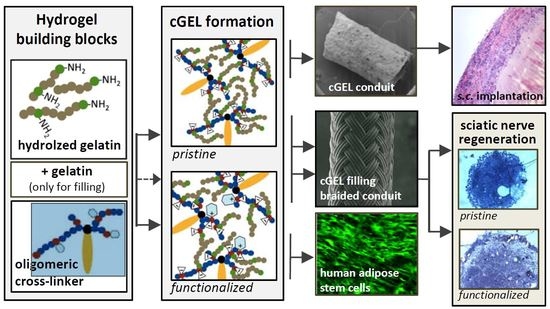
Dual-Component Gelatinous Peptide/Reactive Oligomer Formulations as Conduit Material and Luminal Filler for Peripheral Nerve Regeneration
Abstract
:1. Introduction
2. Results and Discussion
2.1. CGELconduit for Peripheral Nerve Repair
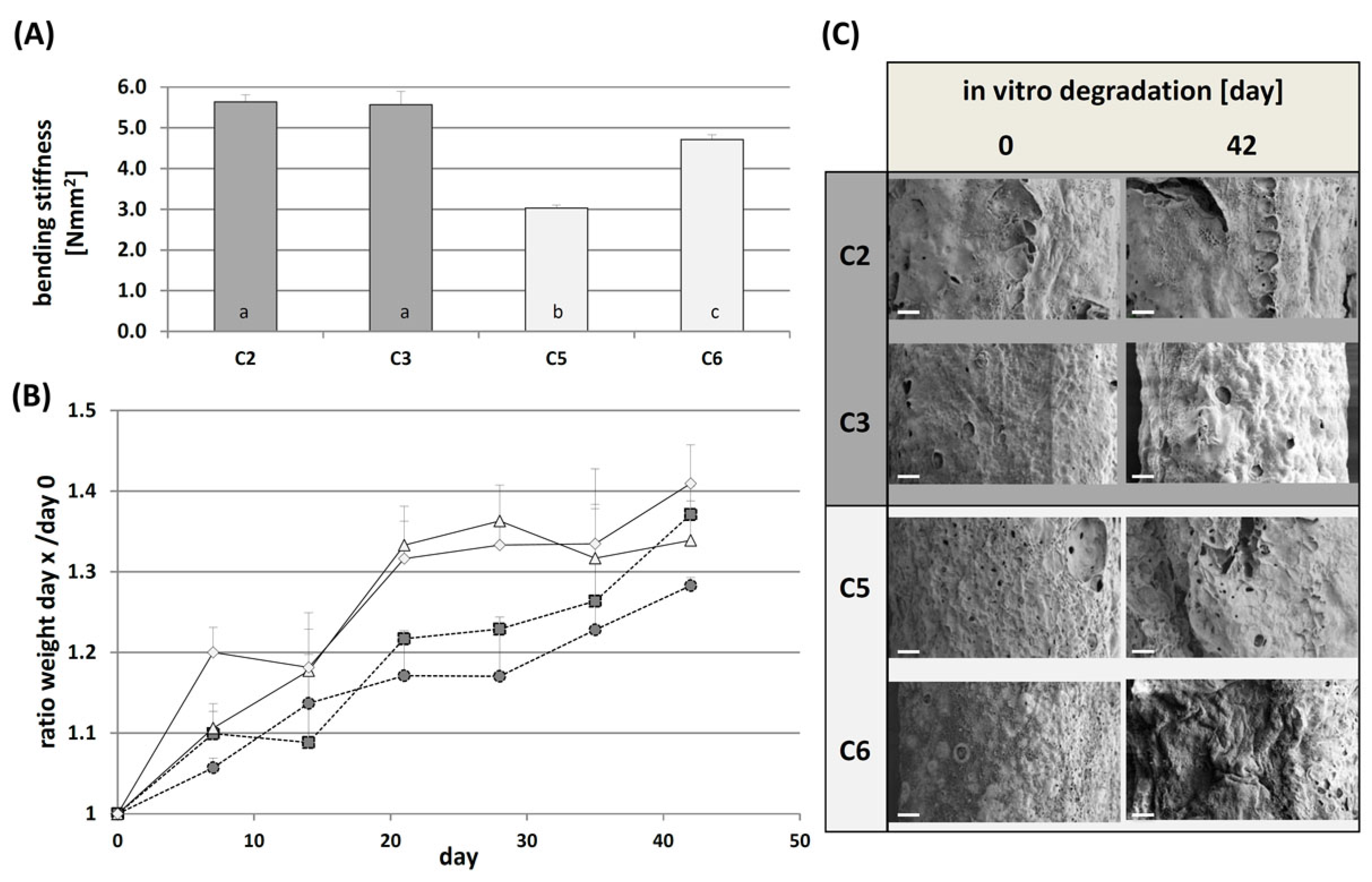
2.1.1. Mechanical Characterization and In Vitro Degradation
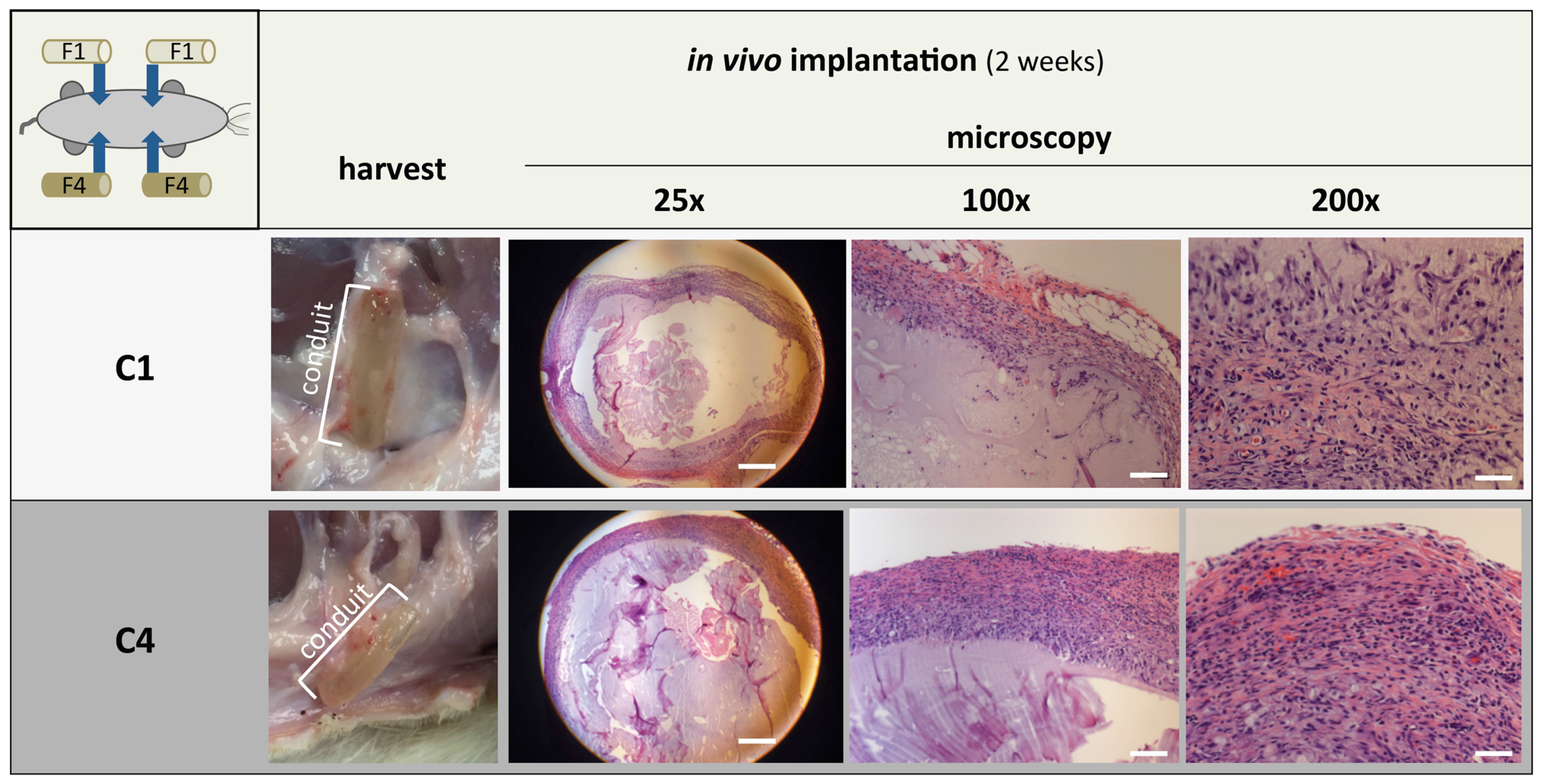
2.1.2. Sub Cutaneous Implantation of cGELconduit
2.2. CGELfiller—Material Development and Characterization
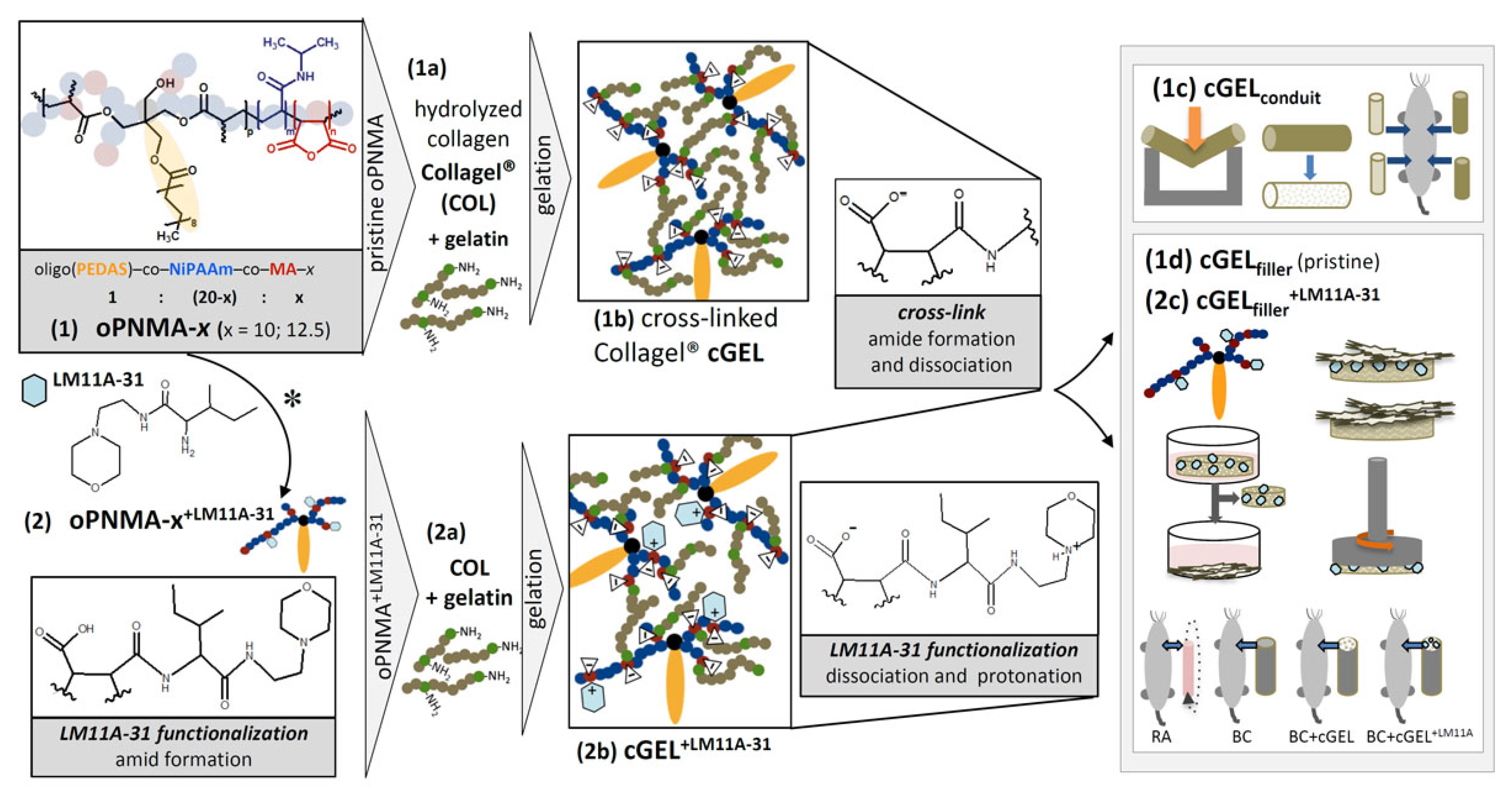
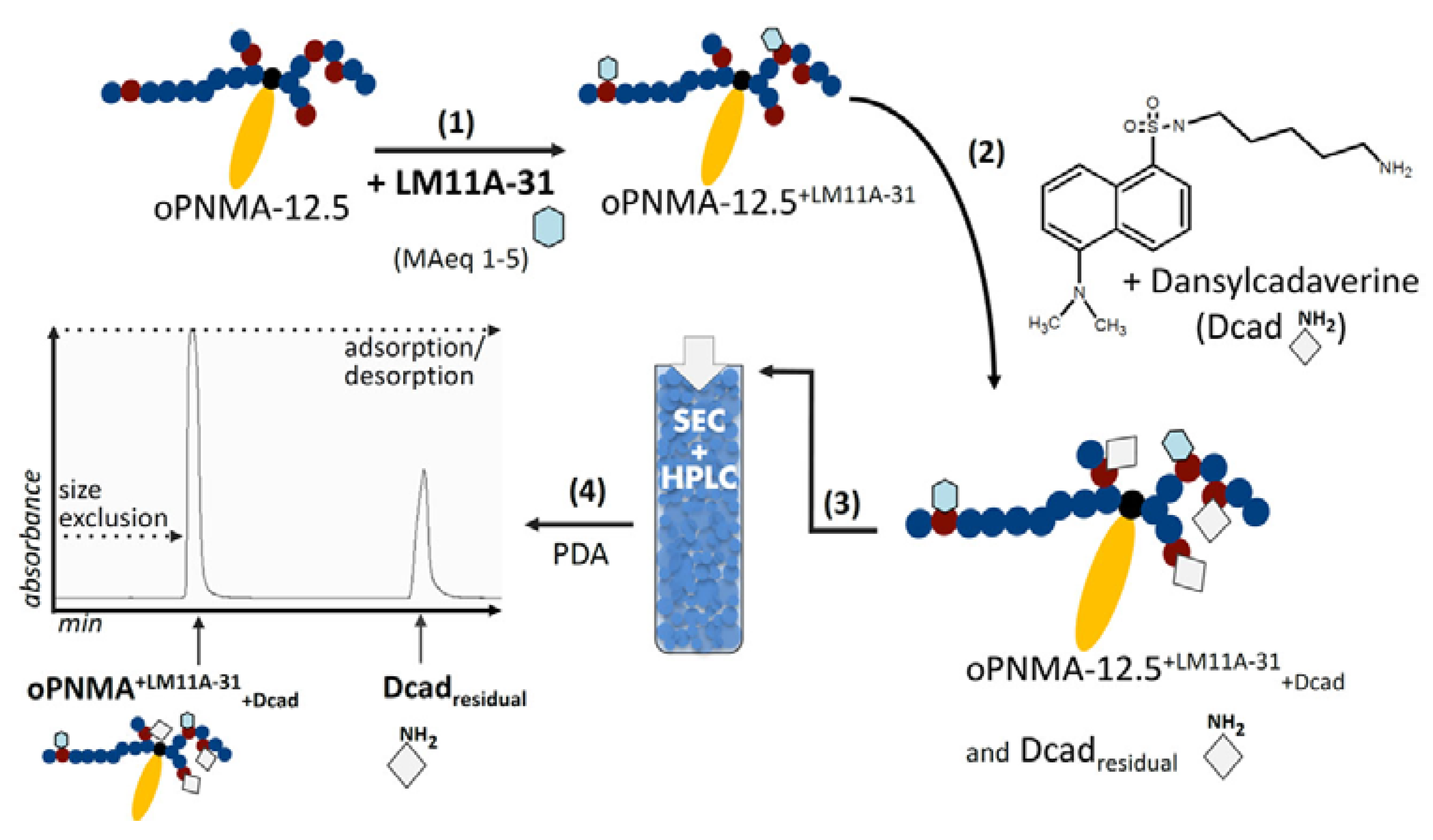
2.2.1. Filler Functionalization Concept
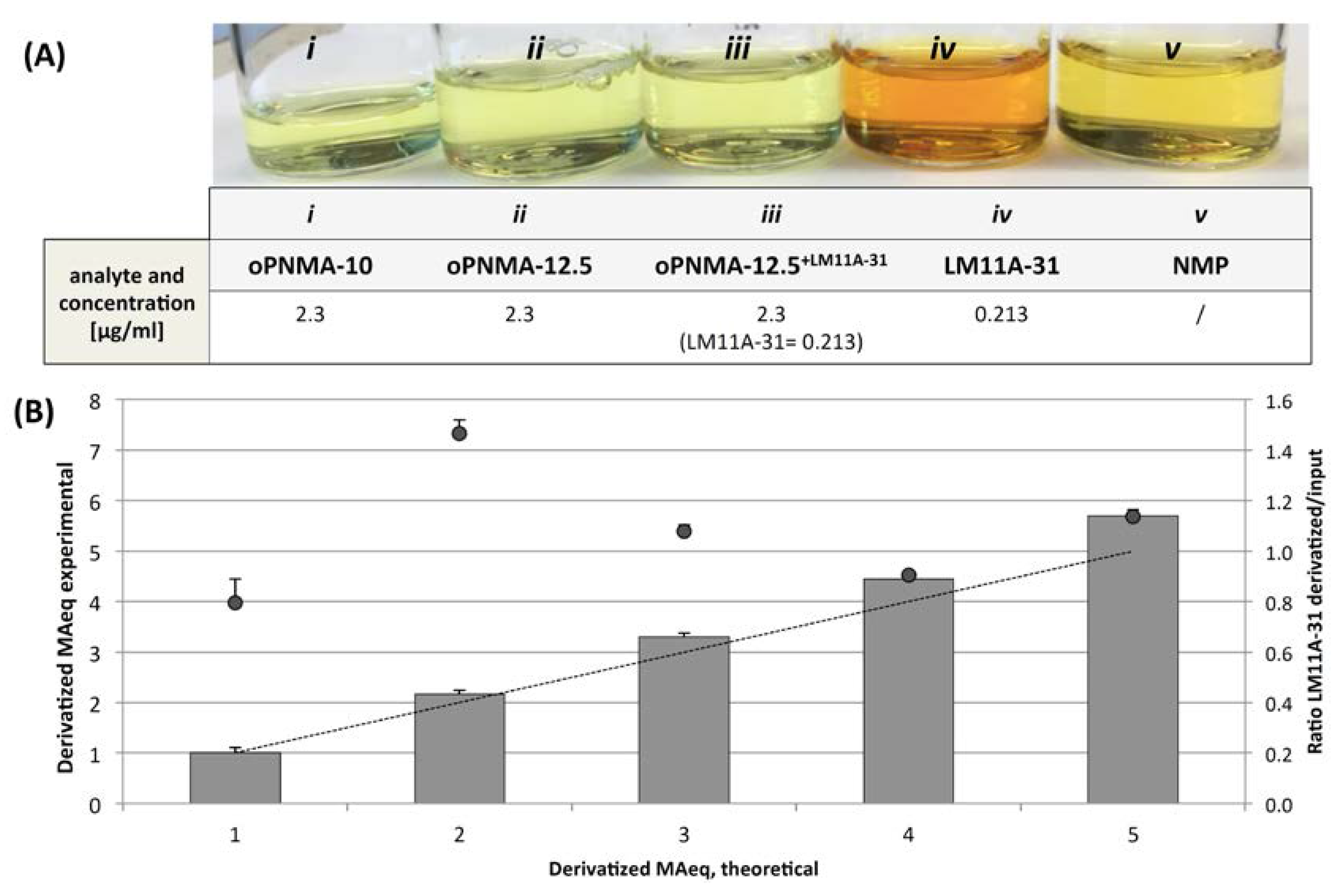
2.2.2. Quantification of LM11A-31 Derivatization
2.2.3. In Vitro Testing of LM11A-31 in Direct Contact with hASC
2.3. CGELfiller–Characterization, In Vitro and In Vivo Testing
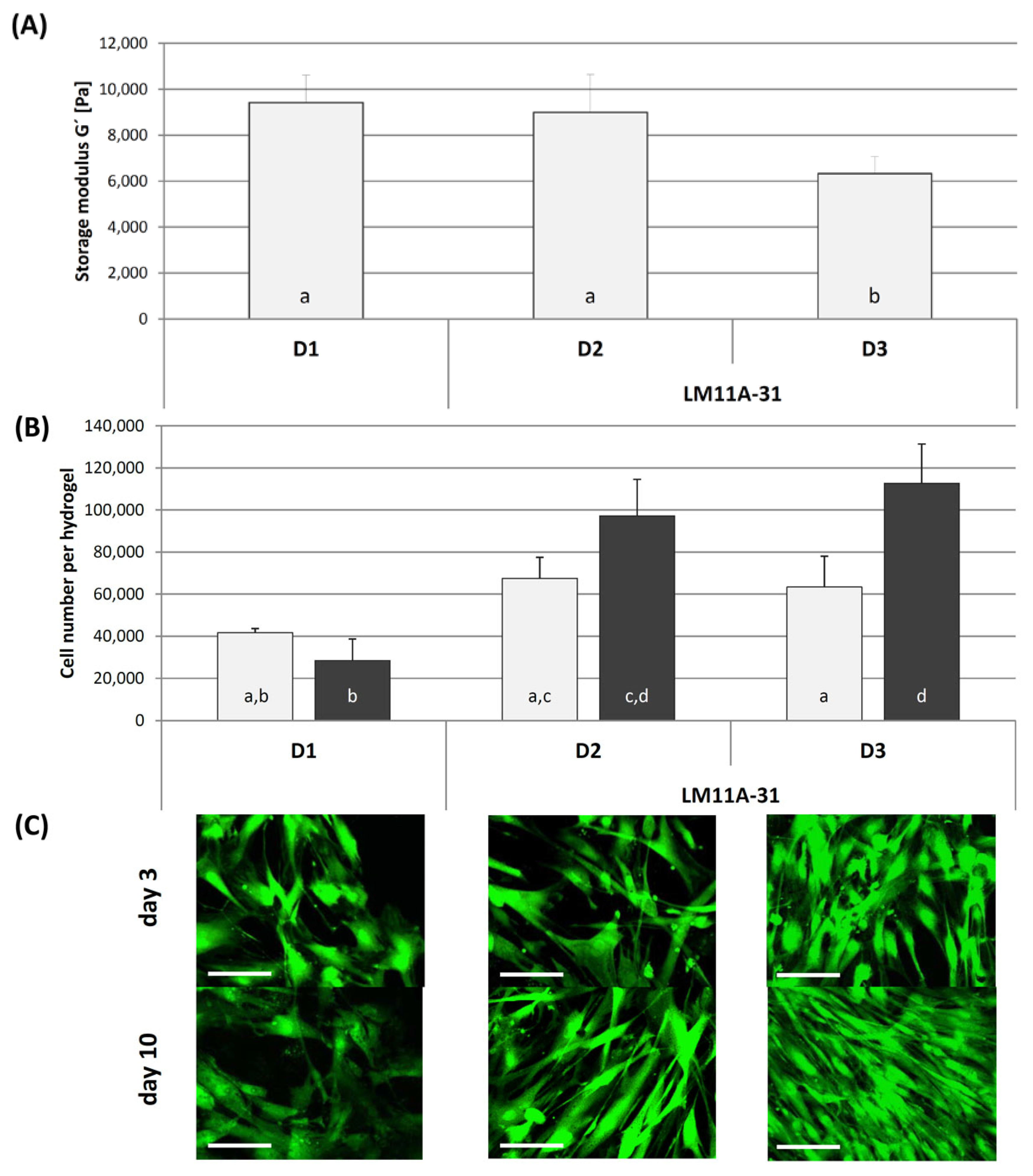
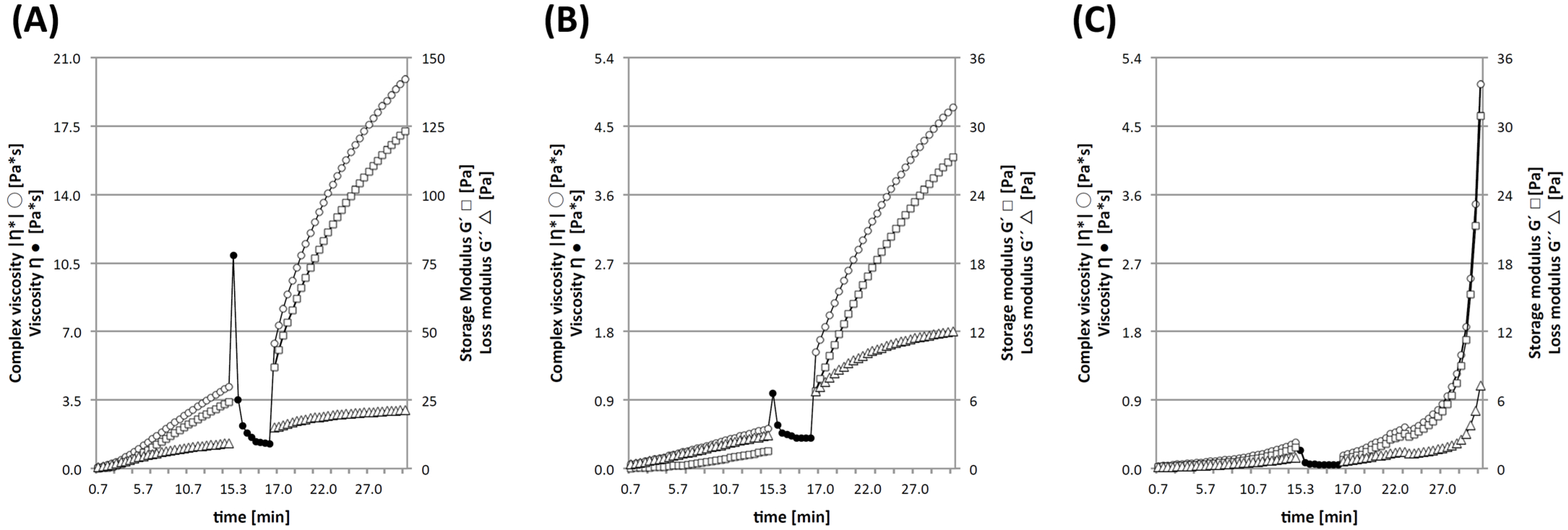
2.3.1. Shear Thinning and Micromechanical Characterization
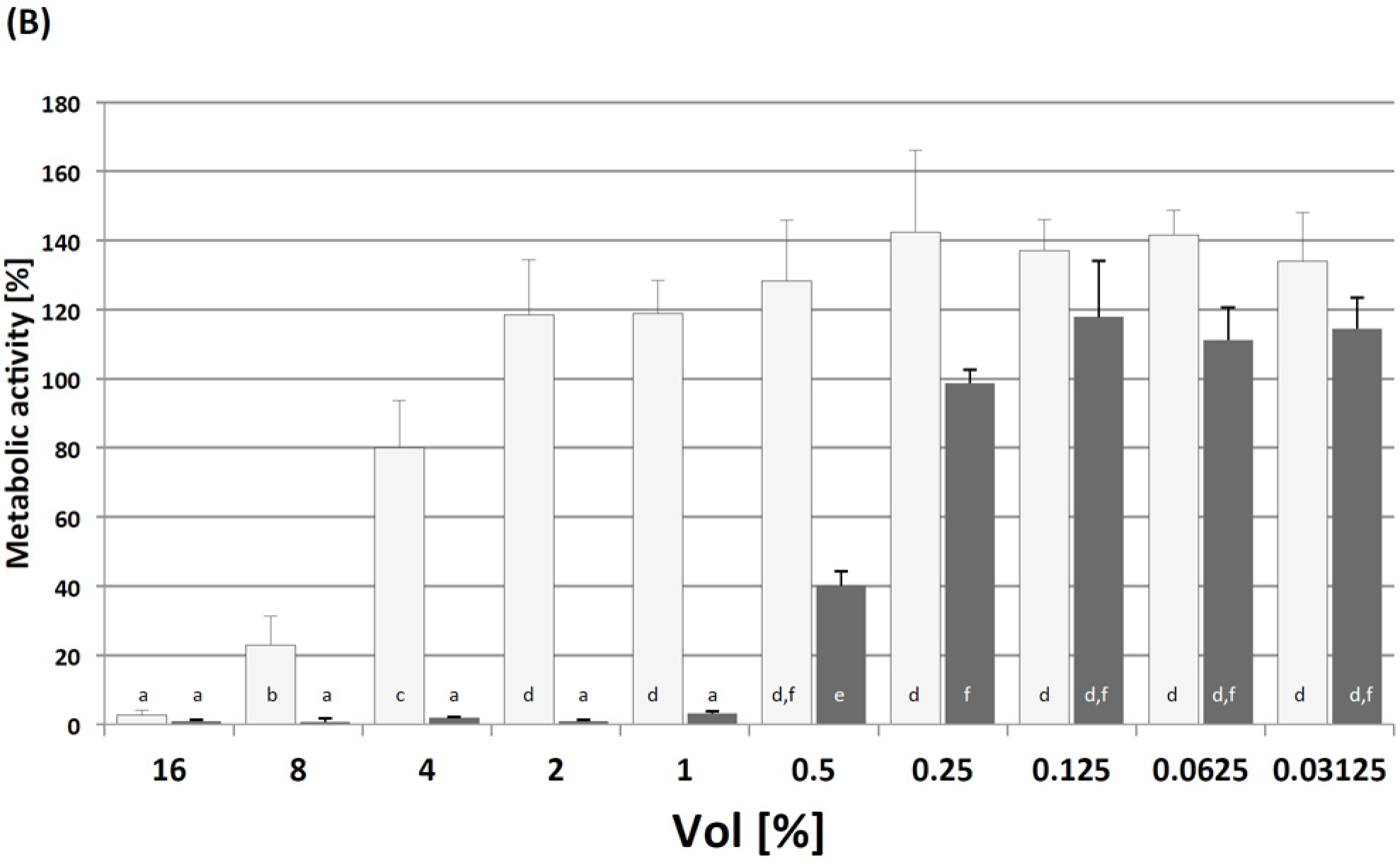
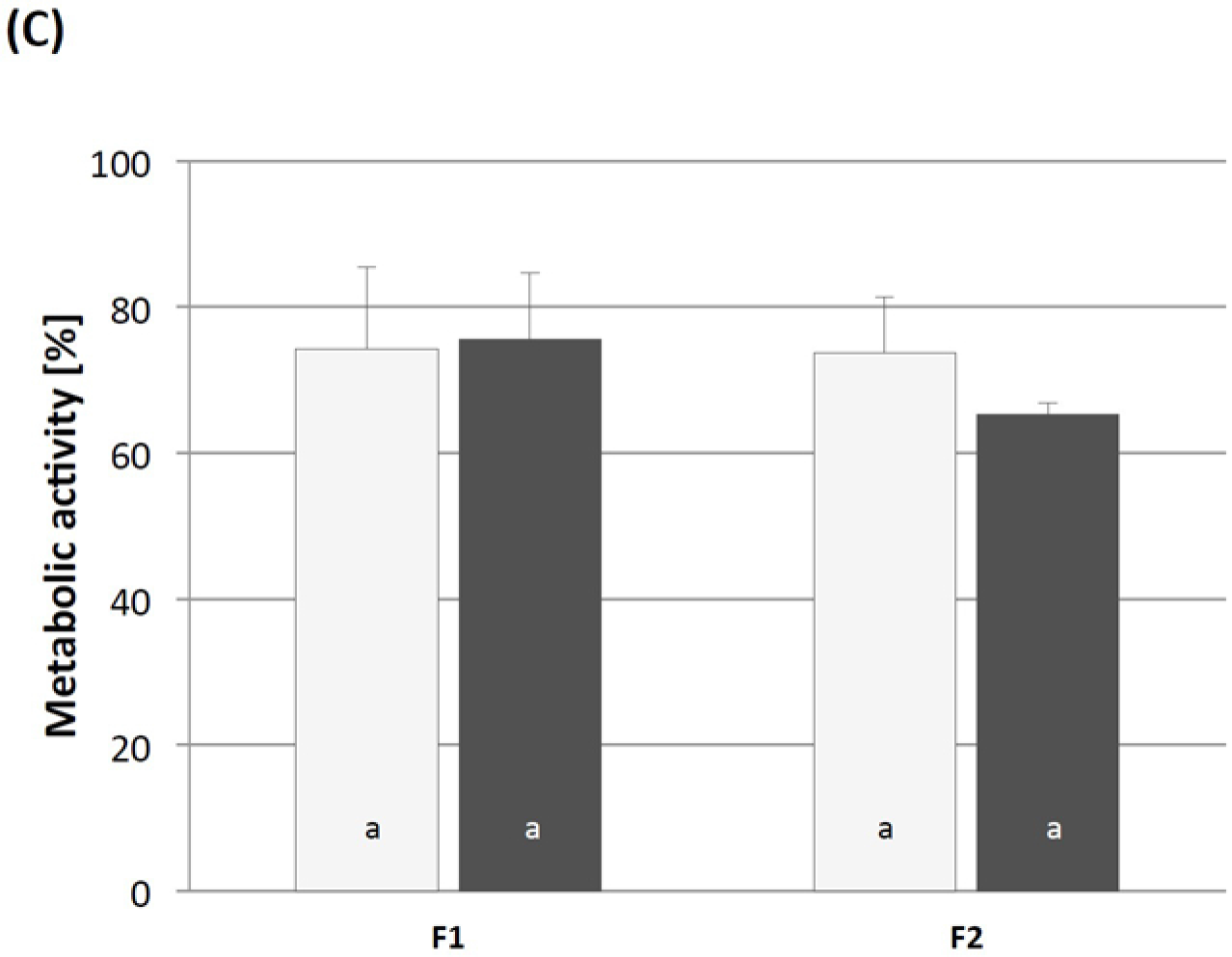
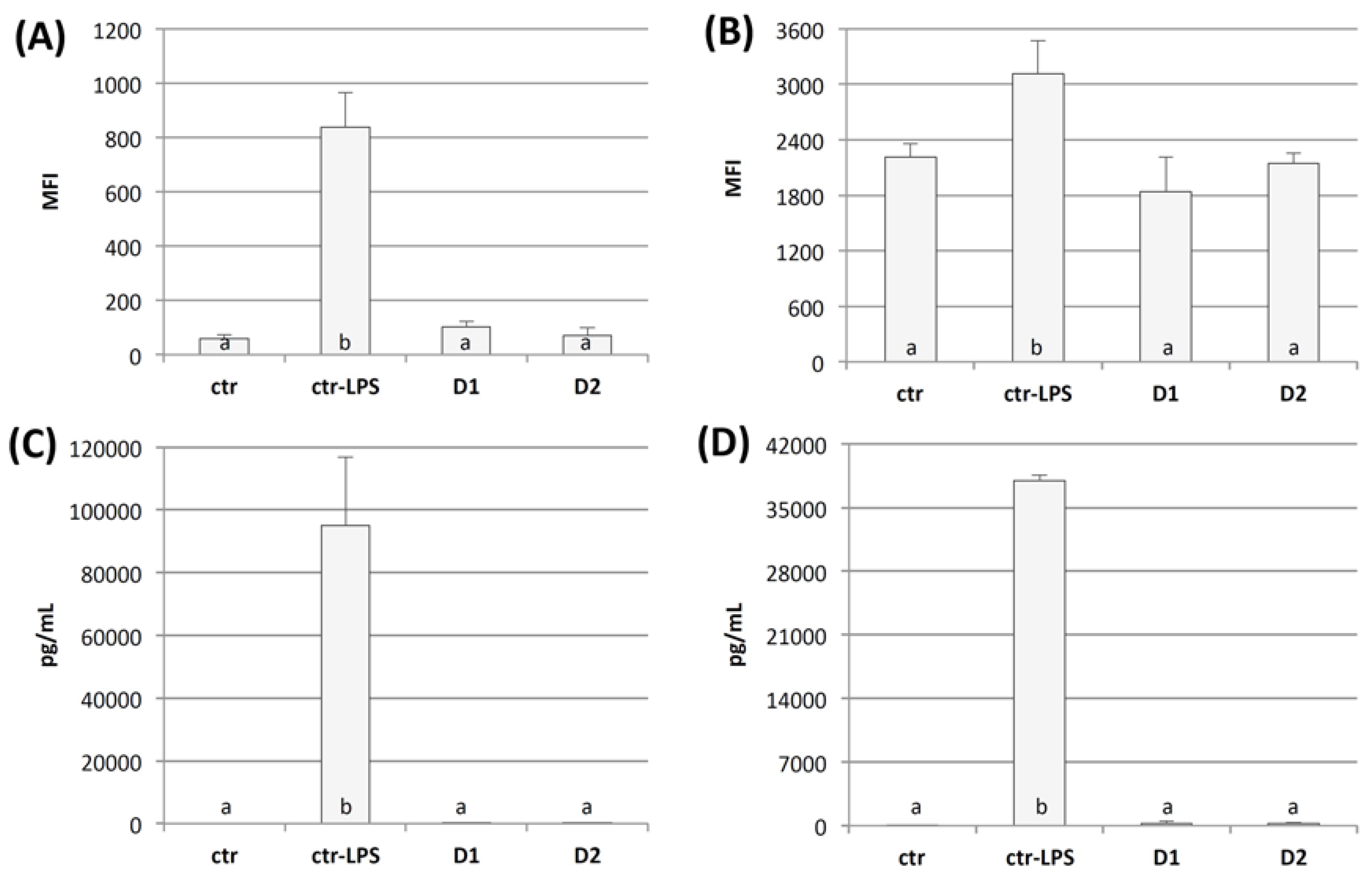
2.3.2. Cytotoxicity Testing and Immunogenicity of cGELfiller Composites
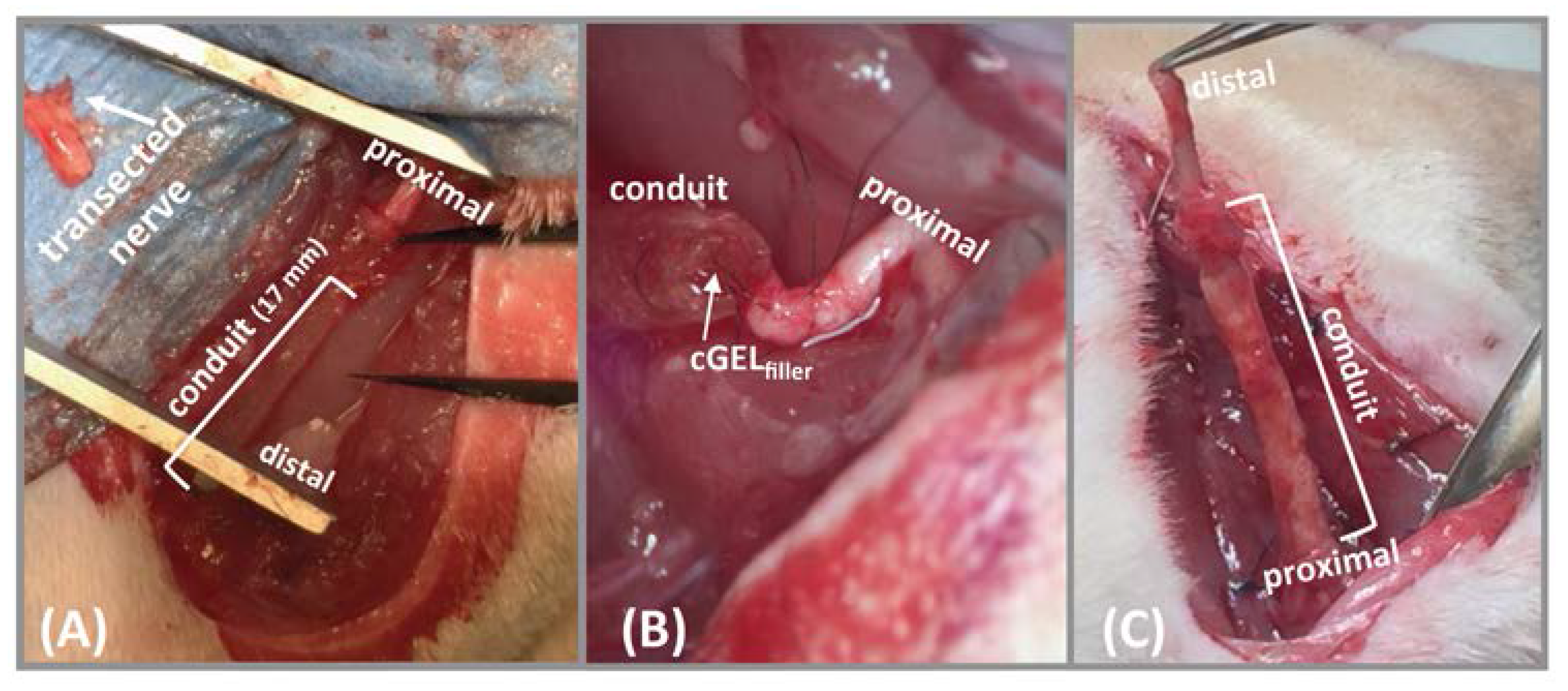
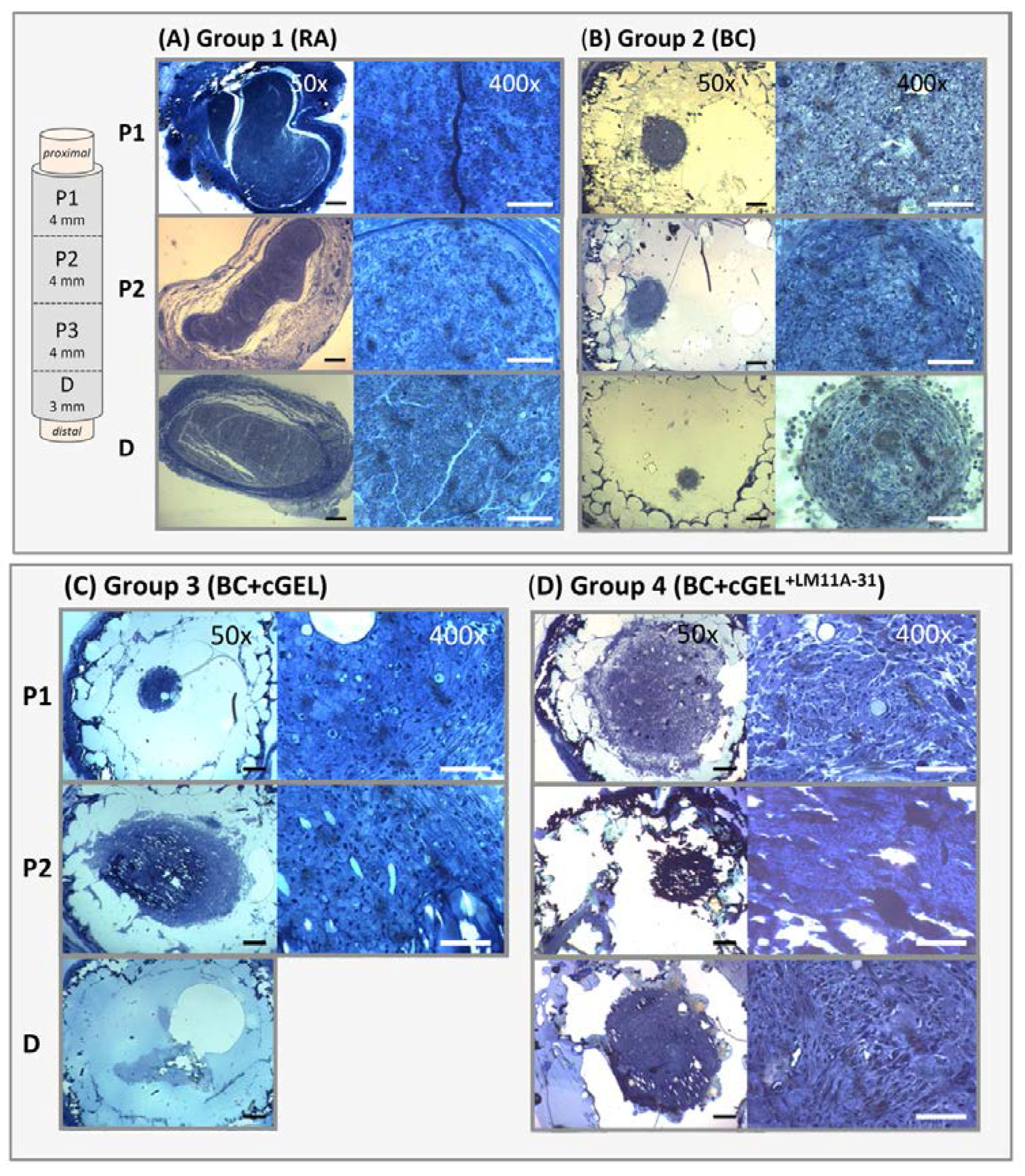
2.3.3. In Vivo Testing of Luminal Fillers
3. Materials and Methods
3.1. Materials
3.2. Oligomeric Cross-Linker Synthesis and Characterization
3.3. Functionalization of cGEL with LM11A-31
3.3.1. Procedure
3.3.2. Colorimetric Staining
3.3.3. Quantification
3.4. Hydrogel Fabrication
3.4.1. Conduits
3.4.2. Discs
3.4.3. Filler Fabrication and Injection
3.5. Characterization of Cross-Linking and cGEL Hydrogel
3.5.1. Mechanical Characterization of cGELdisc
3.5.2. Gelation Profile and Shear Thinning of cGELfiller
3.5.3. Micromechanical Characterization of cGELfiller
3.5.4. Cross-Linking Degree
3.5.5. Bending Stiffness of cGELconduit
3.5.6. In Vitro Degradation of cGELconduit
3.6. In Vitro and In Vivo Compatibility
3.6.1. Subcutaneous Implantation of cGELconduit
3.6.2. Cytotoxicity of NMP and NMPO
3.6.3. Indirect Cytocompatibility Test with cGELfiller Extracts
3.6.4. Immunogenicity of cGELdisc
3.6.5. Direct In Vitro Cell Contact to cGELdisc
3.7. CGELfiller in a Rat Sciatic Nerve Injury Model
3.7.1. Surgical Method and Animal Groups
3.7.2. Histomorphometric Analysis
3.8. Statistics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AIBN | 2,2-azobis(2-methylpropionitrile) |
| λabs | absorption wavelength of Dcad |
| EI | bending stiffness |
| BC | braided conduit |
| calcein-AM | calcein-acetoxymethyl |
| COL | Collagel® |
| cGEL | oligomeric cross-linked Collagel® |
| cGELconduit | cGEL derived conduit |
| cGELdisc | cGEL derived disc |
| cGELfiller | cGEL derived filler |
| |η*| | complex viscosity |
| CD80 | cluster of differentiation 80 |
| CLD | cross-linking degree |
| CM | culture medium |
| LM11A-31 | (2S,3S)-2-amino-3-methyl-N-[2,[2-(4-morpholinyl)ethyl] pentanamide |
| Dcad | dansylcadaverine |
| DMEM | Dulbecco’s modified eagle medium |
| DRG | dorsal root ganglia |
