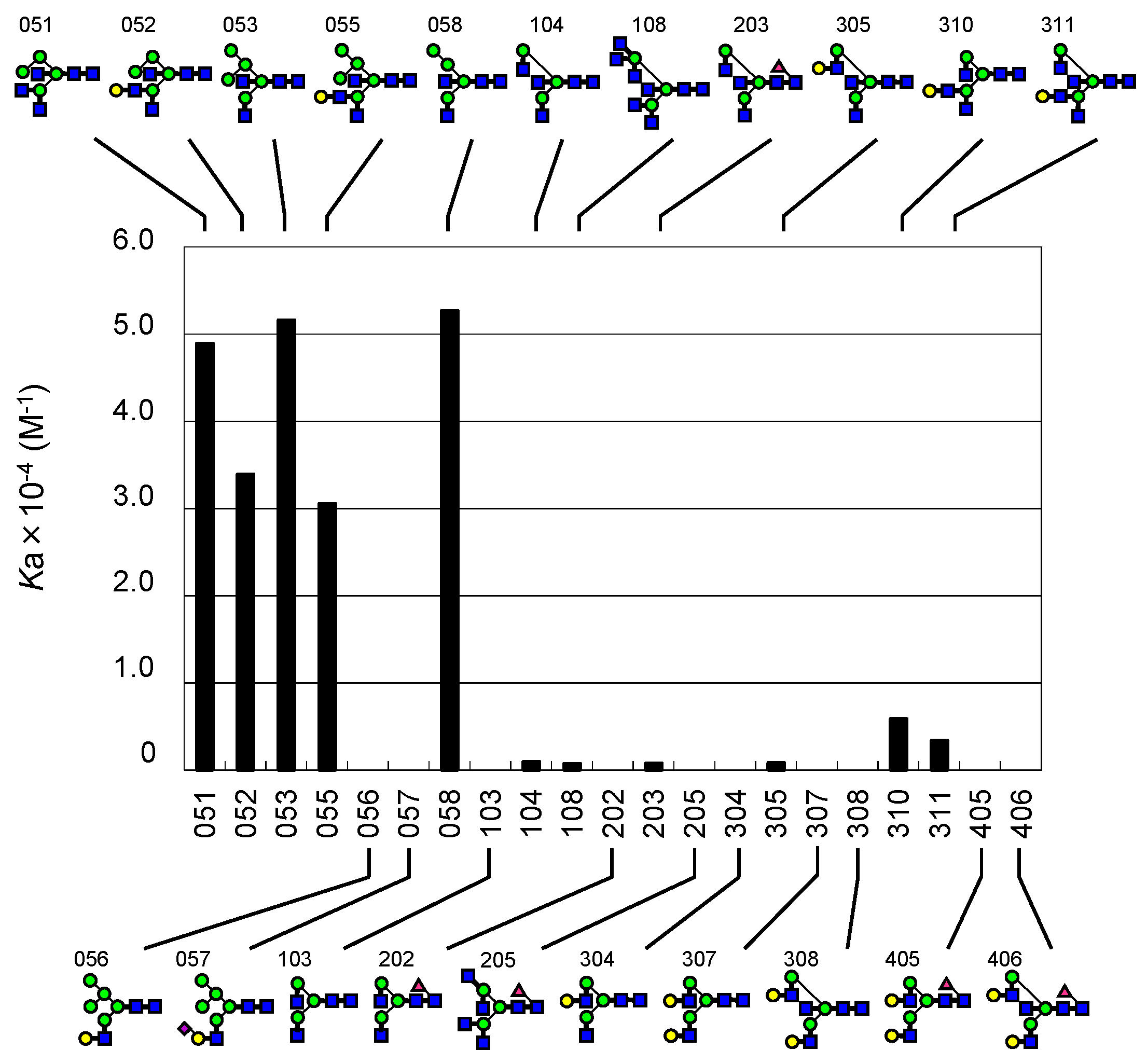
2.3. Global Features of Sugar-Binding Properties of Chitin-Binding Lectins
2.4. Depicted Features of Individual Lectins
2.4.1. DSA
2.4.2. LEL
2.4.3. STL
2.4.4. PWM
2.4.5. UDA
2.4.6. WGA
2.5. Specificity for Multivalent Oligosaccharides: Binding Features on Glycoconjugate Microarray
2.5.1. Binding Features to Synthetic Glycans Conjugated to PAA
2.5.2. Binding Features to Glycoproteins
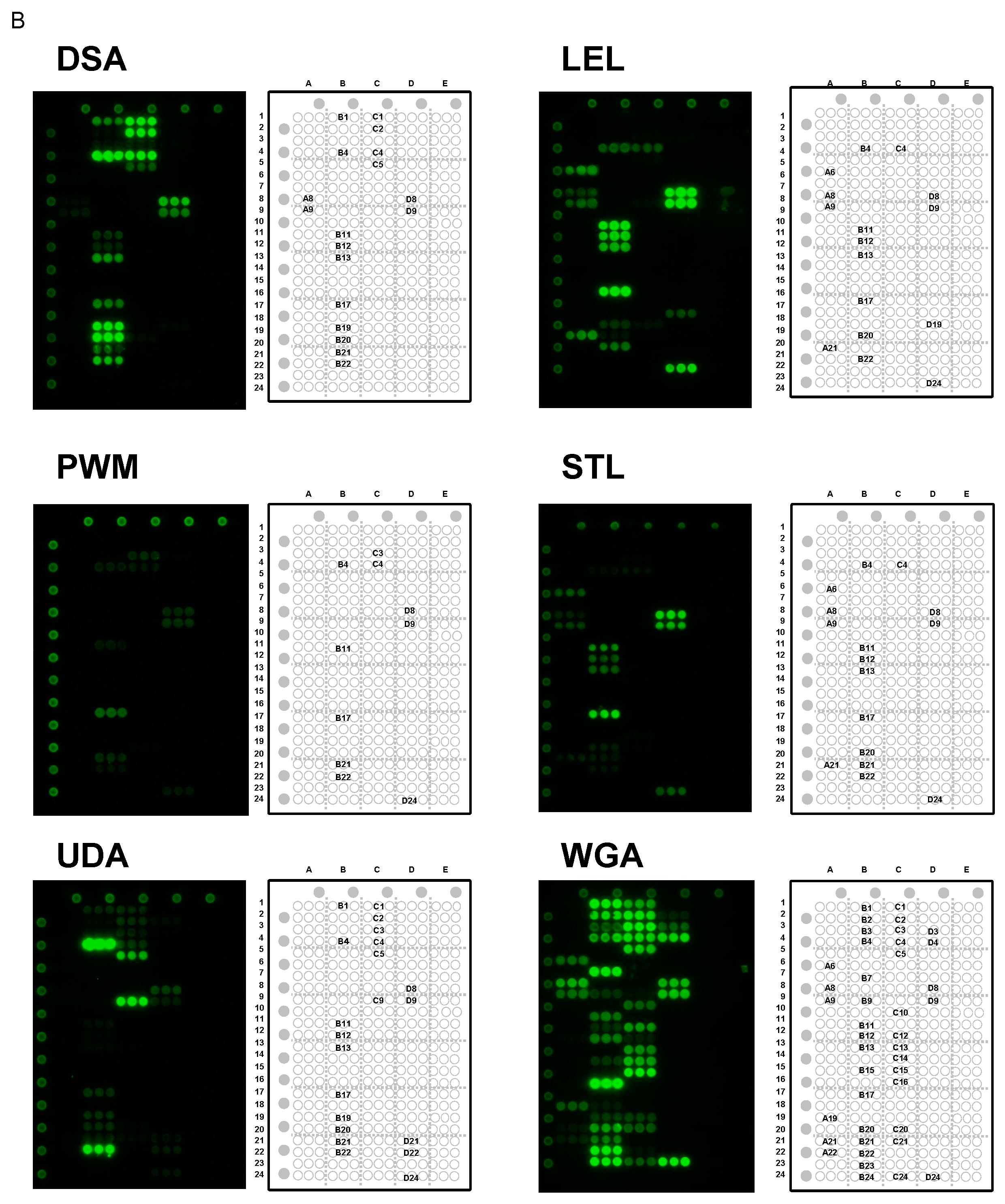
2.6. Clustering Effect of Sialic Acid; Binding Features on a Lectin Microarray
2.7. Future Engineering of Chitin-Binding Proteins as Glycan Probes
3. Materials and Methods
3.1. Materials
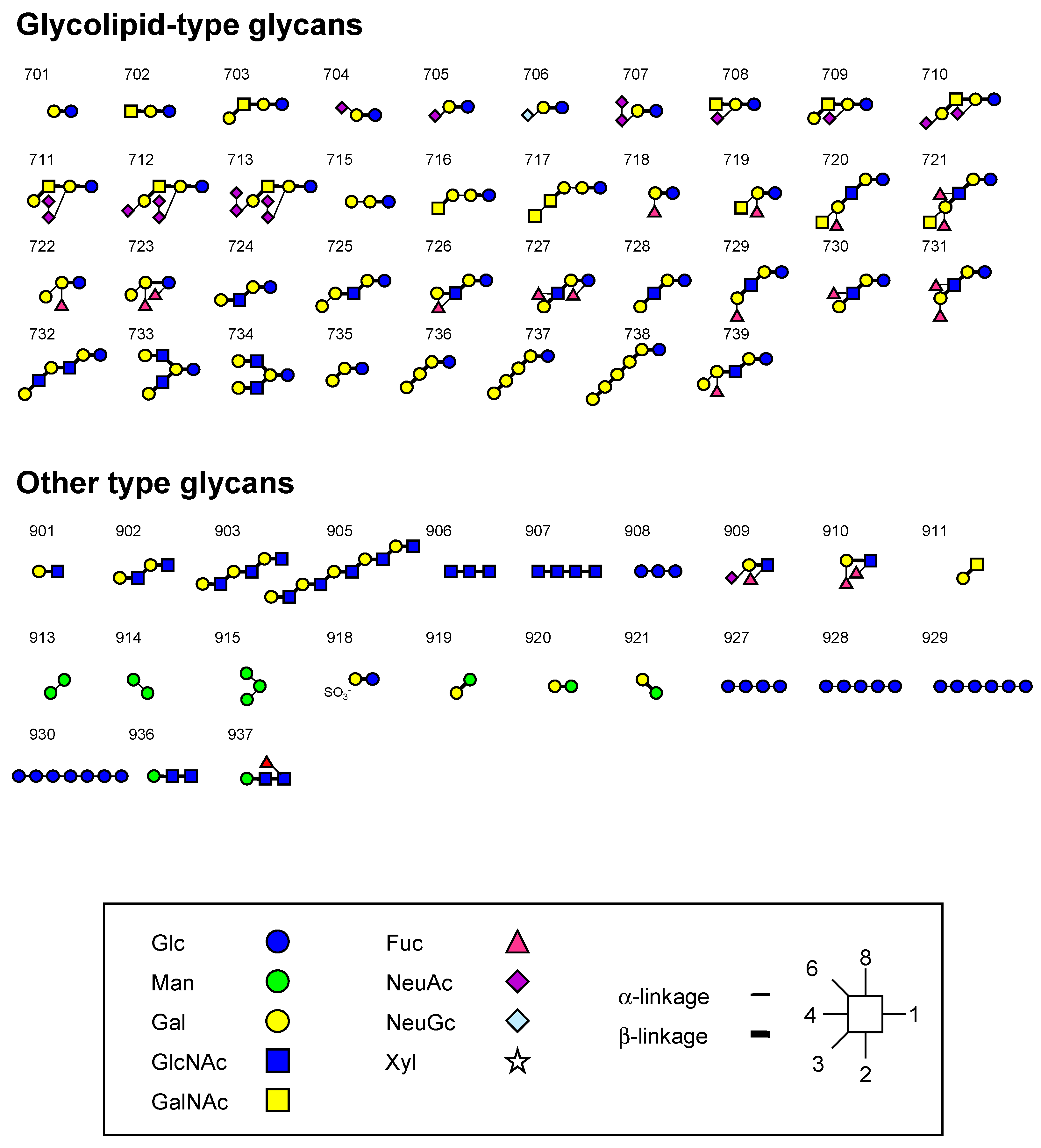
3.2. Oligosaccharides
3.3. Preparation of Lectin Columns
3.4. Frontal Affinity Chromatography (FAC)
3.5. Concentration-Dependent Analysis
3.6. Glycoconjugate-Microarray Analysis
3.7. Lectin Microarray Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AGP | α1-acid glycoprotein |
| BSA | Bovine serum albumin |
| BSM | Bovine submaxillary gland mucin |
| CRD | Carbohydrate-recognition domain |
| CSA | Chondroitin sulfate A |
| CSB | Chondroitin sulfate B |
| DSA | Datura stramonium agglutinin |
| FAC | Frontal affinity chromatography |
| FET | Fetuin |
| GnT | GlcNAc transferase |
| GSL | Griffonia simplicifolia lectin |
| HA | Hyaluronic acid |
| HP | Heparin |
| HS | Heparin sulfate |
| Inver | Invertase |
| KS | Keratin sulfate |
| LDN | LacdiNAc |
| LEL | Lycopersicon esculentum lectin |
| LN | LacNAc |
| OVA | Ovalbumin |
| OVM | Ovomucoid |
| PA | Pyridylaminated |
| pNP | p-nitrophenyl |
| PWM | Phytolacca americana (pokeweed) mitogen |
| RCA | Ricinus communis agglutinin |
| STL | Solanum tuberosum lectin |
| TF | Transferrin |
| TG | Thyroglobulin |
| UDA | Urtica dioica agglutinin |
| WGA | Triticum vulgaris (wheat germ) agglutinin |
References
- Wood, C.; Kabat, E.A.; Murphy, L.A.; Goldstein, I.J. Immunochemical studies of the combining sites of the two isolectins, A4 and B4, isolated from Bandeiraea simplicifolia. Arch. Biochem. Biophys. 1979, 198, 1–11. [Google Scholar] [CrossRef]
- Wu, A.M.; Sugii, S.J. Differential binding properties of GalNAc and/or Ga1 specific lectins. Adv. Exp. Med. Biol. 1988, 228, 205–263. [Google Scholar] [PubMed]
- Wu, A.M.; Wu, J.H.; Chen, Y.Y.; Song, S.C.; Kabat, E.A. Further characterization of the combining sites of Bandeiraea (Griffonia) simplicifolia lectin-I, isolectin A(4). Glycobiology 1999, 9, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Citores, L.; Ferreras, J.M.; Iglesias, R.; Carbajales, M.L.; Arias, F.J.; Jimenez, P.; Rojo, M.A.; Girbes, T. Molecular mechanism of inhibition of mammalian protein synthesis by some four-chain agglutinins. Proposal of an extended classification of plant ribosome-inactivating proteins (rRNA N-glycosidases). FEBS Lett. 1993, 329, 59–62. [Google Scholar] [CrossRef]
