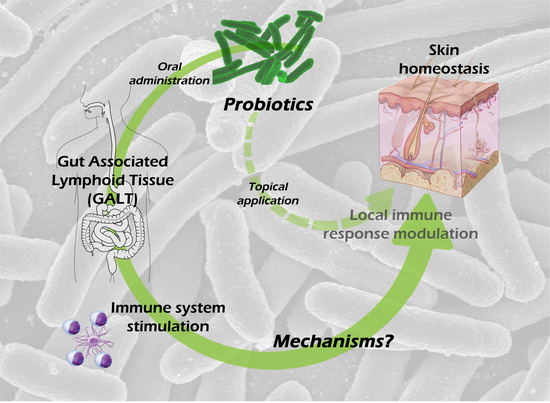
1. Introduction
Three billion microorganisms live within the human body, and they constitute what we know as the microbiota. The largest and most diverse niche is the bowel, which has very special conditions. Many types of microorganisms, each one in a particular amount, can live there without generating an inflammatory microenvironment. In fact, during the last decade, it became clear that the microbiota and the host establish a symbiosis, which has been crucial for our evolution as a species. Thus, our whole physiology depends on the relationship we build with the microbiota. Particularly, the intestinal microbiota is essential for all mammal nutrition, synthesizing vitamins that the host cannot produce by himself/herself, digesting complex carbohydrates and taking part in amino acid homeostasis (especially important in ruminants and coprophage mammals) [
1].
In 1989, Strachan published an epidemiological study where 17,000 individuals were included and followed for 23 years. He found an inverse relationship between hay fever incidence and the number of siblings of the analyzed individuals. Strachan hypothesized that “These observations could, however, be explained if allergic diseases were prevented by infection in early childhood, transmitted by unhygienic contact with older siblings, or acquired prenatally from a mother infected by contact with her older children. Later infection or reinfection by younger siblings might confer additional protection against hay fever” [
2]. This work is considered as the foundation of the “hygiene hypothesis”, which in turn incorporated autoimmune diseases [
3] into allergic disorders as the pathologies modulated by early microbial challenges.
Later in time, many researchers proposed highly industrialized food consumption, anti-microbiotic abuse, as well as commensal parasites’ elimination as factors responsible for altering the intestinal microbiota. They observed that several autoimmune diseases and allergies developed more frequently if those factors were present in the patients. Experiments performed using germ-free animals (animals born and raised in sterile conditions and therefore microbiota-free) have proven that the microbiota is crucial for the development, maturation and normal function of the immune system [
4].
The establishment of a beneficial intestinal microbiota would not just contribute to intestinal homeostasis, but also might have desirable systemic effects by reducing the incidence of certain pathologies. Moreover, it has become clear that there is a relationship between dysbiosis, inflammation and cancer. Mice genetically manipulated to develop inflammatory bowel disease (IBD) can pass on ulcerative colitis (UC) [
5], as well as colorectal cancer [
6] when co-housed with wild-type animals, by horizontal and vertical transmission of pathogenic microbes. At the same time, the microbiota is implicated in the immune response against non-intestinal tumors. In a simple but elegant experiment published in 2015, Sivan and col proved that genetically-identical animals purchased in different laboratories were colonized by different bacteria and responded differently to the B6 melanoma cell line implanted in their skin. In fact, the administration of fecal suspensions equalized the tumor growth between animals [
7]. These results suggest a connection between the intestinal microbiota and immune response against skin tumors.
To summarize, the integrity of intestinal microbiota seems to be one of the key points for the normal development and function of the immune system, while an unbalanced microbiota would predispose to pathology. In the present review article, we will present the current knowledge of the worldwide most-studied strategy to induce a sustained beneficial microbiota: probiotics. Moreover, we will present bibliographic evidence to prove that probiotics could serve as a therapeutic approach for skin conditions
2. Microbiota and Probiotics
The Russian zoologist Ilia Metchnikoff, 1908 Nobel Prize awardee, published in 1907 the book “The Prolongation Of Life: Optimistic Studies”. There, he proposed that large amounts of fermented food consumption could be related to the longevity observed in elderly people of the Caucasus [
8]. He was one of the first researchers that presented lactic bacteria, and fermented food produced by them, as beneficial nutrients and as useful therapeutic tools for many pathologies.
On the other hand, the World Health Organization defined probiotics as live microorganisms that when administered in adequate amounts, confer a benefit to health [
9]. Along these few past years, it has been shown, both in experimental animal models and human individuals, that the beneficial effects depend strongly on the species of the probiotic tested. Just to give some examples,
Lactobacillus reuteri and
Lactobacillus casei, but not
Lactobacillus plantarum, prime monocyte-derived dendritic cells (DC) in a tolerogenic fashion [
10]. Besides, in a dextran-induced ulcerative colitis (UC) model,
Lactobacillus rhamnosus, but not
Bifidobacterium breve, rapidly and effectively improved the dextran-induced bloody diarrhea during the resolution phase [
11]. At last, in a double blind clinical study,
L. rhamnosus HN001, but not
B. animalis, reduced eczema incidence when compared to children administered with placebo [
12].