| E* | effective Young’s Modulus |
| ECM | extra cellular matrix |
| FBS | fetal bovine serum |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| H&E | hematoxylin and eosin |
| HLA-DR | human leukocyte antigen-antigen D related |
| HPLC | high performance liquid chromatography |
| hASC | human adipose tissue-derived stem cells |
| HA | hyaluronic acid |
| IACUC | Institutional Animal Care and Use Committee |
| IL-12 | interleukin-12 |
| ISO | International Organization of Standardization |
| LPS | lipopolysaccharide |
| LSM | laser scanning microscopy |
| cGEL+LM11A-31 | LM11A-31 functionalized cGEL |
| cGELfiller+LM11A-31 | LM11A-31 functionalized filler cGEL |
| G’ | storage modulus |
| G” | loss modulus |
| MA | maleic anhydride |
| MAeq | equivalents of intact maleic anhydride |
| p75NTR | neurotrophin 75 receptors |
| NGF | nerve growth factor |
| NGCs | nerve guidance conduits |
| NiPAAm | N-isopropylacrylamide |
| NMPO | N-methylpiperidin-3-ol |
| NMP | N-methylpyrrolidon |
| DMF | N,N-dimethylformamide |
| oPNMA | oligomeric cross-linker oligo(PEDAS-co-NiPAAm-co-MA) |
| oPNMA+LM11A-31 | oPNMA covalently functionalized with LM11A-31 |
| oPNMA+LM11A-31+Dcad | Dcad covalently bound to oligomer oPNMA+LM11A-31 |
| P/S | penicillin/streptomycin |
| PEDAS | pentaerythritol diacrylate monostearate |
| PNR | peripheral nerve regeneration |
| PBS | phosphate buffered saline |
| RA | reverse autograft |
| SC | Schwann cells |
| SEM | scanning electron microscopy |
| SEC | size exclusion chromatography |
| SD | standard deviations |
| s.c. | subcutaneous |
| TNBS | 2,4,6-trinitrobenzenesulfonic acid |
| TNF | tumor necrosis factor |
| USP/NF | United States Pharmacopeia/National Formulary |
References
- Chiono, V.; Tonda-Turo, C. Trends in the design of nerve guidance channels in peripheral nerve tissue engineering. Prog. Neurobiol. 2015, 131, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.F.; Malahias, M.; Hindocha, S.; Khan, W.S. Peripheral nerve injury: Principles for repair and regeneration. Open Orthop. J. 2014, 8, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: Bridging the peripheral nerve gap and enhancing functional recovery. J. R. Soc. Interface 2012, 9, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Zhang, M.; Spilker, M.H. Standardized criterion to analyze and directly compare various materials and models for peripheral nerve regeneration. J. Biomater. Sci. Polym. Ed. 2012, 18, 943–966. [Google Scholar] [CrossRef] [PubMed]
- Mukhatyar, V.J.; Salmerón-Sánchez, M.; Rudra, S.; Mukhopadaya, S.; Barker, T.H.; García, A.J.; Bellamkonda, R.V. Role of fibronectin in topographical guidance of neurite extension on electrospun fibers. Biomaterials 2011, 32, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, L.J.; Yannas, I.V.; Hsu, H.P.; Strichartz, G.; Spector, M. Collagen-GAG substrate enhances the quality of nerve regeneration through collagen tubes up to level of autograft. Exp. Neurol. 