- Sweeney, E.C.; Tonevitsky, A.G.; Temiakov, D.E.; Agapov, I.I.; Saward, S.; Palmer, R.A. Preliminary crystallographic characterization of ricin agglutinin. Proteins 1997, 28, 586–589. [Google Scholar] [CrossRef]
- Wu, J.H.; Singh, T.; Herp, A.; Wu, A.M. Carbohydrate recognition factors of the lectin domains present in the Ricinus communis toxic protein (ricin). Biochimie 2006, 88, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Baenziger, J.U.; Fiete, D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J. Biol. Chem. 1979, 254, 9795–9799. [Google Scholar] [PubMed]
- Green, E.D.; Brodbeck, R.M.; Baenziger, J.U. Lectin affinity high-performance liquid chromatography. Interactions of N-glycanase-released oligosaccharides with Ricinus communis agglutinin I and Ricinus communis agglutinin II. J. Biol. Chem. 1987, 262, 12030–12039. [Google Scholar] [PubMed]
- Itakura, Y.; Nakamura-Tsuruta, S.; Kominami, J.; Sharon, N.; Kasai, K.; Hirabayashi, J. Systematic comparison of oligosaccharide specificity of Ricinus communis agglutinin I and Erythrina lectins: a search by frontal affinity chromatography. J. Biochem. 2007, 142, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.A.; Goldstein, I.J. Five alpha-D-galactopyranosyl-binding isolectins from Bandeiraea simplicifolia seeds. J. Biol. Chem. 1977, 252, 4739–4742. [Google Scholar] [PubMed]
- Tempel, W.; Tschampel, S.; Woods, R.J. The xenograft antigen bound to Griffonia simplicifolia lectin 1-B(4). X-ray crystal structure of the complex and molecular dynamics characterization of the binding site. J. Biol. Chem. 2002, 277, 6615–6621. [Google Scholar] [CrossRef] [PubMed]
- Asensio, J.L.; Canada, F.J.; Bruix, M.; Rodriguez-Romero, A.; Jimenez-Barbero, J. The interaction of hevein with N-acetylglucosamine-containing oligosaccharides. Solution structure of hevein complexed to chitobiose. Eur. J. Biochem. 1995, 230, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Asensio, J.L.; Canada, F.J.; Siebert, H.C.; Laynez, J.; Poveda, A.; Nieto, P.M.; Soedjanaamadja, U.M.; Gabius, H.J.; Jimenez-Barbero, J. Structural basis for chitin recognition by defense proteins: GlcNAc residues are bound in a multivalent fashion by extended binding sites in hevein domains. Chem. Biol. 2000, 7, 529–543. [Google Scholar] [CrossRef]
- Hayashida, M.; Fujii, T.; Hamasu, M.; Ishiguro, M.; Hata, Y. Similarity between protein-protein and protein-carbohydrate interactions, revealed by two crystal structures of lectins from the roots of pokeweed. J. Mol. Biol. 2003, 334, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.; Peumans, W.J.; Barre, A.; Rouge, P. A composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Plant Lectins 1998, 17, 575–692. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Kasai, K. The family of metazoan metal-independent β-galactoside-binding lectins: Structure, function and molecular evolution. Glycobiology 1993, 3, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bhavanandan, V.P.; Katlic, A.W. The interaction of wheat germ agglutinin with sialoglycoproteins. The role of sialic acid. J. Biol. Chem. 1979, 254, 4000–4008. [Google Scholar] [PubMed]