Once ingested, viable probiotics have to reach the intestinal lumen, colonize and, from that location, produce their effects. Despite that probiotics may not alter the microbiota composition in healthy adults [
13,
14], they appear to re-establish the microbiota more rapidly after antimicrobial therapy [
15]. Besides, in elderly individuals, certain probiotics affect the microbiota metabolism, like flagellar motility, chemotaxis and the adhesion of intestinal bacteria [
16]. The question of whether those effects do impact on human health is still open.
3. Probiotics and Gut-Associated Lymphoid Tissue
The gut-associated lymphoid tissue (GALT) is the most complex of all local immune compartments. It is composed of an epithelial barrier constituted mainly by enterocytes, intraepithelial T lymphocytes, M cells and Paneth cells, a specialized connective tissue called lamina propria, constituted by highly diverse cell lineages (like macrophages, DC, plasmocytes, T-cells), known as the effector compartment of the GALT, and Peyer’s patches (PP) and mesenteric lymph nodes (MLN) known as the induction compartment of the GALT.
There are many articles that explore the effects of probiotics on the GALT; some of them are reviewed by Corthésy [
17]. Briefly, the author summarizes that the effects observed are due to whole bacteria, as well as soluble factors that impact on enterocyte pattern recognition receptors (PRRs), like Toll-like receptors. Upon recognition, enterocytes secrete TGF-β and IL-8, which in turn modulate the immune response. Despite the fact that the WHO dictates that microorganisms must be viable to be considered as probiotics, there is evidence that shows that inactivated or heat-killed bacteria, as well as isolated purified molecules, can exert effects on the GALT [
18,
19,
20,
21,
22,
23]. In fact, it has been suggested to name these soluble probiotic-derived molecules as “probiotaceuticals” [
24].
Besides the enterocytes, DC are crucial for probiotic-host interactions by extending interdigitating dendrites to intestinal lumen and sensing microbial antigens. Afterwards, they mature, migrate and prime T-cells in PP and MLN, inducing different activation profiles. These activated T-cells promote, ultimately, B-cell switching and IgA secretion to the intestinal lumen. Once more, the effects will depend on the microorganisms analyzed and the experimental model used. Young mice administered orally with
L. acidophilus show an upregulation of co-stimulatory molecules in MLN DC. Moreover, when injected in non-treated animals, these DC show an increase in their migration rate to MLN and colon. Finally, when mice were challenged with
Citrobacter rodentium, an intestinal pathogen, these DC significantly reduced the infection susceptibility [
25].
In conclusion, probiotics generate immunomodulation in many tissues and cells of the GALT. Broadly speaking, probiotics induce a protective state against intestinal infections by modulating enterocytes, DC, T- and B-cells.
4. Skin Microbiota and the Skin Immune System
The skin is the largest tissue in the human body and, similar to the intestine, is colonized by millions of microorganisms, around one million bacteria per square centimeter. On the one side, the skin immune system (SIS) is similar to the GALT, as it is a local and organized part of the immune system and is composed of an epithelium, an effector and an induction compartment, as well. On the other side, there are differences in each tissue of the SIS. The epithelium is composed mainly by keratinocytes, but it also contains melanocytes, Langerhans cells (LC) and intraepithelial T-cells; the effector compartment, the dermis, as the lamina propria, is constituted by many different cell types; and the induction of the response happens in the skin draining lymph nodes (SLN).
As in the GALT, the SIS homeostasis depends strongly on the skin microbiota. In fact, the skin is exposed to a great amount of physical, chemical and biological agents that could alter the balance between the microbiota and the SIS. Skin dysbiosis is associated not only with opportunistic microbial infection, but also with chronic skin conditions, especially atopic dermatitis (AD) [
26] and psoriasis [
27]. Recently, a study published in
Nature demonstrated that dysbiosis is also present in a non-inflammatory skin pathology, vitiligo [
28]. Thus, it is becoming clear that alternative therapeutical strategies to preserve the normal skin microbiota are potentially interesting approaches to treat diverse skin conditions. It is important to remark that there is still no evidence that could clearly establish if the dysbiosis is a cause or a consequence of skin pathologies here mentioned.
Apart from preventing microbial infection, the SIS is involved in inducing an anti-inflammatory or tolerogenic state in normal conditions that allows the tissue to coexist with the skin microbiota peacefully. The hyperactivity of different cell populations in the SIS, like keratinocytes, DC and specific T-cells, is present in different skin conditions, like psoriasis [
29], atopic eczema (AE) [
30] and contact dermatitis (CD) [
31,
32].
The first clinical evidence that has shown connections between skin pathology and intestinal microbiota alterations emerged more than a decade ago. Small intestine bacterial overgrowth (SIBO) is more frequent in rosacea patients than in healthy individuals. Moreover, the treatment and eradication of SIBO in rosacea patients resolve rosacea manifestations in 26 of 28 of the individuals analyzed [
33]. On the other hand, inflammatory bowel diseases, like UC and Crohn’s disease, often present skin manifestations. A review article published in 2012 summarizes this issue and presents some of the mechanisms involved [
34].
