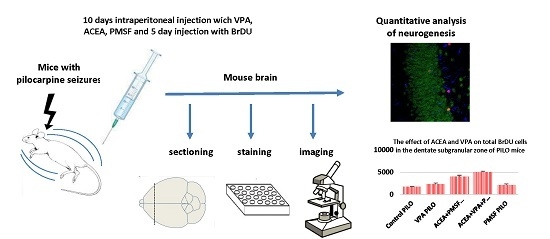
A Long-Term Treatment with Arachidonyl-2′-Chloroethylamide Combined with Valproate Increases Neurogenesis in a Mouse Pilocarpine Model of Epilepsy
Abstract
:1. Introduction
2. Results
2.1. The Effect of Pilocarpine on Proliferation of Newborn Cells
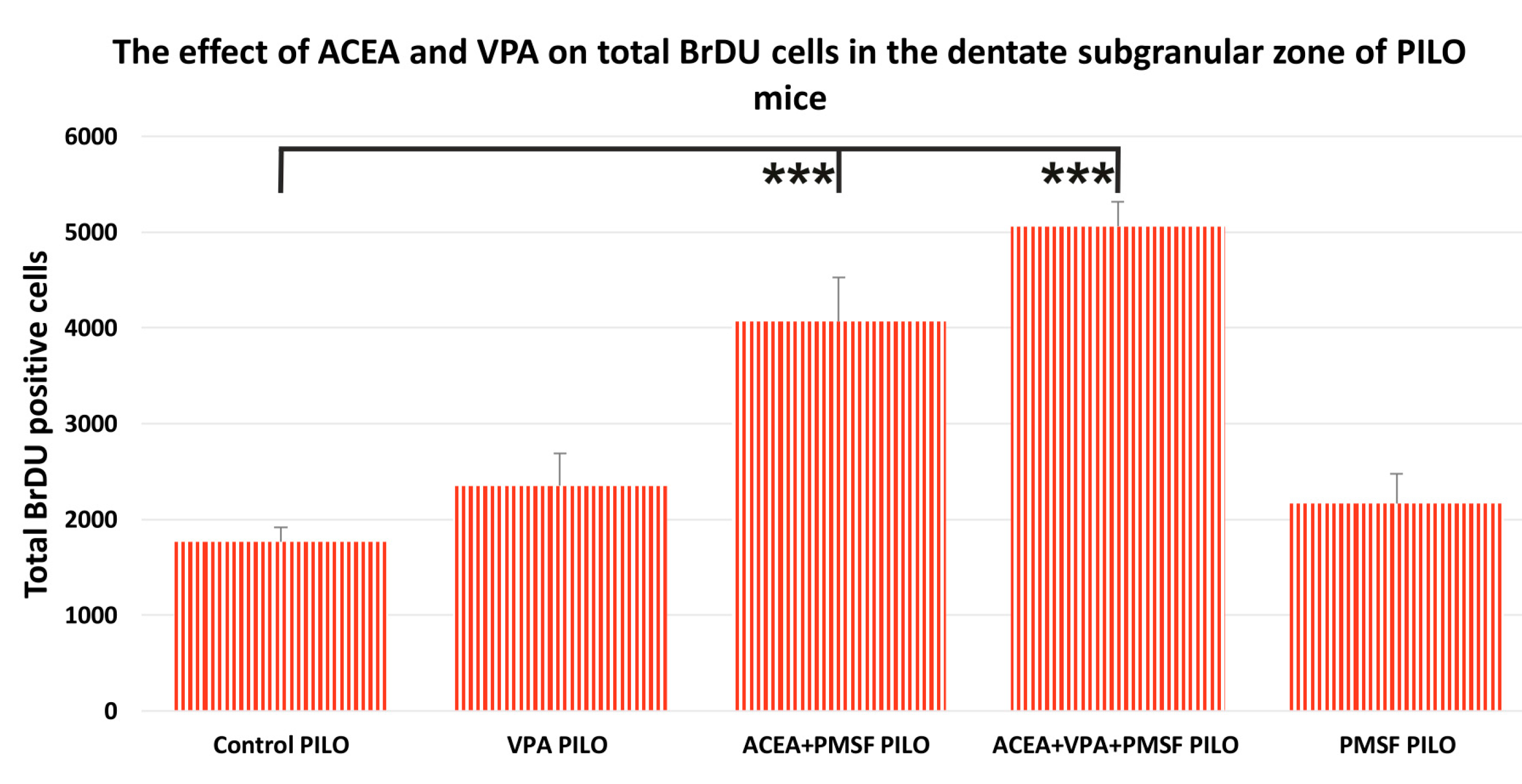
2.2. The Impact of Synthetic Cannabinoid Arachidonyl-2′-Chloroethylamide (ACEA) and Valproic Acid (VPA) on Total Newborn Cells in the Dentate Subgranular Zone of Pilocarpine (PILO) Mice
2.3. The Impact of ACEA and VPA on Newborn Neurons in the Dentate Subgranular Zone of PILO Mice
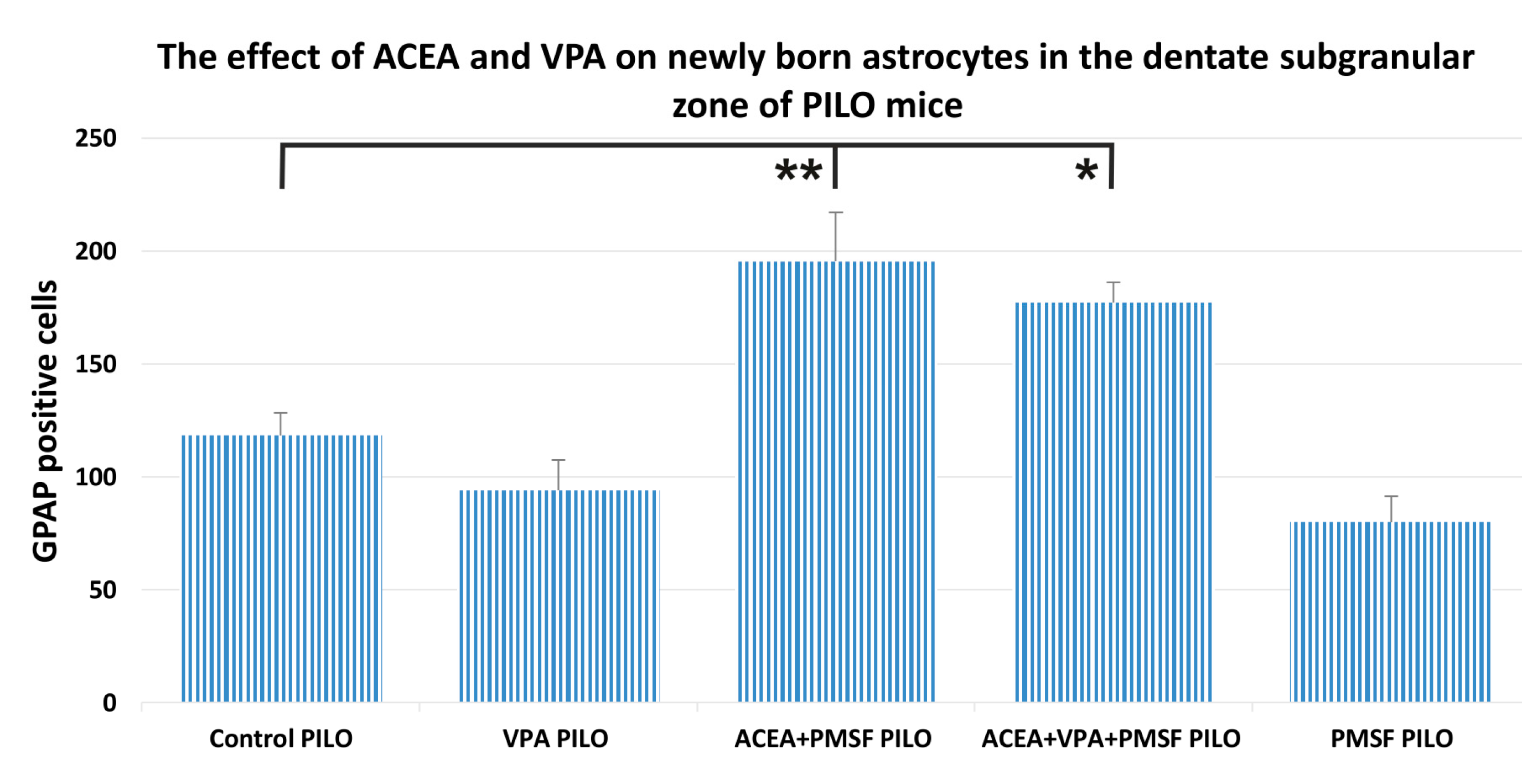
2.4. The Impact of ACEA and VPA on Newborn Astrocytes in the Dentate Subgranular Zone of PILO Mice
3. Discussion
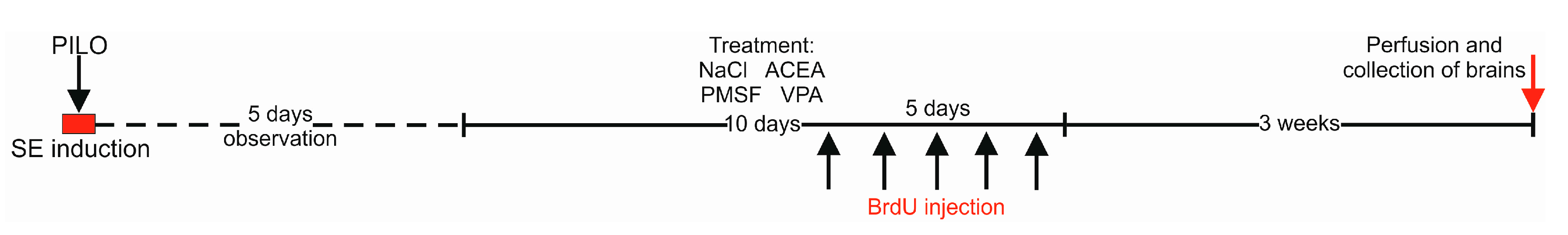
4. Materials and Methods
4.1. Animals and Experimental Conditions
4.2. Drugs
4.3. Pilocarpine-Induced Convulsions
4.4. Drug Administration
4.5. Tissue Preparation
4.6. Immunohistochemical Staining
4.7. Confocal Microscopy and Cell Counting
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TLE | Temporal lobe epilepsy |
| BrdU | 5-Bromo-2-Deoxyuridine |
| NeuN | Neuronal nuclei |
| SGZ | Subgranular zone |
| GFAP | Glial fibrillary acidic protein |
| IP | Intraperitoneal |
| PMSF | Phenylmethylsulfonyl fluoride |
| PILO | Pilocarpine |
| ACEA | Arachidonyl-2′-chloroethylamide |
| VPA | Valproic acid |
| AEDs | Antiepileptic dgrus |
| PTZ | Pentylenetetrazole |
| MES | Maximal electroshock seizure |
| NSPCs | Neural stem/precursor cells |
| DCX | Doublecortin |
References
- Andres-Mach, M.; Haratym-Maj, A.; Zagaja, M.; Luszczki, J.J. Additive interactions between 1-methyl-1,2,3,4-tetrahydroisoquinoline and clobazam in the mouse maximal electroshock-induced tonic seizure model—An isobolographic analysis for parallel dose-response relationship curves. Pharmacology 2014, 93, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Luszczki, J.J.; Czuczwar, P.; Cioczek-Czuczwar, A.; Czuczwar, S.J. Arachidonyl-2′-chloroethylamide, a highly selective cannabinoid CB1 receptor agonist, enhances the anticonvulsant action of valproate in the mouse maximal electroshock-induced seizure model. Eur. J. Pharmacol. 2006, 547, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Luszczki, J.J.; Czuczwar, P.; Cioczek-Czuczwar, A.; Dudra-Jastrzebska, M.; Andres-Mach, M.; Czuczwar, S.J. Effect of arachidonyl-2′-chloroethylamide, a selective cannabinoid CB1 receptor agonist, on the protective action of the various antiepileptic drugs in the mouse maximal electroshock-induced seizure model. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Luszczki, J.J.; Andres-Mach, M.; Barcicka-Klosowska, B.; Florek-Luszczki, M.; Haratym-Maj, A.; Czuczwar, S.J. Effects of WIN 55,212-2 mesylate (a synthetic cannabinoid) on the protective action of clonazepam, ethosuximide, phenobarbital and valproate against pentylenetetrazole-induced clonic seizures in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, K.; Zagaja, M.; Łuszczki, J.J.; Rapacz, A.; Andres-Mach, M.; Latacz, G.; Kieć-Kononowicz, K. Design, synthesis, and anticonvulsant activity of new hybrid compounds derived from 2-(2,5-dioxopyrrolidin-1-yl)propanamides and 2-(2,5-dioxopyrrolidin-1-yl)butanamides. J. Med. Chem. 2015, 58, 5274–5286. [Google Scholar] [CrossRef] [PubMed]
- Curia, G.; Lucchi, C.; Vinet, J.; Gualtieri, F.; Marinelli, C.; Torsello, A.; Costantino, L.; Biagini, G. Pathophysiogenesis of mesial temporal lobe epilepsy: Is prevention of damage antiepileptogenic? Curr. Med. Chem. 2014, 21, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Stefovska, V.G.; Uckermann, O.; Czuczwar, M.; Smitka, M.; Czuczwar, P.; Kis, J.; Kaindl, A.M.; Turski, L.; Turski, W.A.; Ikonomidou, C. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann. Neurol. 2008, 64, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, Y.; Maru, E.; Kudo, K.; Shibasaki, T.; Kato, N. Levetiracetam suppresses development of spontaneous EEG seizures and aberrant neurogenesis following kainate-induced status epilepticus. Brain Res. 2010, 1352, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Quan, Q.Y.; Yang, F.; Wang, Y.; Wang, J.C.; Zhao, G.; Jiang, W. Effects of lamotrigine and topiramate on hippocampal neurogenesis in experimental temporal-lobe epilepsy. Brain Res. 2010, 1313, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Sondossi, K.; Majdzadeh, M.; Ghaeli, P.; Ghahremani, M.H.; Shafaroodi, H.; Paknejad, B.; Ostad, S.N. Analysis of the antiepileptic, ethosuximide impacts on neurogenesis of rat forebrain stem cells. Fundam. Clin. Pharmacol. 2014, 28, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Andres-Mach, M.; Haratym-Maj, A.; Zagaja, M.; Rola, R.; Maj, M.; Chrościńska-Krawczyk, M.; Luszczki, J.J. ACEA (a highly selective cannabinoid CB1 receptor agonist) stimulates hippocampal neurogenesis in mice treated with antiepileptic drugs. Brain Res. 2015, 1624, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Nakashima, K.; Clemenson, G.D., Jr.; Mejia, E.; Mathews, E.; Ure, K.; Ogawa, S.; Sinton, C.M.; Gage, F.H.; Hsieh, J. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J. Neurosci. 2013, 27, 5967–5975. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.J.; Nagel, D.A.; O’Neil, J.D.; Torr, E.; Woehrling, E.K.; Devitt, A.; Coleman, M.D. Effects of lithium and valproic acid on gene expression and phenotypic markers in an NT2 neurosphere model of neural development. PLoS ONE 2013, 8, e58822. [Google Scholar] [CrossRef] [PubMed]
- Brunn, J.; Wiroth, V.; Kowalski, M.; Runge, U.; Sabolek, M. Valproic acid in normal therapeutic concentration has no neuroprotective or differentiation influencing effects on long term expanded murine neural stem cells. Epilepsy Res. 2014, 108, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Umka, J.; Mustafa, S.; ElBeltagy, M.; Thorpe, A.; Latif, L.; Bennett, G.; Wigmore, P.M. Valproic acid reduces spatial working memory and cell proliferation in the hippocampus. Neuroscience 2010, 166, 15–22. [Google Scholar] [CrossRef] [PubMed]
- James, E.J.; Gu, J.; Ramirez-Vizcarrondo, C.M.; Hasan, M.; Truszkowski, T.L.; Tan, Y.; Oupravanh, P.M.; Khakhalin, A.S.; Aizenman, C.D. Valproate-induced neurodevelopmental deficits in Xenopus laevis tadpoles. J. Neurosci. 2015, 18, 3218–3229. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.J.; Blair, R.E.; Falenski, K.W.; Martin, B.R.; DeLorenzo, R.J. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J. Pharmacol. Exp. Ther. 2015, 307, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Andres-Mach, M.; Zolkowska, D.; Barcicka-Klosowska, B.; Haratym-Maj, A.; Florek-Luszczki, M.; Luszczki, J.J. Effect of ACEA—A selective cannabinoid CB1 receptor agonist on the protective action of different antiepileptic drugs in the mouse pentylenetetrazole-induced seizure model. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, R.; Cannon, J.R.; Greenamyre, J.T. Post-status epilepticus treatment with the cannabinoid agonist WIN 55,212-2 prevents chronic epileptic hippocampal damage in rats. Neurobiol. Dis. 2015, 73, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Florek-Luszczki, M.; Zagaja, M.; Luszczki, J.J. Influence of arachidonyl-2′-chloroethylamide, a selective cannabinoid CB1 receptor agonist, on the anticonvulsant and acute side-effect potentials of clobazam, lacosamide, and pregabalin in the maximal electroshock-induced seizure model and chimney test in mice. Fundam. Clin. Pharmacol. 2013, 29, 382–393. [Google Scholar]