- Peters, B.P.; Ebisu, S.; Goldstein, I.J.; Flashner, M. Interaction of wheat germ agglutinin with sialic acid. Biochemistry 1979, 18, 5505–5511. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Sueyoshi, S.; Li, H.; Yamamoto, K.; Osawa, T. Carbohydrate binding specificities of several poly-N-acetyllactosamine-binding lectins. Glycoconj. J. 1990, 7, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Dias-Baruffi, M.; Penttila, L.; Renkonen, O.; Nyame, A.K.; Cummings, R.D. Human galectin-1 recognition of poly-N-acetyllactosamine and chimeric polysaccharides. Glycobiology 2004, 14, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Kostrominova, T.Y. Application of WGA lectin staining for visualization of the connective tissue in skeletal muscle, bone, and ligament/tendon studies. Microsc. Res. Tech. 2011, 74, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Mula, J.; Lee, J.D.; Liu, F.; Yang, L.; Peterson, C.A. Automated image analysis of skeletal muscle fiber cross-sectional area. J. Appl. Physiol. 2013, 114, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Benton, R.L.; Maddie, M.A.; Worth, C.A.; Mahoney, E.T.; Hagg, T.; Whittemore, S.R. Transcriptomic screening of microvascular endothelial cells implicates novel molecular regulators of vascular dysfunction after spinal cord injury. J. Cereb. Blood Flow Metab. 2008, 28, 1771–1785. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.T.; Levine, S.T.; Haynes, S.M.; Gutierrez, P.; Baratta, J.L.; Tan, Z.; Longmuir, K.J. Use of labeled tomato lectin for imaging vasculature structures. Histochem. Cell Biol. 2015, 143, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Imai, S.; Haga, S.; Nagaike, C.; Morimura, Y.; Hatake, K. Localization of binding sites of Ulex europaeus I, Helix pomatia and Griffonia simplicifolia I-B4 lectins and analysis of their backbone structures by several glycosidases and poly-N-acetyllactosamine-specific lectins in human breast carcinomas. Histochem. Cell Biol. 1996, 106, 331–339. [Google Scholar] [PubMed]
- Jacobs, K.; Lakes-Harlan, R. Lectin histochemistry of the metathoracic ganglion of the locust schistocerca gregaria before and after axotomy of the tympanal nerve. J. Comp. Neurol. 1997, 387, 255–265. [Google Scholar] [CrossRef]
- Allen, A.K.; Neuberger, A.; Sharon, N. The purification, composition and specificity of wheat-germ agglutinin. Biochem. J. 1973, 131, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Drenth, J.; Low, B.W.; Richardson, J.S.; Wright, C.S. The toxin-agglutinin fold. A new group of small protein structures organized around a four-disulfide core. J. Biol. Chem. 1980, 255, 2652–2655. [Google Scholar] [PubMed]
- LeVine, D.; Kaplan, M.J.; Greenaway, P.J. The purification and characterization of wheat-germ agglutinin. Biochem. J. 1972, 129, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Burger, M.M. Wheat germ agglutinin. Isolation and crystallization. J. Biol. Chem. 1972, 247, 2248–2250. [Google Scholar] [PubMed]