5. Skin Immune System and Probiotics
On the basis of the above, probiotics can result in a potentially interesting therapeutic strategy for skin clinical conditions. For that purpose, probiotics may be used topically or orally. Despite the fact that there are some articles that show beneficial effects of probiotics on human keratinocytes in vitro [
35,
36,
37] and on mouse skin [
38], there are only a few clinical trials showing effectiveness of topical treatment. One of them proved that
L. plantarum HY7714 applied during 12 weeks on the skin of volunteers aged 41 to 59 years, with dry skin and wrinkles, improved photoaging parameters [
39]. In addition, a preliminary study shows that topical application of
L. plantarum in burn wounds reduces skin infection risk. This constitutes a novel bacteriotherapy for this condition [
40].
On the other hand, orally-administered probiotics have been studied profusely for different skin conditions. Regarding AD, there are more clinical trials performed in children than in adult patients, probably because this pathology is more prevalent in this group [
41]. Meneghin and col gathered together clinical trials where different probiotics were used to prevent or treat AD in children: 13 out of 17 studies showed effectiveness in the prevention of AD, while 15 out of 20 showed effectiveness in the treatment of this condition [
42]. The differences observed in each clinical trial may be explained by the diversity of the probiotic organisms used (including different species of the
Lactobacillus and
Bifidobacterium genus and combinations of them), the clinical conditions and the parameters evaluated. Kukkonen and col performed a double-blind, randomized clinical trial administering placebo or capsules containing probiotics to 1223 pregnant women, carrying children with high risk for allergy, for two to four weeks. These capsules include a combination of
L. rhamnosus GG,
L. rhamnosus LC705,
B. breve Bb99 and
Propionibacterium freudenreichii ssp.
shermanii JS. The newborns received the same capsules, but with the prebiotics galacto-oligosaccharides or placebo, during six months. The study revealed that there were no differences in allergic skin conditions, while the incidence of AE in two-year-old children was reduced [
43]. Similar results were obtained with
L. rhamnosus alone [
12], but opposed to the latter,
L. reuteri has been shown to reduce the incidence of IgE-dependent eczema in children [
44]. When tested in adult AD patients,
B. animalis subsp.
lactis LKM512 [
45], heat-killed
L. acidophilus L-92 [
46] and
L. salivarius LS01 [
47] were each shown to have improved AD symptoms.
6. Probiotics and Cancer
In relation to cancer therapy, the clinical use of probiotics is controversial. To date, there are no clinical trials where probiotics were used as adjuvant therapy for cancer, even though many original articles have been written about probiotics’ effects in experimental models, both in vitro and in vivo. Most of the work on humans is epidemiologic. There, the incidence of colorectal cancer, in the first place, and bladder and mammary cancer, in the second, is correlated with nutritional habits, including the consumption of fermented food [
48].
The Lancet published an epidemiological study performed in Denmark and Finland, which showed a reduced incidence of colon cancer in a rural population, a fact that could be explained by higher milk product consumption, including fermented food. In fact, the low-incidence population had higher numbers of
Lactobacillus in feces [
49]. Moreover, an epidemiological study performed in the USA showed a correlation between higher yogurt consumption and a reduced colon cancer incidence [
50]. At last, another U.S. epidemiological study suggests that cultured milk consumption in adults could be protective for colon cancer development [
51].
There are, also, reports of probiotics’ efficacy in the treatment of extra-intestinal cancer in animal models. Balb/C mice orally administered with
L. acidophilus, before and after mammary carcinoma cell line injection, showed a higher survival rate compared to the placebo group [
52]. Furthermore,
L. helveticus yogurt was shown to reduce and abolish the tumor development in a mammary cancer mouse model, after seven, but not two, days of probiotic treatment [
53].
However, what is the state of knowledge about oral probiotic therapy in skin cancer? Sadly, there are few original articles that explore this field. In a brilliant recent paper, Silvan and col demonstrated the effectiveness of an oral
Bifidobacterium cocktail for melanoma treatment, in a mouse model. They have shown that the oral probiotic is as efficient as PDL-1-specific monoclonal antibody (mAb), the clinically-approved checkpoint blockade therapy. In fact, the co-administration of both the anti-PDL1 mAb and the
Bifidobacterium cocktail almost abolished tumor growth. This is the first report of a mixed treatment of oral probiotics and regular chemotherapy, in an effort to improve the efficacy of the latter [
7]. Furthermore, in our laboratory, we have demonstrated that oral administration of purified lipoteichoic acid (LTA) from
L. rhamnosus GG retards and reduces UV-induced skin squamous cell carcinomas, in a mouse model of chronic UV irradiation [
54].
7. Ultraviolet Radiation, Probiotics and Cancer
The biological effects of ultraviolet radiation (UVr), in particular the UVB fraction, have been deeply studied during the last century. Briefly, it is widely known that the most important direct effects on exposed cells are the DNA pyrimidine dimer formation and the isomerization of urocanic acid. Moreover, reactive oxygen and nitrogen species are important byproducts induced after UV irradiation. The most important distant or systemic effect is the photo-immunosuppression described for the first time by the group of Dr. Margaret Kripke [
55,
56]. Thanks to their work in the late 1970s, knowledge has been built about UVr inducing an immunosuppressive state, which is both local (at the skin level), as well as systemic. This leads to an impaired immune surveillance, which, in turn, can favor cancer development [
57]. In addition, there is clinical evidence that shows that UVr is strongly related to photoaging [
58,
59,
60]. In the last few years, oral probiotic treatment has been proposed as an interesting alternative approach for photoprotection, which may be useful in reducing photoaging, UV-induced immunosuppression and cancer development.