- Campos, A.C.; Ortega, Z.; Palazuelos, J.; Fogaça, M.V.; Aguiar, D.C.; Díaz-Alonso, J.; Ortega-Gutiérrez, S.; Vázquez-Villa, H.; Moreira, F.A.; Guzmán, M.; et al. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: Involvement of the endocannabinoid system. Int. J. Neuropsychopharmacol. 2013, 16, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Albayram, O.; Alferink, J.; Pitsch, J.; Piyanova, A.; Neitzert, K.; Poppensieker, K.; Mauer, D.; Michel, K.; Legler, A.; Becker, A.; et al. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc. Natl. Acad. Sci. USA 2011, 108, 11256–11261. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.A.; Bick-Sander, A.; Fabel, K.; Leal-Galicia, P.; Tauber, S.; Ramirez-Rodriguez, G.; Müller, A.; Melnik, A.; Waltinger, T.P.; Ullrich, O.; et al. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell. Commun. Signal. 2010, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Parent, J.M.; Kron, M.M. Neurogenesis and Epilepsy. In Jasper’s Basic Mechanisms of the Epilepsies, 4th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Zhong, Q.; Ren, B.X.; Tang, F.R. Neurogenesis in the hippocampus of patients with temporal lobe epilepsy. Curr. Neurol. Neurosci. Rep. 2016, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.O.; Lybrand, Z.R.; Ito, N.; Brulet, R.; Tafacory, F.; Zhang, L.; Good, L.; Ure, K.; Kernie, S.G.; Birnbaum, S.G.; et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat. Commun. 2015, 6, 6606. [Google Scholar] [CrossRef] [PubMed]
- Danzer, S.C. Neurogenesis in Epilepsy: Better to Burn Out or Fade Away? Epilepsy Curr. 2016, 16, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Creson, T.; Zhang, L.; Li, P.; Du, F.; Yuan, P.; Gould, T.D.; Manji, H.K.; Chen, G. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J. Neurosci. 2004, 24, 6590–6599. [Google Scholar] [CrossRef] [PubMed]
- Hosák, L.; Libiger, J. Antiepileptic drugs in schizophrenia: A review. Eur. Psychiatry 2002, 17, 371–378. [Google Scholar] [CrossRef]
- Smith, L.A.; Cornelius, V.R.; Azorin, J.M.; Perugi, G.; Vieta, E.; Young, A.H.; Bowden, C.L. Valproate for the treatment of acute bipolar depression: Systematic review and meta-analysis. J. Affect. Disord. 2010, 122, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yurekli, V.A.; Akhan, G.; Kutluhan, S.; Uzar, E.; Koyuncuoglu, H.A.; Gultekin, F. The effect of sodium valproate on chronic daily headache and its subgroups. J. Headache Pain 2008, 9, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, D.; Sun, B.; O’Brien, P.; Hansen, M. Intravenous sodium valproate for acute pediatric headache. J. Emerg. Med. 2015, 49, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Borowicz, K.K.; Piskorska, B.; Stępniak, B.; Czuczwar, S.J. Effects of fluoxetine on the anticonvulsant action of valproate and ethosuximide in mouse model of myoclonic convulsions. Ann. Agric. Environ. Med. 2012, 19, 487–490. [Google Scholar]