- Nagata, Y.; Burger, M.M. Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J. Biol. Chem. 1974, 249, 3116–3122. [Google Scholar] [PubMed]
- Wright, C.S.; Olafsdottir, S. Structural differences in the two major wheat germ agglutinin isolectins. J. Biol. Chem. 1986, 261, 7191–7195. [Google Scholar] [PubMed]
- Yamaguchi, K.; Mori, A.; Funatsu, G. The complete amino acid sequence of lectin-C from the roots of pokeweed (Phytolacca americana). Biosci. Biotechnol. Biochem. 1995, 59, 1384–1385. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Mori, A.; Funatsu, G. Amino acid sequence and some properties of lectin-D from the roots of pokeweed (Phytolacca americana). Biosci. Biotechnol. Biochem. 1996, 60, 1380–1382. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Yurino, N.; Kino, M.; Ishiguro, M.; Funatsu, G. The amino acid sequence of mitogenic lectin-B from the roots of pokeweed (Phytolacca americana). Biosci. Biotechnol. Biochem. 1997, 61, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Beintema, J.J.; Peumans, W.J. The primary structure of Stinging nettle (Urtica dioica) agglutinin. A two-domain member of the hevein family. FEBS Lett. 1992, 299, 131–134. [Google Scholar] [CrossRef]
- Does, M.P.; Ng, D.K.; Dekker, H.L.; Peumans, W.J.; Houterman, P.M.; Van Damme, E.J.; Cornelissen, B.J. Characterization of Urtica dioica agglutinin isolectins and the encoding gene family. Plant Mol. Biol. 1999, 39, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Harata, K.; Muraki, M. Crystal structures of Urtica dioica agglutinin and its complex with tri-N-acetylchitotriose. J. Mol. Biol. 2000, 297, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.K.; Bolwell, G.P.; Brown, D.S.; Sidebottom, C.; Slabas, A.R. Potato lectin: A three-domain glycoprotein with novel hydroxyproline-containing sequences and sequence similarities to wheat-germ agglutinin. Int. J. Biochem. Cell Biol. 1996, 28, 1285–1291. [Google Scholar] [CrossRef]
- Desai, N.N.; Allen, A.K.; Neuberger, A. Some properties of the lectin from Datura stramonium (thorn-apple) and the nature of its glycoprotein linkages. Biochem. J. 1981, 197, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Kieliszewski, M.J.; Showalter, A.M.; Leykam, J.F. Potato lectin: A modular protein sharing sequence similarities with the extensin family, the hevein lectin family, and snake venom disintegrins (platelet aggregation inhibitors). Plant J. 1994, 5, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, K.; Tanaka, K.; Murakami, T.; Nakashita, H.; Sakamoto, H.; Oguri, S. Datura stramonium agglutinin: Cloning, molecular characterization and recombinant production in arabidopsis thaliana. Glycobiology 2015, 25, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.; Barre, A.; Rouge, P.; Peumans, W.J. Potato lectin: An updated model of a unique chimeric plant protein. Plant J. 2004, 37, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Oguri, S.; Amano, K.; Nakashita, H.; Nagata, Y.; Momonoki, Y.S. Molecular structure and properties of lectin from tomato fruit. Biosci. Biotechnol. Biochem. 2008, 72, 2640–2650. [Google Scholar] [CrossRef] [PubMed]