Pequet-Navarro and col performed a clinical study with 54 healthy volunteers, who received
L. johnsonii or placebo during six weeks prior to sun-simulated-UV irradiation. They reported that Langerhans cells from probiotic-treated individuals showed an increased repopulation of the skin, as well as a functional recovery four days after irradiation, compared to the placebo group [
61]. In another study,
L. johnsonii was mixed with carotenoids and subsequently administered to healthy individuals, before they were exposed to “non-extreme UV with high UVA level”. As a result, inflammatory dermal cells (CD45+) were reduced, Langerhans cells’ reduction was avoided and dermal dendrocytes (XIIIa+ cells) were increased in treated individuals, compared to the placebo-administered group [
62]. However, it is not possible to distinguish if the effect observed was due to the probiotics, to the carotenoids or to the combination of both. The same probiotic strain,
L. johnsonii, was employed by Guéniche and col in an animal model of UV-induced immunosuppression. They demonstrated that treatment with the probiotic prior to the irradiation challenge results in a restoration of the cellular immune response.
Less studied are the UVr effects on skin microbiota. Each microorganism in the skin has particular susceptibilities to UVr, which depend also on the time and intensity of the radiation. This could alter the microbiota composition in exposed individuals [
63].
8. Possible Mechanisms Involved in the Skin Effects of Oral Probiotic Treatment
Given the intestinal barrier impermeability to microbial cells and microbial-derived molecules, and therefore the improbable passage of those antigens to the blood, it is feasible to hypothesize the following mechanisms involved in transmitting probiotic signals to the host: (a) probiotics or probiotic-derived molecules could modulate the systemic immune system by activating DC [
7,
61], stimulating NK cells [
62,
63] and inducing different T-cell differentiation profiles/subsets [
64,
65]; or (b) they could modulate the intestine-brain-skin axis.
We will focus on those original articles that explore the skin effects of oral-administered probiotics, setting aside the enormous bibliography that proves in vitro probiotic effects.
8.1. Mechanisms Involved in the Systemic Immunomodulation by Oral Probiotics
Scientists have tried to understand probiotic-host interactions at the skin level by using either contact hypersensitivity (CHS) or delayed-type hypersensitivity (DTH).
L. casei DN-114 001 has been shown to reduce both dinitrofluorobenzene (DNFB)-induced CHS, as well as Ovoalbumin-induced DTH in healthy animals. The mechanism described is related to a reduction in cytotoxic T-cells’ chemotaxis to challenged skin, an increase in regulatory T-cells’ (Tregs) differentiation and an augmented recruitment of these regulatory cells to the challenged skin. In addition, Tregs purified from spleen and draining lymph nodes of probiotic-treated and DNFB-sensitized animals showed a three-fold increase in IL-10 secretion after in vitro polyclonal stimulation. By an adoptive transfer experiment, CD8+ T-cells isolated from probiotic-treated animals were shown to produce a decreased CHS reaction in CD3
−/− recipients compared to CD8+ T-cells from placebo-treated group. On the contrary, a co-transfer experiment showed no differences in CHS reaction between Tregs from the probiotic-treated group and from the control animals in CD3
−/− recipients. Altogether, these results demonstrate that this particular probiotic induces an impaired CD8+ T-cell function, but did not alter Tregs’ effector function [
66]. In an AD mouse model, where the pathology was already established, an oral cocktail containing
L. casei,
L. plantarum,
L. rhamnosus and
B. lactis managed to reduce IgE levels, as well as IL-4 and IL-5, while increasing IL-12p40 and IFN-γ. Treated mice also showed reduced mastocyte infiltration and degranulation [
67]. This evidence shows that probiotics are able not only to modulate T-cell subsets’ activation during normal immune responses, but they also may modulate soluble mediators in established pathological conditions.
In relation to the mechanisms that could explain the reduction in UVr-induced skin damage by oral probiotics, as mentioned above, it has been shown that
L. johnsonii treatment could re-establish LC turn over after irradiation in humans [
61] and abolish UV-induced immunosuppression in mice [
68]. Both effects could be explained, at least in part, by a reduction in IL-10 blood level after UVr in probiotic-treated animals. On the other side,
L. plantarum administration could decrease MMP-13 expression and activity in hairless mice, thus reducing UVr-induced photoaging [
69].
Regarding the DC role in oral probiotic skin effects, Sivan and col demonstrated that melanoma implanted animals administered with a
Bifidobacterium cocktail had more activated DC (increased MHCII expression) infiltrating the tumor microenvironment than saline solution-treated ones. In addition, those DC were able to activate cytotoxic T-cells more efficiently and induced higher IFN-γ levels in vitro. The authors concluded that signals produced by
Bifidobacterium, but not the bacteria itself, could induce DC activation in the steady state. In turn, this could lead to an improved specific cytotoxic T-cell function. They suggest that this effect could be occurring in a non-antigen-specific fashion [
7]. Similarly, in our laboratory, we have proven that LTA from
L. rhamnosus GG administered to chronically UV-irradiated animals increases CD4+ and CD8+ T-cells, as well as it increases IFN-γ secretion in SLN [
54].