- Wu, T.; Nagaya, Y.; Hanada, T. Pharmacodynamic and pharmacokinetic interactions of perampanel and other antiepileptic drugs in a rat amygdala kindling model. Seizure 2014, 23, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Tomson, T.; Battino, D.; Bonizzoni, E.; Craig, J.; Lindhout, D.; Perucca, E.; Sabers, A.; Thomas, S.V.; Vajda, F. Dose-dependent teratogenicity of valproate in mono- and polytherapy: An observational study. Neurology 2015, 85, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Kawanai, T.; Ago, Y.; Watanabe, R.; Inoue, A.; Taruta, A.; Onaka, Y.; Hasebe, S.; Hashimoto, H.; Matsuda, T.; Takuma, K. Prenatal exposure to histone deacetylase inhibitors affects gene expression of autism-related molecules and delays neuronal maturation. Neurochem. Res. 2016, 41, 2574–2584. [Google Scholar] [CrossRef] [PubMed]
- Semmler, A.; Frisch, C.; Bleul, C.; Smith, D.; Bigler, L.; Prost, J.C.; Blom, H.; Linnebank, M. Intrauterine valproate exposure is associated with alterations in hippocampal cell numbers and folate metabolism in a rat model of valproate teratogenicity. Seizure 2017, 46, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Monti, B.; Polazzi, E.; Contestabile, A. Biochemical, molecular and epigenetic mechanisms of valproic acid neuroprotection. Curr. Mol. Pharmacol. 2009, 2, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Qing, H.; He, G.; Ly, P.T.; Fox, C.J.; Staufenbiel, M.; Cai, F.; Zhang, Z.; Wei, S.; Sun, X.; Chen, C.H.; et al. Valproic acid inhibits Aβ production, neuritic plaque formation, and behavioral deficits in Alzheimer’s disease mouse models. J. Exp. Med. 2008, 205, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Nalivaeva, N.N.; Belyaev, N.D.; Lewis, D.I.; Pickles, A.R.; Makova, N.Z.; Bagrova, D.I.; Dubrovskaya, N.M.; Plesneva, S.A.; Zhuravin, I.A.; Turner, A.J.; et al. Effect of sodium valproate administration on brain neprilysin expression and memory in rats. J. Mol. Neurosci. 2012, 46, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Monti, B.; Gatta, V.; Piretti, F.; Raffaelli, S.S.; Virgili, M.; Contestabile, A. Valproic acid is neuroprotective in the rotenone rat model of Parkinson’s disease: Involvement of alpha-synuclein. Neurotox. Res. 2010, 17, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Zádori, D.; Geisz, A.; Vámos, E.; Vécsei, L.; Klivényi, P. Valproate ameliorates the survival and the motor performance in a transgenic mouse model of Huntington’s disease. Pharmacol. Biochem. Behav. 