- Peumans, W.J.; Rouge, P.; van Damme, E.J. The tomato lectin consists of two homologous chitin-binding modules separated by an extensin-like linker. Biochem. J. 2003, 376, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Arata, Y.; Kasai, K. Frontal affinity chromatography as a tool for elucidation of sugar recognition properties of lectins. Methods Enzymol. 2003, 362, 353–368. [Google Scholar] [PubMed]
- Kasai, K.; Oda, Y.; Nishikata, M.; Ishii, S. Frontal affinity chromatography: Theory for its application to studies on specific interactions of biomolecules. J. Chromatogr. 1986, 376, 33–47. [Google Scholar] [CrossRef]
- Nakamura-Tsuruta, S.; Uchiyama, N.; Hirabayashi, J. High-throughput analysis of lectin-oligosaccharide interactions by automated frontal affinity chromatography. Methods Enzymol. 2006, 415, 311–325. [Google Scholar] [PubMed]
- Tateno, H.; Mori, A.; Uchiyama, N.; Yabe, R.; Iwaki, J.; Shikanai, T.; Angata, T.; Narimatsu, H.; Hirabayashi, J. Glycoconjugate microarray based on an evanescent-field fluorescence-assisted detection principle for investigation of glycan-binding proteins. Glycobiology 2008, 18, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Uchiyama, N.; Koseki-Kuno, S.; Ebe, Y.; Takashima, S.; Yamada, M.; Hirabayashi, J. Evanescent-field fluorescence-assisted lectin microarray: A new strategy for glycan profiling. Nat. Methods 2005, 2, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, N.; Kuno, A.; Tateno, H.; Kubo, Y.; Mizuno, M.; Noguchi, M.; Hirabayashi, J. Optimization of evanescent-field fluorescence-assisted lectin microarray for high-sensitivity detection of monovalent oligosaccharides and glycoproteins. Proteomics 2008, 8, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Nakamura-Tsuruta, S.; Hirabayashi, J. Frontal affinity chromatography: Sugar-protein interactions. Nat. Protoc. 2007, 2, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Debray, H.; Decout, D.; Strecker, G.; Spik, G.; Montreuil, J. Specificity of twelve lectins towards oligosaccharides and glycopeptides related to N-glycosylproteins. Eur. J. Biochem. 1981, 117, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, A.; Seno, N.; Matsumoto, I. Tryptophan residues and the sugar binding site of potato lectin. J. Biochem. 1984, 95, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, Y.; Yamamoto, K.; Tsuji, T.; Osawa, T. Structural requirements for the binding of high-mannose-type glycopeptides to immobilized pokeweed PA-2 lectin. Carbohydr. Res. 1983, 120, 283–292. [Google Scholar] [CrossRef]
- Kilpatrick, D.C.; Jeffree, C.E.; Lockhart, C.M.; Yeoman, M.M. Immunological evidence for structural similarity among lectins from species of the solanaceae. FEBS Lett. 1980, 113, 129–133. [Google Scholar] [CrossRef]