Besides the articles here reviewed, many original papers have been written, which describe probiotics’ effects in the GALT. However, to date, there is no work centered on oral probiotic mechanisms that could explain, on one side, the effects seen in the GALT and, on the other side, the modulation of the SIS. Here, some questions that are still open are presented, with regards to the mechanisms mentioned above:
- (1)
Are the mechanisms working in a non-antigen-specific fashion and therefore due to the innate immune system, or, on the contrary, are there antigens both present in damaged skin and in probiotic-conditioned intestine that are being presented in the GALT and therefore responding in an antigen-specific fashion?
- (2)
The effector cells, as DC and T-cells, appear to be involved in the effects observed in the skin after probiotic oral treatment. Are these cells coming from the GALT, or are there just cytokines that are secreted in the GALT, reaching the skin through blood and activating those cells in the SIS?
- (3)
Are there bone marrow-derived DC or inflammatory DC taking part in these effects?
- (4)
How is it explained that the same probiotic bacteria can, on one side, reduce inflammatory levels during an established AD and, on the other side, improve a pro-inflammatory antitumoral response?
8.2. Mechanisms Involving the Intestine-Brain-Skin Axis
The intestine-brain-skin axis hypothesis was first proposed 80 years ago by the dermatologists John H. Stokes and Donald M. Pillsbury [
70] and has recently become of interest once again. Briefly, the hypothesis is centered on the capacity of emotional states (e.g., depression and anxiety) to alter normal intestinal microbiota, to increase intestinal permeability and to contribute to systemic inflammation. They were also among the first ones to propose the use of probiotic
L. acidophilus cultures for human health. Even if the present review article focus is the link between oral probiotics and skin, the brain could be considered an organ taking an important part in this crosstalk [
71].
It has been postulated that substance P (SP) could be involved in the skin-intestine crosstalk. This could be explained by: (a) skin conditions like AD are characterized by a great amount of neuroinflammation with high levels of SP in the skin [
72]; (b) oral antibiotic therapy induces SP release in the enteric nervous system, while
L. paracasei oral treatment reduces this release [
73]. Although there are well-known similarities and connections between the skin and intestine nervous systems and both of them together with the brain have a common embryologic origin, more evidence is needed in order to fully understand the relation between oral probiotics and the intestine-brain axis.
9. Conclusions
Fermented food has been consumed by worldwide cultures since at least the year 6000 B.C. Scientific evidence around the beneficial effects of its consumption is not a novelty. However, the mechanisms involved in the probiotic-host interactions are still poorly understood. After years of deep research, it has become clear that the effects observed depend profusely on the particular microorganisms evaluated. In the future, it may be possible to choose the precise microorganism or combination for each pathology. Last, but not least, probiotics may be potentially explored as an adjuvant treatment to improve the effectiveness of different therapeutic approaches.
Acknowledgments
This study was funded by grants from Universidad de Buenos Aires (UBACyT 2011 to 2014 and 2013 to 2016), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) Proyectos de Investigación Plurianuales (PIP 2011–2013) and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Proyectos de Investigación Científica y Tecnológica (PICT 2012). The authors are members of the CONICET Research Career Program.
Author Contributions
Adrián D. Friedrich, Daniel H. González Maglio and Mariela L. Paz conceived and designed the manuscript. Adrián D. Friedrich collected, assembled, analyzed and interpreted the data. Adrián D. Friedrich, Daniel H. González Maglio and Mariela L. Paz wrote the manuscript. All the authors mentioned and Juliana Leoni contributed with the corrections of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hooper, L.V.; Midtvedt, T.; Gordon, J.I. How host-microbial interactions shape the nutrient enviroment of the mammalian intestine. Annu. Rev. Nutr. 2002, 22, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.F. The Effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 2002, 347, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Cebra, J.J. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 1999, 69, 1046S–1051S. [Google Scholar] [PubMed]
- Garrett, W.S.; Lord, G.M.; Punit, S.; Lugo-Villarino, G.; Mazmanian, S.K.; Ito, S.; Glickman, J.N.; Glimcher, L.H. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 2007, 131, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Elinav, E.; Huber, S.; Strowig, T.; Hao, L.; Hafemann, A.; Jin, C.; Wunderlich, C.; Wunderlich, T.; Eisenbarth, S.C.; et al. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 9862–9867. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Metchnikoff, E. The prolongation of life. Bristol Med. Chir. J. (1883) 1908, 26, 74. [Google Scholar]
