Valproic Acid Induces Endocytosis-Mediated Doxorubicin Internalization and Shows Synergistic Cytotoxic Effects in Hepatocellular Carcinoma Cells
Abstract
:1. Introduction
2. Results
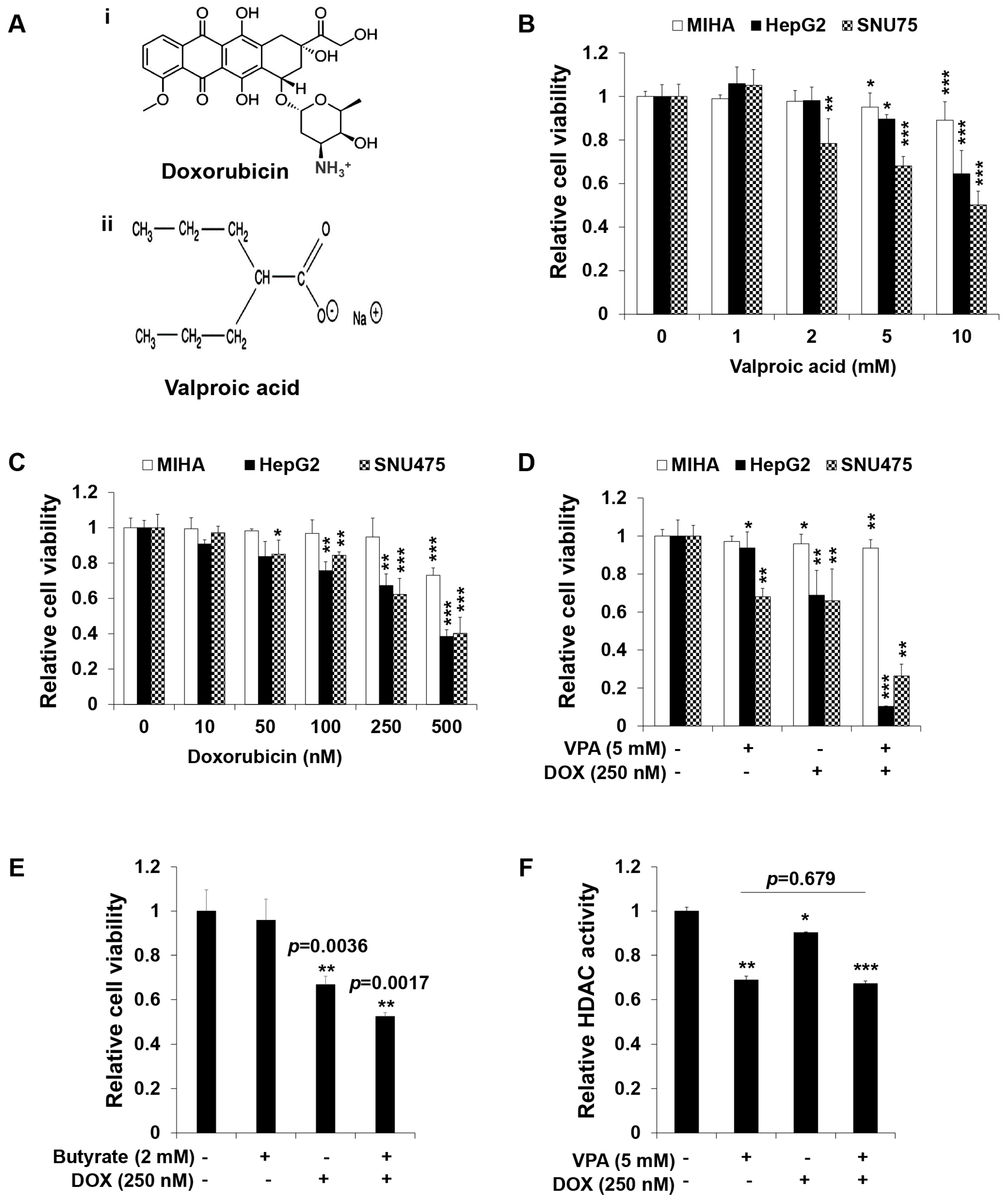
2.1. Combination Treatment of VPA and DOX Synergistically Inhibits the Viability of Human HCC Cells