- Kilpatrick, D.C.; Yeoman, M.M. Purification of the lectin from Datura stramonium. Biochem. J. 1978, 175, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.T.; Gabius, H.J.; Lee, Y.C. Thermodynamic parameters of the interaction of Urtica dioica agglutinin with N-acetylglucosamine and its oligomers. Glycoconj. J. 1998, 15, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, I.; Jimbo, A.; Mizuno, Y.; Seno, N.; Jeanloz, R.W. Purification and characterization of potato lectin. J. Biol. Chem. 1983, 258, 2886–2891. [Google Scholar] [PubMed]
- Van Damme, E.J.; Broekaert, W.F.; Peumans, W.J. The Urtica dioica agglutinin is a complex mixture of isolectins. Plant Physiol. 1988, 86, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D.; Kornfeld, S. The distribution of repeating [Gal β 1,4GlcNAc β 1,3] sequences in asparagine-linked oligosaccharides of the mouse lymphoma cell lines BW5147 and PHAR 2.1. J. Biol. Chem. 1984, 259, 6253–6260. [Google Scholar] [PubMed]
- Merkle, R.K.; Cummings, R.D. Relationship of the terminal sequences to the length of poly-N-acetyllactosamine chains in asparagine-linked oligosaccharides from the mouse lymphoma cell line BW5147. Immobilized tomato lectin interacts with high affinity with glycopeptides containing long poly-N-acetyllactosamine chains. J. Biol. Chem. 1987, 262, 8179–8189. [Google Scholar] [PubMed]
- Yamashita, K.; Totani, K.; Ohkura, T.; Takasaki, S.; Goldstein, I.J.; Kobata, A. Carbohydrate binding properties of complex-type oligosaccharides on immobilized Datura stramonium lectin. J. Biol. Chem. 1987, 262, 1602–1607. [Google Scholar] [PubMed]
- Irimura, T.; Nicolson, G.L. Interaction of pokeweed mitogen with poly(N-acetyllactosamine)-type carbohydrate chains. Carbohydr. Res. 1983, 120, 187–195. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Uechi, M.; Katakura, Y.; Oda, T.; Ishiguro, M. Mitogenic properties of pokeweed lectin-d isoforms on human peripheral blood lymphocytes: Non-mitogen PL-D1 and mitogen PL-D2. Biosci. Biotechnol. Biochem. 2004, 68, 1591–1593. [Google Scholar] [CrossRef] [PubMed]
- Bodger, M.P.; McGiven, A.R.; Fitzgerald, P.H. Mitogenic proteins of pokeweed. I. Purification, characterization and mitogenic activity of two proteins from pokeweed (Phytolacca octandra). Immunology 1979, 37, 785–792. [Google Scholar] [PubMed]
- Kilpatrick, D.C. Datura stramonium lectin: Agglutinating and mitogenic activities of a purified preparation. Plant Sci. Lett. 1979, 15, 279–284. [Google Scholar] [CrossRef]
- Gordts, S.C.; Renders, M.; Ferir, G.; Huskens, D.; van Damme, E.J.; Peumans, W.; Balzarini, J.; Schols, D. Nictaba and UDA, two GlcNAc-binding lectins with unique antiviral activity profiles. J. Antimicrob. Chemother. 2015, 70, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.S. Crystal structure of a wheat germ agglutinin/glycophorin-sialoglycopeptide receptor complex. Structural basis for cooperative lectin-cell binding. J. Biol. Chem. 1992, 267, 14345–14352. [Google Scholar] [PubMed]