2009, 94, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Sugai, F.; Yamamoto, Y.; Miyaguchi, K.; Zhou, Z.; Sumi, H.; Hamasaki, T.; Goto, M.; Sakoda, S. Benefit of valproic acid in suppressing disease progression of ALS model mice. Eur. J. Neurosci. 2004, 11, 3179–3183. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Orsi, S.A.; Zhang, M.; Grill, R.J.; Pati, S.; Zhao, J.; Moore, A.N. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS ONE 2010, 5, e11383. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.W.; Yang, S.; Lee, C.H.; Son, H. A critical time window for the survival of neural progenitor cells by HDAC inhibitors in the hippocampus. Mol. Cells 2011, 31, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Vukićević, V.; Qin, N.; Balyura, M.; Eisenhofer, G.; Wong, M.L.; Licinio, J.; Bornstein, S.R.; Ehrhart-Bornstein, M. Valproic acid enhances neuronal differentiation of sympathoadrenal progenitor cells. Mol. Psychiatry 2015, 20, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Juliandi, B.; Tanemura, K.; Igarashi, K.; Tominaga, T.; Furukawa, Y.; Otsuka, M.; Moriyama, N.; Ikegami, D.; Abematsu, M.; Sanosaka, T.; et al. Reduced adult hippocampal neurogenesis and cognitive impairments following prenatal treatment of the antiepileptic drug valproic acid. Stem Cell Rep. 2015, 8, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Boku, S.; Nakagawa, S.; Masuda, T.; Nishikawa, H.; Kato, A.; Toda, H.; Song, N.; Kitaichi, Y.; Inoue, T.; Koyama, T. Effects of mood stabilizers on adult dentate gyrus-derived neural precursor cells. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Yuan, J.; Huang, L.; Xiang, X.; Zhu, H.; Chen, F.; Lin, J.; Feng, H. Valproic acid arrests proliferation but promotes neuronal differentiation of adult spinal NSPCs from SCI rats. Neurochem. Res. 2015, 40, 1472–1486. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Ahmad-Molaei, L.; Mazar-Atabaki, A.; Ronaghi, A.; Shirazi-zand, Z.; Motiei-Langroudi, S.M.; Eslahkar, S. L-type calcium channel mediates anticonvulsant effect of cannabinoids in acute and chronic murine models of seizure. Neurochem. Res. 2012, 37, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Shafaroodi, H.; Moezi, L.; Bahremand, A.; Dehpour, A.R. The role of α₂-adrenoceptors in the anti-convulsant effects of cannabinoids on pentylenetetrazole-induced seizure threshold in mice. Eur. J. Pharmacol. 