1998, 154, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Glasby, M.A.; Gschmeissner, S.G.; Hitchcock, R.J.I.; Huang, C.L.H. The dependence of nerve regeneration through muscle grafts in the rat on the availability and orientation of basement membrane. J. Neurocytol. 1986, 15, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Höke, A. Mechanisms of Disease: What factors limit the success of peripheral nerve regeneration in humans? Nat. Clin. Pract. Neurol. 2006, 2, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, L.J.; Yannas, I.V.; Hsu, H.P.; Spector, M. Connective tissue response to tubular implants for peripheral nerve regeneration: The role of myofibroblasts. J. Comp. Neurol. 2000, 417, 415–430. [Google Scholar] [CrossRef]
- Lu, M.C.; Hsiang, S.-W.; Lai, T.Y.; Yao, C.-H.; Lin, L.-Y.; Chen, Y.-S. Influence of cross-linking degree of a biodegradable genipin-cross-linked gelatin guide on peripheral nerve regeneration. J. Biomater. Sci. Polym. Ed. 2007, 18, 843–863. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-C.; Yang, Y.-C.; Liu, B.-S. Peripheral nerve repair of transplanted undifferentiated adipose tissue-derived stem cells in a biodegradable reinforced nerve conduit. J. Biomed. Mater. Res. Part A 2012, 100, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Kokol, V. Collagen- vs. gelatine-based biomaterials and their biocompatibility: Review and perspectives. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; InTech: Rijeka, Croatia, 2011; pp. 17–52. [Google Scholar]
- Tonda Turo, C.; Gnavi, S.; Ruini, F.; Gambarotta, G.; Gioffredi, E.; Chiono, V.; Perroteau, I.; Ciardelli, G. Development and characterization of novel agar and gelatin injectable hydrogel as filler for peripheral nerve guidance channels. J. Tissue Eng. Regen. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-S.; Yao, C.-H.; Hsu, S.-H.; Yeh, T.-S.; Chen, Y.-S.; Kao, S.-T. A novel use of genipin-fixed gelatin as extracellular matrix for peripheral nerve regeneration. J. Biomater. Appl. 2004, 19, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; Zoubos, A.B.; Soucacos, P.N. Regeneration and repair of peripheral nerves. Injury 2005, 36, S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Gulati, A.K.; Rai, D.R.; Ali, A.M. The influence of cultured Schwann cells on regeneration through acellular basal lamina grafts. Brain Res. 1995, 705, 118–124. [Google Scholar] [CrossRef]
- Stoll, G.; Griffin, J.W.; Li, C.Y.; Trapp, B.D. Wallerian degeneration in the peripheral nervous system: Participation of both Schwann cells and macrophages in myelin degradation. J. Neurocytol. 1989, 18, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.; van Neerven, S.G.A.; Claeys, K.G.; O’Dey, D.M.; Sudhoff, A.; Brook, G.A.; Sellhaus, B.; Schulz, J.B.; Weis, J.; Pallua, N. The proximal medial sural nerve biopsy model: A standardised and reproducible baseline clinical model for the translational evaluation of bioengineered nerve guides. BioMed Res. Int. 2014, 2014, 121452. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, F.; Udina, E.; Navarro, X. Extracellular Matrix Components in Peripheral Nerve Regeneration. Int. Rev. Neurobiol. 2013, 108, 257–275. [Google Scholar] [PubMed]