- FAO and WHO. Guidelines for the Evaluation of Probiotics in Food; Indian Council of Medical Research: New Delhi, India, 2002. [Google Scholar]
- Smits, H.H.; Engering, A.; van der Kleij, D.; de Jong, E.C.; Schipper, K.; van Capel, T.M.M.; Zaat, B.A.J.; Yazdanbakhsh, M.; Wierenga, E.A.; van Kooyk, Y.; et al. Selective probiotic bacteria induce IL-10–producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell–specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 2005, 115, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; van Bergenhenegouwen, J.; van de Kant, H.J.G.; Folkerts, G.; Garssen, J.; Vos, A.P.; Morgan, M.E.; Kraneveld, A.D. Specific probiotic dietary supplementation leads to different effects during remission and relapse in murine chronic colitis. Benef. Microbes 2016, 7, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Wickens, K.; Black, P.N.; Stanley, T.V.; Mitchell, E.; Fitzharris, P.; Tannock, G.W.; Purdie, G.; Crane, J.; Probiotic Study Group. A differential effect of 2 probiotics in the prevention of eczema and atopy: A double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2008, 122, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- McNulty, N.P.; Yatsunenko, T.; Hsiao, A.; Faith, J.J.; Muegge, B.D.; Goodman, A.L.; Henrissat, B.; Oozeer, R.; Cools-Portier, S.; Gobert, G.; et al. The impact of a consortium of fermented milk strains on the gut microbiome of gnotobiotic mice and monozygotic twins. Sci. Transl. Med. 2011, 3, 106ra106. [Google Scholar] [CrossRef] [PubMed]
- Engelbrektson, A.; Korzenik, J.R.; Pittler, A.; Sanders, M.E.; Klaenhammer, T.R.; Leyer, G.; Kitts, C.L. Probiotics to minimize the disruption of faecal microbiota in healthy subjects undergoing antibiotic therapy. J. Med. Microbiol. 2009, 58, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Eloe-Fadrosh, E.A.; Brady, A.; Crabtree, J.; Drabek, E.F.; Ma, B.; Mahurkar, A.; Ravel, J.; Haverkamp, M.; Fiorino, A.M.; Botelho, C.; et al. Functional dynamics of the gut microbiome in elderly people during probiotic consumption. MBio 2015, 6, e00231-15. [Google Scholar] [CrossRef] [PubMed]
- Corthésy, B.; Gaskins, H.R.; Mercenier, A. Cross-talk between probiotic bacteria and the host immune system. J. Nutr. 2007, 137 (Suppl. S2), 781S–790S. [Google Scholar]
- Cela, E.M.; Weill, F.S.; Paz, M.L.; Leoni, J.; Maglio, D.H.G. Lipoteichoic acid challenge induces higher inflammatory responses than lipopolysaccharide in UV-irradiated keratinocytes. Photodermatol. Photoimmunol. Photomed. 2015, 31, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Heuvelin, E.; Lebreton, C.; Bichara, M.; Cerf-Bensussan, N.; Heyman, M. A bifidobacterium probiotic strain and its soluble factors alleviate chloride secretion by human intestinal epithelial cells. J. Nutr. 2010, 140, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Prisciandaro, L.; Geier, M.; Butler, R.; Cummins, A.; Howarth, G. Probiotics and their derivatives as treatments for inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.H.; Lin, C.K.; Sheu, S.J.; Hwang, C.F.; Ye, W.T.; Hwang, W.Z.; Tsen, H.Y. Antagonistic Activity of spent culture supernatants of lactic acid bacteria against Helicobacter Pylori growth and Infection in human gastric epithelial AGS cells. J. Food Sci. 2009, 74, M225–M230. [Google Scholar] [CrossRef] [PubMed]
- Ewaschuk, J.B.; Diaz, H.; Meddings, L.; Diederichs, B.; Dmytrash, A.; Backer, J.; Langen, M.L.; Madsen, K.L. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1025–G1034. [Google Scholar] [CrossRef] [PubMed]
- Bär, F.; von koschitzky, H.; Roblick, U.; Bruch, H.P.; Schulze, L.; Sonnenborn, U.; Böttner, M.; Wedel, T. Cell-free supernatants of Escherichia coli Nissle 1917 modulate human colonic motility: Evidence from an in vitro organ bath study. Neurogastroenterol. Motil. 2009, 21, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Howarth, G.S. Probiotic-derived factors: Probiotaceuticals? J. Nutr. 2010, 140, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chiu, C.H.; Lin, T.Y.; Shi, H.N.; Walker, W.A. Effect of probiotics Lactobacillus acidophilus on Citrobacter rodentium Colitis: The role of dendritic cells. Pediatr. Res. 2009, 65, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Seite, S.; Bieber, T. Barrier function and microbiotic dysbiosis in atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2015, 8, 479. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Tseng, C.; Strober, B.E.; Pei, Z.; Blaser, M.J. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS ONE 2008, 3, e2719. [Google Scholar] [CrossRef] [PubMed]