2013, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kozan, R.; Ayyildiz, M.; Agar, E. The effects of intracerebroventricular AM-251, a CB1-receptor antagonist, and ACEA, a CB1-receptor agonist, on penicillin-induced epileptiform activity in rats. Epilepsia 2009, 50, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Arslan, G.; Alici, S.K.; Ayyildiz, M.; Agar, E. The role of CB1-receptors in the proconvulsant effect of leptin on penicillin-induced epileptiform activity in rats. CNS Neurosci. Ther. 2013, 19, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Arslan, G.; Ayyildiz, M.; Agar, E. The interaction between ghrelin and cannabinoid systems in penicillin-induced epileptiform activity in rats. Neuropeptides 2014, 48, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, Y.; Xiao, L.; van Cleemput, J.; Ji, S.P.; Bai, G.; Zhang, X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Investig. 2005, 115, 3104–3116. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; Blanco, E.; Bindila, L.; Alen, F.; Vargas, A.; Rubio, L.; Pavón, F.J.; Serrano, A.; Lutz, B.; Rodríguez de Fonseca, F.; et al. Pharmacological activation of CB2 receptors counteracts the deleterious effect of ethanol on cell proliferation in the main neurogenic zones of the adult rat brain. Front. Cell. Neurosci. 2015, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Hutch, C.R.; Hegg, C.C. Cannabinoid receptor signaling induces proliferation but not neurogenesis in the mouse olfactory epithelium. Neurogenesis 2016, 3, e1118177. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, L.V.; van Rijn, C.M. Long-term disease-modifying effect of the endocannabinoid agonist WIN55,212-2 in a rat model of audiogenic epilepsy. Pharmacol. Rep. 2015, 67, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Welbat, J.U.; Chaisawang, P.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Sripanidkulchai, B.; Wigmore, P. Kaempferia parviflora extract ameliorates the cognitive impairments and the reduction in cell proliferation induced by valproic acid treatment in rats. Ann. Anat. 2016, 206, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Welbat, J.U.; Sirichoat, A.; Chaijaroonkhanarak, W.; Prachaney, P.; Pannangrong, W.; Pakdeechote, P.; Sripanidkulchai, B.; Wigmore, P. Asiatic acid prevents the deleterious effects of valproic acid on cognition and hippocampal cell proliferation and survival. Nutrients 2016, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, M.D.; Smith, B.N. Cannabinoid-mediated inhibition of recurrent excitatory circuitry in the dentate gyrus in a mouse model of temporal lobe epilepsy. PLoS ONE 2010, 5, e10683. [Google Scholar] [CrossRef] [PubMed]