- Luca, A.C.; Terenghi, G.; Downes, S. Chemical surface modification of poly-ε-caprolactone improves Schwann cell proliferation for peripheral nerve repair. J. Tissue Eng. Regen. Med. 2014, 8, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Madduri, S.; Gander, B. Growth factor delivery systems and repair strategies for damaged peripheral nerves. J. Control. Release 2012, 161, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J. Effects of nerve growth factor from genipin-crosslinked gelatin in polycaprolactone conduit on peripheral nerve regeneration—In vitro and in vivo. J. Biomed. Mater. Res. Part A 2009, 91, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.M.; Massa, S.M. Small-molecule modulation of neurotrophin receptors: A strategy for the treatment of neurological disease. Nat. Rev. Drug Discov. 2013, 12, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, R.A.; Pundavela, J.; Biarc, J.; Chalkley, R.J.; Burlingame, A.L.; Hondermarck, H. NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling. Adv. Biol. Regul. 2015, 58, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, C.F.; Simi, A. p75 neurotrophin receptor signaling in nervous system injury and degeneration: Paradox and opportunity. Trends Neurosci. 2012, 35, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Kubo, T.; Matsuda, K.; Fujiwara, T.; Yano, K. The neurotrophin receptor p75NTR in Schwann cells is implicated in remyelination and motor recovery after peripheral nerve injury. Glia 2007, 55, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- He, X.-L.; Garcia, K.C. Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science 2004, 304, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.M.; Xie, Y.; Massa, S.M. Neurotrophin small molecule mimetics: Candidate therapeutic agents for neurological disorders. Curr. Med. Chem. Cent. Nerv. Syst. Agents 2005, 5, 29–41. [Google Scholar] [CrossRef]
- Massa, S.M.; Xie, Y.; Yang, T.; Harrington, A.W.; Kim, M.L.; Yoon, S.O.; Kraemer, R.; Moore, L.A.; Hempstead, B.L.; Longo, F.M. Small, nonpeptide p75NTR ligands induce survival signaling and inhibit proNGF-induced death. J. Neurosci. 2006, 26, 5288–5300. [Google Scholar] [CrossRef] [PubMed]
- Kazim, S.F.; Iqbal, K. Neurotrophic factor small-molecule mimetics mediated neuroregeneration and synaptic repair: Emerging therapeutic modality for Alzheimer’s disease. Mol. Neurodegener. 2016, 11, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Loth, T.; Hötzel, R.; Kascholke, C.; Anderegg, U.; Schulz-Siegmund, M.; Hacker, M.C. Gelatin-based biomaterial engineering with anhydride-containing oligomeric cross-linkers. Biomacromolecules 2014, 15, 2104–2118. [Google Scholar] [CrossRef] [PubMed]
- Kohn, C.; Klemens, J.M.; Kascholke, C.; Murthy, N.S.; Kohn, J.; Brandenburger, M.; Hacker, M.C. Dual-component collagenous peptide/reactive oligomer hydrogels as potential nerve guidance materials—From characterization to functionalization. Biomater. Sci. 2016, 4, 1605–1621. [Google Scholar] [CrossRef] [PubMed]