- Yamamoto, K.; Tsuji, T.; Matsumoto, I.; Osawa, T. Structural requirements for the binding of oligosaccharides and glycopeptides to immobilized wheat germ agglutinin. Biochemistry 1981, 20, 5894–5899. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.T.; Lee, Y.C. Rabbit and rat hepatic lectins have two sugar-combining sites per monomeric unit. Biochem. Biophys. Res. Commun. 1988, 155, 1444–1451. [Google Scholar] [CrossRef]
- Doi, A.; Matsumoto, I.; Seno, N. Fluorescence spectral studies on the specific interaction between sulfated glycosaminoglycans and potato lectin. J. Biochem. 1983, 93, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Doi, A.; Jimbo, A.; Matsumoto, I.; Seno, N. Interaction of sulfated glycosaminoglycans with lectins. J. Biol. Chem. 1981, 256, 5345–5349. [Google Scholar] [PubMed]
- Soga, K.; Teruya, F.; Tateno, H.; Hirabayashi, J.; Yamamoto, K. Terminal N-acetylgalactosamine-specific leguminous lectin from Wisteria japonica as a probe for human lung squamous cell carcinoma. PLoS ONE 2013, 8, e83886. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, J.Q.; Kilcoyne, M.; Joshi, L. Microarray evaluation of the effects of lectin and glycoprotein orientation and data filtering on glycoform discrimination. Anal. Methods 2014, 6, 440–449. [Google Scholar] [CrossRef]
- Spiro, R.G. Studies on the monosaccharide sequence of the serum glycoprotein fetuin. J. Biol. Chem. 1962, 237, 646–652. [Google Scholar] [PubMed]
- Spiro, R.G.; Bhoyroo, V.D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J. Biol. Chem. 1974, 249, 5704–5717. [Google Scholar] [PubMed]
- Bertaux, C.; Daelemans, D.; Meertens, L.; Cormier, E.G.; Reinus, J.F.; Peumans, W.J.; Van Damme, E.J.; Igarashi, Y.; Oki, T.; Schols, D.; et al. Entry of hepatitis C virus and human immunodeficiency virus is selectively inhibited by carbohydrate-binding agents but not by polyanions. Virology 2007, 366, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Ward, H.D.; Alroy, J.; Lev, B.I.; Keusch, G.T.; Pereira, M.E. Identification of chitin as a structural component of Giardia cysts. Infect. Immun. 1985, 49, 629–634. [Google Scholar] [PubMed]
- Kumaki, Y.; Wandersee, M.K.; Smith, A.J.; Zhou, Y.; Simmons, G.; Nelson, N.M.; Bailey, K.W.; Vest, Z.G.; Li, J.K.; Chan, P.K.; et al. Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by Stinging nettle lectin, Urtica dioica agglutinin. Antivir. Res. 2011, 90, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vallet, A.; Mesters, J.R.; Thomma, B.P. The battle for chitin recognition in plant-microbe interactions. FEMS Microbiol. Rev. 2015, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Akcapinar, G.B.; Kappel, L.; Sezerman, O.U.; Seidl-Seiboth, V. Molecular diversity of LysM carbohydrate-binding motifs in fungi. Curr. Genet. 2015, 61, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, J.; Audfray, A.; Imberty, A. Binding sugars: From natural lectins to synthetic receptors and engineered neolectins. Chem. Soc. Rev. 2013, 42, 4798–4813. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Tateno, H.; Hirabayashi, J. Lectin engineering, a molecular evolutionary approach to expanding the lectin utilities. Molecules 2015, 20, 7637–7656. [Google Scholar] [CrossRef] [PubMed]