- Mazzuferri, M.; Kumar, G.; Rospo, C.; Kaminski, R.M. Rapid epileptogenesis in the mouse pilocarpine model: Video-EEG, pharmacokinetic and histopathological characterization. Exp. Neurol. 2012, 238, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J.; Gartner, J.G.; Burnham, W.M. Epileptiform activity and neural plasticity in limbic structures. Brain Res. 1972, 47, 262–268. [Google Scholar] [CrossRef]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Vinet, J.; Vainchtein, I.D.; Spano, C.; Giordano, C.; Bordini, D.; Curia, G.; Dominici, M.; Boddeke, H.W.; Eggen, B.J.; Biagini, G. Microglia are less pro-inflammatory than myeloid infiltrates in the hippocampus of mice exposed to status epilepticus. Glia 2016, 64, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Abercrombie, M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946, 94, 239–247. [Google Scholar] [CrossRef] [PubMed]



| Target | Origin | Company | Cat. Number | Dilution |
|---|---|---|---|---|
| Neurons (NeuN) | Mouse | Millipore | MAB377 | 1:200 |
| Mouse IgG | Goat | Jackson Immunoresearch | 715-095-150 | 1:200 |
| Astrocytes (GFAP) | Rabbit | DakoCytomation | Z033401 | 1:500 |
| Rabbit IgG | Goat | Invitrogen | A-21071 | 1:200 |
| S-phase cells (BrdU) | Rat | Accurate Chem | OBT0030S | 1:10 |
| Rat IgG | Donkey | Jackson Immunoresearch | 712-295-153 | 1:200 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andres-Mach, M.; Zagaja, M.; Haratym-Maj, A.; Rola, R.; Maj, M.; Haratym, J.; Dudra-Jastrzębska, M.; Łuszczki, J.J. A Long-Term Treatment with Arachidonyl-2′-Chloroethylamide Combined with Valproate Increases Neurogenesis in a Mouse Pilocarpine Model of Epilepsy. Int. J. Mol. Sci. 2017, 18, 900. https://doi.org/10.3390/ijms18050900
Andres-Mach M, Zagaja M, Haratym-Maj A, Rola R, Maj M, Haratym J, Dudra-Jastrzębska M, Łuszczki JJ. A Long-Term Treatment with Arachidonyl-2′-Chloroethylamide Combined with Valproate Increases Neurogenesis in a Mouse Pilocarpine Model of Epilepsy. International Journal of Molecular Sciences. 2017; 18(5):900. https://doi.org/10.3390/ijms18050900
Chicago/Turabian StyleAndres-Mach, Marta, Mirosław Zagaja, Agnieszka Haratym-Maj, Radosław Rola, Maciej Maj, Joanna Haratym, Monika Dudra-Jastrzębska, and Jarogniew J. Łuszczki. 2017. "A Long-Term Treatment with Arachidonyl-2′-Chloroethylamide Combined with Valproate Increases Neurogenesis in a Mouse Pilocarpine Model of Epilepsy" International Journal of Molecular Sciences 18, no. 5: 900. https://doi.org/10.3390/ijms18050900