- Kascholke, C.; Loth, T.; Kohn-Polster, C.; Möller, S.; Bellstedt, P.; Schulz-Siegmund, M.; Schnabelrauch, M.; Hacker, M.C. Dual-functional hydrazide-reactive and anhydride containing oligomeric hydrogel building blocks. Biomacromolecules 2017, 18, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Clements, B.A.; Bushman, J.; Murthy, N.S.; Ezra, M.; Pastore, C.M.; Kohn, J. Design of barrier coatings on kink-resistant peripheral nerve conduits. J. Tissue Eng. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, G.C.; Onyeneho, I.A.; Liang, E.T.; Moore, M.J.; Knight, A.M.; Malessy, M.J.A.; Spinner, R.J.; Lu, L.; Currier, B.L.; Yaszemski, M.J.; et al. Methods for in vitro characterization of multichannel nerve tubes. J. Biomed. Mater. Res. Part A 2008, 84, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Billiar, K.L.; Windebank, A.J.; Pandit, A. Multichanneled collagen conduits for peripheral nerve regeneration: Design, fabrication, and characterization. Tissue Eng. Part C 2010, 16, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Koch, D.; Rosoff, W.J.; Jiang, J.; Geller, H.M.; Urbach, J.S. Strength in the periphery: Growth cone biomechanics and substrate rigidity response in peripheral and central nervous system neurons. Biophys. J. 2012, 102, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Sundararaghavan, H.G.; Monteiro, G.A.; Firestein, B.L.; Shreiber, D.I. Neurite growth in 3D collagen gels with gradients of mechanical Properties. Biotechnol. Bioeng. 2009, 102, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ji, Y.; Zhao, Y.; Liu, Y.; Ding, F.; Gu, X.; Yang, Y. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials 2012, 33, 6672–6681. [Google Scholar] [CrossRef] [PubMed]
- Willits, R.K.; Skornia, S.L. Effect of collagen gel stiffness on neurite extension. J. Biomater. Sci. Polym. Ed. 2012, 15, 1521–1531. [Google Scholar] [CrossRef]
- Dendunnen, W.; Schakenraad, J.M.; Zondervan, G.J.; Pennings, A.J.; Vanderlei, B.; Robinson, P.H. A new PLLA/PCL copolymer for nerve regeneration. J. Mater. Sci. Mater. Med. 1993, 4, 521–525. [Google Scholar] [CrossRef]
- Williams, D.F. There is no such thing as a biocompatible material. Biomaterials 2014, 35, 10009–10014. [Google Scholar] [CrossRef] [PubMed]
- Loth, T.; Hennig, R.; Kascholke, C.; Hötzel, R.; Hacker, M.C. Reactive and stimuli-responsive maleic anhydride containing macromers—Multi-functional cross-linkers and building blocks for hydrogel fabrication. React. Funct. Polym. 2013, 73, 1480–1492. [Google Scholar] [CrossRef]
- Song, X.Y.; Zhou, F.H.H.; Zhong, J.H.; Wu, L.L.Y.; Zhou, X.F. Knockout of p75NTR impairs re-myelination of injured sciatic nerve in mice. J. Neurochem. 2006, 96, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Lu, J.; Sheen, V.; Wang, S. Optimal poly(l-lysine) grafting density in hydrogels for promoting neural progenitor cell functions. Biomacromolecules 2012, 13, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Osada, S.; Narita, T.; Oishi, Y.; Kodama, H. Promotion of cell adhesion by low-molecular-weight hydrogel by Lys based amphiphile. Mater. Sci. Eng. C 2015, 47, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Dadsetan, M.; Knight, A.M.; Lu, L.; Windebank, A.J.; Yaszemski, M.J. Stimulation of neurite outgrowth using positively charged hydrogels. Biomaterials 2009, 30, 3874–3881. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Abou-Seri, S.M.; Hanna, M.M.; Abdalla, M.M.; El Sayed, N.A. Design, synthesis and biological evaluation of novel condensed pyrrolo[1,2-c]pyrimidines featuring morpholine moiety as PI3Kα inhibitors. Eur. J. Med. Chem. 2015, 99, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Z.; Liu, L.; Zhao, C.; Xiong, F.; Zhou, C.; Li, Y.; Shan, Y.; Peng, F.; Zhang, C. Neurospheres from rat adipose-derived stem cells could be induced into functional Schwann cell-like cells in vitro. BMC Neurosci. 2008, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Lu, J.; Sheen, V.; Wang, S. Promoting nerve cell functions on hydrogels grafted with poly(l-lysine). Biomacromolecules 2012, 13, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Yasuda, K. Chondrogenesis on sulfonate-coated hydrogels is regulated by their mechanical properties. J. Mech. Behav. Biomed. Mater. 2013, 17, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Georges, P.C.; Janmey, P.A. Cell type-specific response to growth on soft materials. J. Appl. Physiol. 2005, 98, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Jouyban, A.; Fakhree, M.A.A.; Shayanfar, A. Review of pharmaceutical applications of N-methyl-2-pyrrolidone. J. Pharm. Pharm. Sci. 2010, 13, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Shortt, J.; Hsu, A.K.; Martin, B.P.; Doggett, K.; Matthews, G.M.; Doyle, M.A.; Ellul, J.; Jockel, T.E.; Andrews, D.M.; Hogg, S.J.; et al. The drug vehicle and solvent N-methylpyrrolidone Is an immunomodulator and antimyeloma compound. Cell Rep. 2014, 7, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Green, B.T.; Lee, S.T.; Panter, K.E.; Brown, D.R. Piperidine alkaloids: Human and food animal teratogens. Food Chem. Toxicol. 2012, 50, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Molyneux, R.J.; Panter, K.E.; Chang, C.-W.T.; Gardner, D.R.; Pfister, J.A.; Garrossian, M. Ammodendrine and N-methylammodendrine enantiomers: Isolation, optical rotation, and toxicity. J. Nat. Prod. 2005, 68, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; Soucacos, P.N. Nerve repair: Experimental and clinical evaluation of biodegradable artificial nerve guides. Injury 2008, 39, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Ezra, M.; Bushman, J.; Shreiber, D.I.; Schachner, M.; Kohn, J. Porous and non-porous nerve conduits: The effects of a hydrogel luminal filler with and without a neurite-promoting moiety. Tissue Eng. Part A 2016, 22, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Kawabuchi, M. Myelin phagocytosis by macrophages and nonmacrophages during Wallerian degeneration. Microsc. Res. Tech. 2002, 57, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Fleetwood, A.J.; Dinh, H.; Cook, A.D.; Hertzog, P.J.; Hamilton, J.A. GM-CSF- and M-CSF-dependent macrophage phenotypes display differential dependence on Type I interferon signaling. J. Leukoc. Biol. 2009, 86, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Adanali, G.; Verdi, M.; Tuncel, A.; Erdogan, B.; Kargi, E. Effects of hyaluronic acid-carboxymethylcellulose membrane on extraneural adhesion formation and peripheral nerve regeneration. J. Reconstr. Microsurg. 