- Ganju, P.; Nagpal, S.; Mohammed, M.; Kumar, P.N.; Pandey, R.; Natarajan, V.T.; Mande, S.S.; Gokhale, R.S.; Grice, E.A.; Segre, J.A.; et al. Microbial community profiling shows dysbiosis in the lesional skin of Vitiligo subjects. Sci. Rep. 2016, 6, 18761. [Google Scholar] [CrossRef] [PubMed]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Prim. 2016, 2, 16082. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J. Molecular mechanisms in atopic eczema: Insights gained from genetic studies. J. Pathol. 2017, 241, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.F.; Jakob, T. From innate to adaptive immune responses in contact hypersensitivity. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Gober, M.D.; Gaspari, A.A. Allergic contact dermatitis. In Dermatologic Immunity; KARGER: Basel, Switzerland, 2008; Volume 10, pp. 1–26. [Google Scholar]
- Parodi, A.; Paolino, S.; Greco, A.; Drago, F.; Mansi, C.; Rebora, A.; Parodi, A.; Savarino, V. Small intestinal bacterial overgrowth in Rosacea: Clinical effectiveness of its eradication. Clin. Gastroenterol. Hepatol. 2008, 6, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.L.; Chandra, S.; Shih, D.Q. Skin manifestations of inflammatory bowel disease. Front. Physiol. 2012, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Mohammedsaeed, W.; Cruickshank, S.; McBain, A.J.; O’Neill, C.A. Lactobacillus rhamnosus GG lysate increases re-epithelialization of keratinocyte scratch assays by promoting migration. Sci. Rep. 2015, 5, 16147. [Google Scholar] [CrossRef] [PubMed]
- Prince, T.; McBain, A.J.; O’Neill, C.A. Lactobacillus reuteri protects epidermal keratinocytes from staphylococcus aureus-induced cell death by competitive exclusion. Appl. Environ. Microbiol. 2012, 78, 5119–5126. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; McBain, A.J.; O’Neill, C.A. Strain-dependent augmentation of tight-junction barrier function in human primary epidermal keratinocytes by Lactobacillus and Bifidobacterium lysates. Appl. Environ. Microbiol. 2013, 79, 4887–4894. [Google Scholar] [CrossRef] [PubMed]
- Argenta, A.; Satish, L.; Gallo, P.; Liu, F.; Kathju, S. Local application of probiotic bacteria prophylaxes against sepsis and death resulting from burn wound infection. PLoS ONE 2016, 11, e0165294. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Huh, C.S.; Ra, J.; Choi, I.D.; Jeong, J.W.; Kim, S.H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.H.; et al. Clinical evidence of effects of lactobacillus plantarum hy7714 on skin aging: A randomized, double blind, placebo-controlled study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Peral, M.C.; Martinez, M.A.H.; Valdez, J.C. Bacteriotherapy with Lactobacillus plantarum in burns. Int. Wound J. 2009, 6, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Meneghin, F.; Fabiano, V.; Mameli, C.; Zuccotti, G.V. Probiotics and atopic dermatitis in children. Pharmaceuticals 2012, 5, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, K.; Savilahti, E.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Kuitunen, M. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: A randomized, double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, T.; Böttcher, M.F.; Fredrikson, M.; Jenmalm, M.C.; Björkstén, B.; Oldaeus, G. Probiotics in prevention of IgE-associated eczema: A double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2007, 119, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Ebata, T.; Hirooka, J.; Hosoya, R.; Inoue, N.; Itami, S.; Tsuji, K.; Yaginuma, T.; Muramatsu, K.; Nakamura, A.; et al. Antipruritic effects of the probiotic strain LKM512 in adults with atopic dermatitis. Ann. Allergy Asthma Immunol. 2014, 113, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Kambara, T.; Murata, N.; Komori-Yamaguchi, J.; Matsukura, S.; Takahashi, Y.; Ikezawa, Z.; Aihara, M. Effects of oral administration of Lactobacillus acidophilus L-92 on the symptoms and serum cytokines of atopic dermatitis in Japanese adults: A double-blind, randomized, clinical trial. Int. Arch. Allergy Immunol. 2015, 165, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Iemoli, E.; Rodighiero, V.; Nicola, L.; de Vecchi, E.; Piconi, S. Effects of Lactobacillus salivarius LS01 (DSM 22775) treatment on adult atopic dermatitis: A randomized placebo-controlled study. Int. J. Immunopathol. Pharmacol. 2011, 24, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Capurso, G.; Marignani, M.; Fave, G.D. Probiotics and the incidence of colorectal cancer: When evidence is not evident. Dig. Liver Dis. 2006, 38, S277–S282. [Google Scholar] [CrossRef]
- Maclennan, R.; Jensen, O.M. Dietary fibre, transit-time, faecal bacteria, steroids, and colon cancer in two Scandinavian populations. Report from the International Agency for Research on Cancer Intestinal Microecology Group. Lancet 1977, 2, 207–211. [Google Scholar] [PubMed]
- Peters, R.K.; Pike, M.C.; Garabrant, D.; Mack, T.M. Diet and colon cancer in Los Angeles County, California. Cancer Causes Control 1992, 3, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Young, T.B.; Wolf, D.A. Case-control study of proximal and distal colon cancer and diet in Wisconsin. Int. J. Cancer 1988, 42, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Fooladi, A.A.I.; Yazdi, M.H.; Pourmand, M.R.; Mirshafiey, A.; Hassan, Z.M.; Azizi, T.; Mahdavi, M.; Dallal, M.M.S. Th1 cytokine production induced by Lactobacillus acidophilus in BALB/c mice bearing transplanted breast tumor. Jundishapur J. Microbiol. 2015, 8, e17354. [Google Scholar]