- Hase, S.; Ikenaka, T.; Matsushima, Y. Structure analyses of oligosaccharides by tagging of the reducing end sugars with a fluorescent compound. Biochem. Biophys Res. Commun. 1978, 85, 257–263. [Google Scholar] [CrossRef]
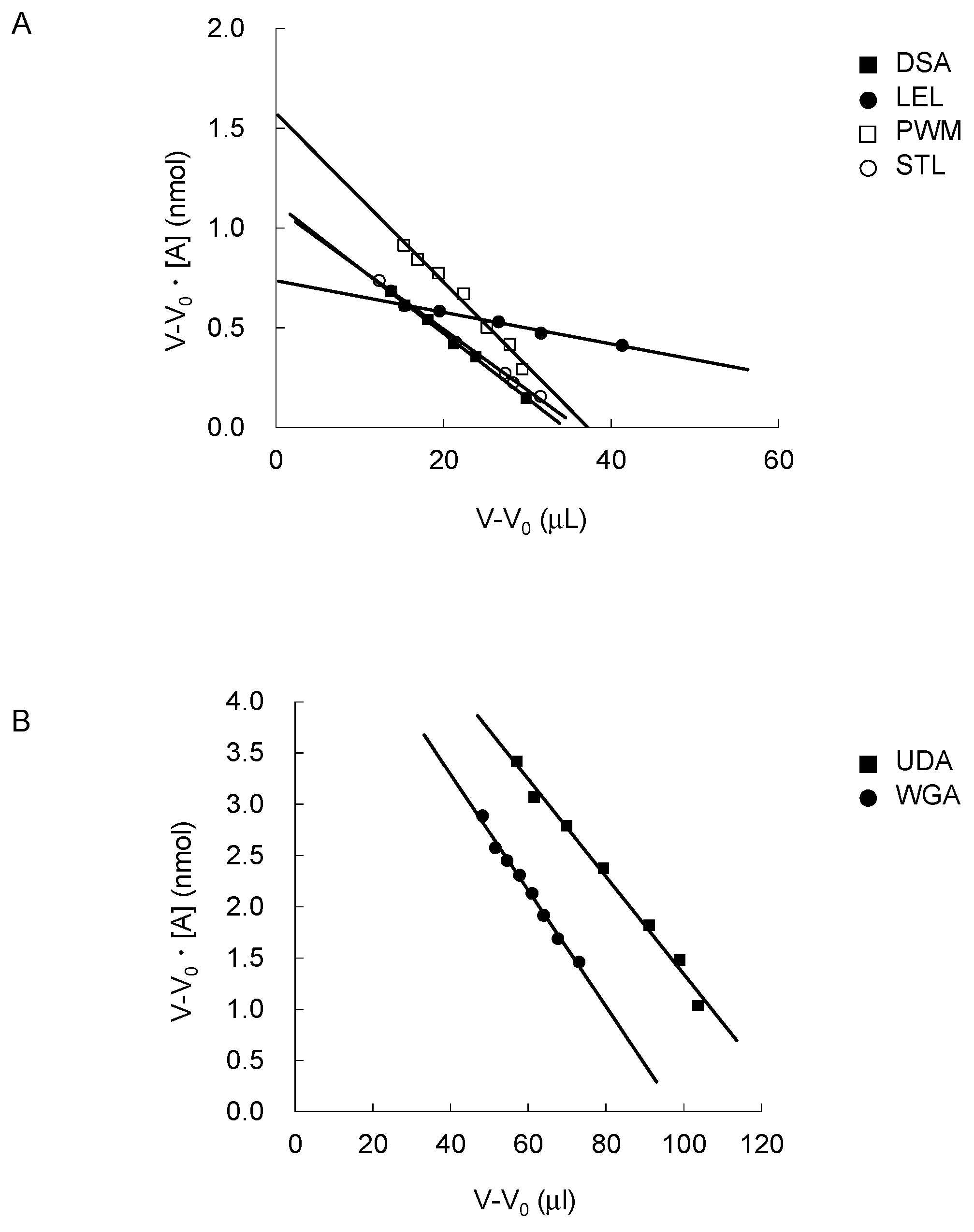


| Lectin Name | Origin | Organ | Immobilized (mg/mL Resin) | Bt (nmol) | r2 a | Glycan-pNP Used for Specification | Kd (μM) |
|---|---|---|---|---|---|---|---|
| DSA | Datura stramonium | Seed | 6.0 | 1.12 | 1.00 | chitotetraose-β-pNP | 33 |
| LEL | Solanum lycopersicum | Fruit | 6.0 | 0.74 | 0.99 | LacNAc-β-pNP | 7.9 |
| 0.5 | 0.11 | 0.96 | chitopentaose-β-pNP b | 4.6 | |||
| PWM | Phytolacca Americana | Plant root | 4.6 | 1.58 | 0.96 | chitopentaose-β-pNP | 42 |
| STL | Solanum tuberosum | Plant tuber | 3.0 | 1.10 | 1.00 | chitopentaose-β-pNP | 31 |
| UDA | Urtica dioica | Rhizome | 6.0 | 6.10 | 0.99 | LacNAc-β-pNP | 48 |
| WGA | Triticum vulgaris | Germ | 7.0 | 5.55 | 0.99 | LacNAc-β-pNP | 57 |
| Lectin | DSA a | LEL b | PWM c | STL d | UDA e | WGA f |
|---|---|---|---|---|---|---|
| Common features: Binding to chito-oligo | ||||||
| chitotriose-PA (906) | N.D. | 4.6 μM | N.D. | N.D. | 57 μM | 4.7 μM |
| chitotetraose-PA (907) | 43 μM | 0.64 μM | 53 μM | 12 μM | 3.8 μM | 4.1 μM |
| Binding to LNH/LNnH | ||||||
| LNnH (type II+ type II; 733) | 43 μM | 6.7 μM | 130 μM | 130 μM | 29 μM | 93 μM |
| LNH (type I + type II; 734) | 19 μM | 3.5 μM | 93 μM | 220 μM | 35 μM | 110 μM |
| Unique features: | ||||||
| 3 other best PA-oligosaccharides | 4.0 μM (323) | 2.9 μM (905) | 150 μM (004) | LacdiNAc-PAA (Glycoconjugate microarray) | 3.7 μM (016) | 19 μM (053) |
| 4.1 μM (418) | 10 μM (903) | 300 μM (005) | 4.0 μM (014) | 19 μM (058) | ||
| 5.2 μM (420) | 39 μM (902) | 300 μM (007) | 5.5 μM (011) | 20 μM (051) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itakura, Y.; Nakamura-Tsuruta, S.; Kominami, J.; Tateno, H.; Hirabayashi, J. Sugar-Binding Profiles of Chitin-Binding Lectins from the Hevein Family: A Comprehensive Study. Int. J. Mol. Sci. 2017, 18, 1160. https://doi.org/10.3390/ijms18061160
Itakura Y, Nakamura-Tsuruta S, Kominami J, Tateno H, Hirabayashi J. Sugar-Binding Profiles of Chitin-Binding Lectins from the Hevein Family: A Comprehensive Study. International Journal of Molecular Sciences. 2017; 18(6):1160. https://doi.org/10.3390/ijms18061160
Chicago/Turabian StyleItakura, Yoko, Sachiko Nakamura-Tsuruta, Junko Kominami, Hiroaki Tateno, and Jun Hirabayashi. 2017. "Sugar-Binding Profiles of Chitin-Binding Lectins from the Hevein Family: A Comprehensive Study" International Journal of Molecular Sciences 18, no. 6: 1160. https://doi.org/10.3390/ijms18061160