2003, 19, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zor, F.; Deveci, M.; Kilic, A.; Ozdag, M.F.; Kurt, B.; Sengezer, M.; Sönmez, T.T. Effect of vegf gene therapy and hyaluronic acid film sheath on peripheral nerve regeneration. Microsurgery 2014, 34, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Daly, W.T.; Yao, L.; Abu-rub, M.T.; O’Connell, C.; Zeugolis, D.I.; Windebank, A.J.; Pandit, A.S. The effect of intraluminal contact mediated guidance signals on axonal mismatch during peripheral nerve repair. Biomaterials 2012, 33, 6660–6671. [Google Scholar] [CrossRef] [PubMed]
- Stang, F.; Fansa, H.; Wolf, G.; Reppin, M.; Keilhoff, G. Structural parameters of collagen nerve grafts influence peripheral nerve regeneration. Biomaterials 2005, 26, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kempen, D.H.R.; de Ruiter, G.C.W.; Cai, L.; Spinner, R.J.; Windebank, A.J.; Yaszemski, M.J.; Lu, L. Molecularly engineered biodegradable polymer networks with a wide range of stiffness for bone and peripheral nerve regeneration. Adv. Funct. Mater. 2015, 25, 2715–2724. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Martorina, F.; Casale, C.; Urciuolo, F.; Netti, P.A.; Imparato, G. In vitro activation of the neuro-transduction mechanism in sensitive organotypic human skin model. Biomaterials 2017, 113, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Moshtagh, P.R.; Pouran, B.; Korthagen, N.M.; Zadpoor, A.A.; Weinans, H. Guidelines for an optimized indentation protocol for measurement of cartilage stiffness: The effects of spatial variation and indentation parameters. J. Biomech. 2016, 49, 3602–3607. [Google Scholar] [CrossRef] [PubMed]
- Dawlee, S.; Sugandhi, A.; Balakrishnan, B.; Labarre, D.; Jayakrishnan, A. Oxidized chondroitin sulfate-cross-linked gelatin matrixes: A new class of hydrogels. Biomacromolecules 2005, 6, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N. Multiple applications of alamar blue as an indicator of metabolic function and cellular health in cell viability Bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Franz, S.; Allenstein, F.; Kajahn, J.; Forstreuter, I.; Hintze, V.; Moeller, S.; Simon, J.C. Artificial extracellular matrices composed of collagen I and high-sulfated hyaluronan promote phenotypic and functional modulation of human pro-inflammatory M1 macrophages. Acta Biomater. 2013, 9, 5621–5629. [Google Scholar] [CrossRef] [PubMed]
- Canan, S.; Bozkurt, H.H.; Acar, M.; Vlamings, R.; Aktas, A.; Sahin, B.; Temel, Y.; Kaplan, S. An efficient stereological sampling approach for quantitative assessment of nerve regeneration. Neuropathol. Appl. Neurobiol. 2008, 34, 638–649. [Google Scholar] [CrossRef] [PubMed]