- De LeBlanc, A.d.; Matar, C.; LeBlanc, N.; Perdigón, G. Effects of milk fermented by Lactobacillus helveticus R389 on a murine breast cancer model. Breast Cancer Res. 2005, 7, R477–R486. [Google Scholar] [CrossRef] [PubMed]
- Weill, F.S.; Cela, E.M.; Paz, M.L.; Ferrari, A.; Leoni, J.; Maglio, D.H.G. Lipoteichoic acid from Lactobacillus rhamnosus GG as an oral photoprotective agent against UV-induced carcinogenesis. Br. J. Nutr. 2013, 109, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Kripke, M.L. Antigenicity of murine skin tumors induced by ultraviolet light. J. Natl. Cancer Inst. 1974, 53, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Kripke, M.L.; Fisher, M.S. Immunologic parameters of ultraviolet carcinogenesis. J. Natl. Cancer Inst. 1976, 57, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.S.; Kripke, M.L. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc. Natl. Acad. Sci. USA 1977, 74, 1688–1692. [Google Scholar] [CrossRef] [PubMed]
- Sartorelli, P.; Romeo, R.; Paolucci, V.; Puzzo, V.; di Simplicio, F.; Barabesi, L. Skin photoaging in farmers occupationally exposed to ultraviolet radiation. Med. Lav. 2013, 104, 24–29. [Google Scholar] [PubMed]
- Lastowiecka-Moras, E.; Bugajska, J.; Młynarczyk, B. Occupational exposure to natural UV radiation and premature skin ageing. Int. J. Occup. Saf. Ergon. 2014, 20, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, M.; Kuechmeister, B.; Ohnemus, U.; Baur, X.; Moll, I. Extrinsic skin ageing symptoms in seafarers subject to high work-related exposure to UV radiation. Eur. J. Dermatol. 2014, 23, 663–670. [Google Scholar]
- Peguet-Navarro, J.; Dezutter-Dambuyant, C.; Buetler, T.; Leclaire, J.; Smola, H.; Blum, S.; Bastien, P.; Breton, L.; Gueniche, A. Supplementation with oral probiotic bacteria protects human cutaneous immune homeostasis after UV exposure-double blind, randomized, placebo controlled clinical trial. Eur. J. Dermatol. 2008, 18, 504–511. [Google Scholar] [PubMed]
- Bouilly-Gauthier, D.; Jeannes, C.; Maubert, Y.; Duteil, L.; Queille-Roussel, C.; Piccardi, N.; Montastier, C.; Manissier, P.; Piérard, G.; Ortonne, J.P. Clinical evidence of benefits of a dietary supplement containing probiotic and carotenoids on ultraviolet-induced skin damage. Br. J. Dermatol. 2010, 163, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Patra, V.; Byrne, S.N.; Wolf, P. The skin microbiome: Is it affected by UV-induced immune suppression? Front. Microbiol. 2016, 7, 1235. [Google Scholar] [CrossRef] [PubMed]
- Takagi, A.; Matsuzaki, T.; Sato, M.; Nomoto, K.; Morotomi, M.; Yokokura, T. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis 2001, 22, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Horinaka, M.; Yoshida, T.; Kishi, A.; Akatani, K.; Yasuda, T.; Kouhara, J.; Wakada, M.; Sakai, T. Lactobacillus strains induce TRAIL production and facilitate natural killer activity against cancer cells. FEBS Lett. 2010, 584, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Hacini-Rachinel, F.; Gheit, H.; le Luduec, J.B.; Dif, F.; Nancey, S.; Kaiserlian, D. Oral probiotic control skin inflammation by acting on both effector and regulatory T cells. PLoS ONE 2009, 4, e4903. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.E.; Yoon, Y.S.; Seo, J.G.; Chung, M.J.; Yum, D.Y. A probiotic preparation alleviates atopic dermatitis-like skin lesions in murine models. Toxicol. Res. 2016, 32, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Guéniche, A.; Oréal, L.; Buetler, T.M. Supplementation with oral probiotic bacteria maintains cutaneous immune homeostasis after UV exposure. Eur. J. Dermatol. 2006, 6, 511–517. [Google Scholar]
- HKim, M.; Lee, D.E.; Park, S.D.; Kim, Y.T.; Kim, Y.J.; Jeong, J.W.; Jang, S.S.; Ahn, Y.T.; Sim, J.H.; Huh, C.S.; et al. Oral administration of Lactobacillus plantarum HY7714 protects hairless mouse against ultraviolet B-induced photoaging. J. Microbiol. Biotechnol. 2014, 24, 1583–1591. [Google Scholar]
- Stokes, J.H.; Pillsbury, D.M. The effect on the skin of emotional and nervous states. Arch. Derm. Syphilol. 1930, 22, 962. [Google Scholar] [CrossRef]
- Bowe, W.; Patel, N.B.; Logan, A.C. Acne vulgaris, probiotics and the gut-brain-skin axis: From anecdote to translational medicine. Benef. Microbes 2014, 5, 185–199. [Google Scholar]
- Pavlovic, S.; Daniltchenko, M.; Tobin, D.J.; Hagen, E.; Hunt, S.P.; Klapp, B.F.; Arck, P.C.; Peters, E.M.J. Further exploring the brain-skin connection: Stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J. Investig. Dermatol. 2008, 128, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Verdú, E.F.; Bercik, P.; Verma-Gandhu, M.; Huang, X.X.; Blennerhassett, P.; Jackson, W.; Mao, Y.; Wang, L.; Rochat, F.; Collins, S.M. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 2006, 55, 182–190. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).