| oPNMA | COL (Gelatin) | NMPO | Mechanical Testing | In Vitro | In Vivo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | (%) 1 | +LM11A-31 (MAeq) | (%) 1 | (%) 2 | Three-Point Bending | Rheology | Degradation | Indirect/Direct Cell Contact | s.c. | Sciatic Nerve Model | |
| cGEL conduit | |||||||||||
| C1 | 10 | 3.5 | 12.5 | 4 | + | ||||||
| C2 | 10 | 3.5 | 17.5 | 4 | + | + | |||||
| C3 | 10 | 3.5 | 20 | 2 | + | + | |||||
| C4 | 10 | 3.5 | 20 | 4 | + | ||||||
| C5 | 12.5 | 3.5 | 17.5 | 2 | + | + | |||||
| C6 | 12.5 | 3.5 | 17.5 | 4 | + | + | |||||
| cGEL disc | |||||||||||
| D1 | 10 | 3.5 | 15 | 2 | + | + | |||||
| D2 | 10 | 3.5 | 2.5 | 15 | 2 | + | + | ||||
| D3 | 10 | 3.5 | 5 | 15 | 2 | + | + | ||||
| cGEL filler | |||||||||||
| F1 | 10 | 2.5 | 5.3 (1) | 2 | + | + | + | ||||
| F2 | 12.5 | 2.5 | 2.5 | 5.3 (1) | 2 | + | + | + | |||
| Group | Conduit Material | Filler | Abbreviation | Number of Animals |
|---|---|---|---|---|
| 1 | reverse autograft | - | RA | 5 |
| 2 | braided conduit | - | BC | 5 |
| 3 | cGEL | BC+cGEL | 5 | |
| 4 | cGEL+LM11A-31 | BC+cGEL+LM11A-31 | 5 |
| Group | Histomorphometric Measurements | ||||||
|---|---|---|---|---|---|---|---|
| Number | Material | Number of Animals Recovered | Number of Animals Considered (n out of 5) | Nerve Cable Area (mm2) ‡ | Axonal Density (Axons/mm2) ‡ | G-Ratio ‡ | Nerve Cable Length from Proximal End (mm) |
| 1 | RA | 5 | 5/5 | 2.36 ± 0.27 a | 9646 ± 1856 a | 0.64 ± 0.03 a | 15 |
| 2 | BC | 3 | 3/5 | 1.23 ± 0.06 b,c | 10,980 ± 2673 a | 0.63 ± 0.05 a,b | 4–8 |
| 5/5 | 0.74 ± 0.60 b | 6588 ± 5764 a,b | 0.38 ± 0.31 a,b | ||||
| 3 | BC+cGEL | 1 | 1/5 | 0.10 ‡ | 483 * | 0.78 ‡ | 12 |
| 5/5 | 0.02 ± 0.04 d | 97 ± 193 b | 0.16 ± 0.3 b | ||||
| 4 | BC+cGEL+LM11A-31 | 5 | 5/5 | 1.63 ± 0.18 c | 8153 ± 4731 a | 0.69 ± 0.04 a | 4–12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohn-Polster, C.; Bhatnagar, D.; Woloszyn, D.J.; Richtmyer, M.; Starke, A.; Springwald, A.H.; Franz, S.; Schulz-Siegmund, M.; Kaplan, H.M.; Kohn, J.; et al. Dual-Component Gelatinous Peptide/Reactive Oligomer Formulations as Conduit Material and Luminal Filler for Peripheral Nerve Regeneration. Int. J. Mol. Sci. 2017, 18, 1104. https://doi.org/10.3390/ijms18051104
Kohn-Polster C, Bhatnagar D, Woloszyn DJ, Richtmyer M, Starke A, Springwald AH, Franz S, Schulz-Siegmund M, Kaplan HM, Kohn J, et al. Dual-Component Gelatinous Peptide/Reactive Oligomer Formulations as Conduit Material and Luminal Filler for Peripheral Nerve Regeneration. International Journal of Molecular Sciences. 2017; 18(5):1104. https://doi.org/10.3390/ijms18051104
Chicago/Turabian StyleKohn-Polster, Caroline, Divya Bhatnagar, Derek J. Woloszyn, Matthew Richtmyer, Annett Starke, Alexandra H. Springwald, Sandra Franz, Michaela Schulz-Siegmund, Hilton M. Kaplan, Joachim Kohn, and et al. 2017. "Dual-Component Gelatinous Peptide/Reactive Oligomer Formulations as Conduit Material and Luminal Filler for Peripheral Nerve Regeneration" International Journal of Molecular Sciences 18, no. 5: 1104. https://doi.org/10.3390/ijms18051104