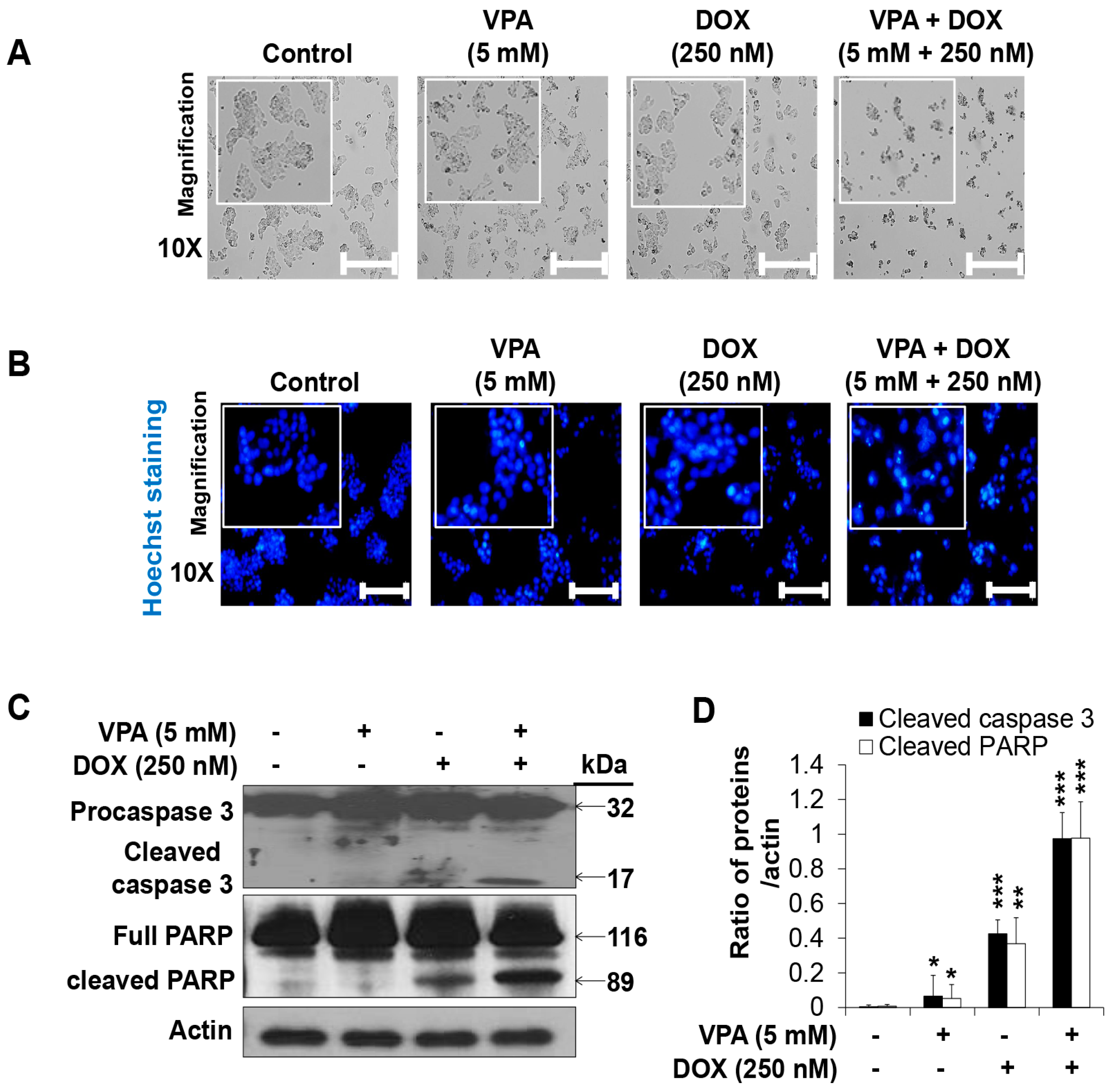
2.2. Combination Treatment of VPA and DOX Synergistically Induces Apoptotic Cell Death in HepG2 Cells
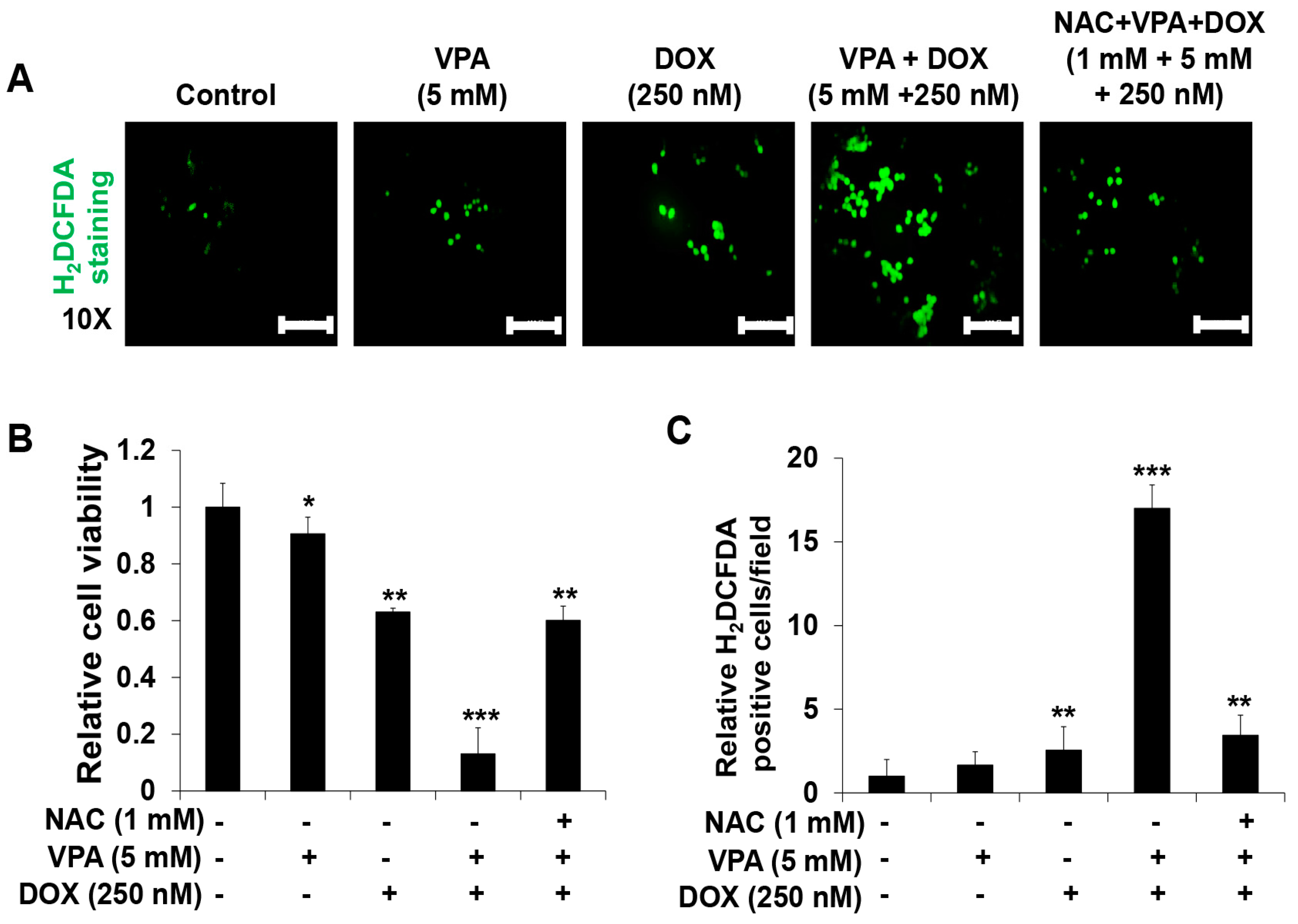
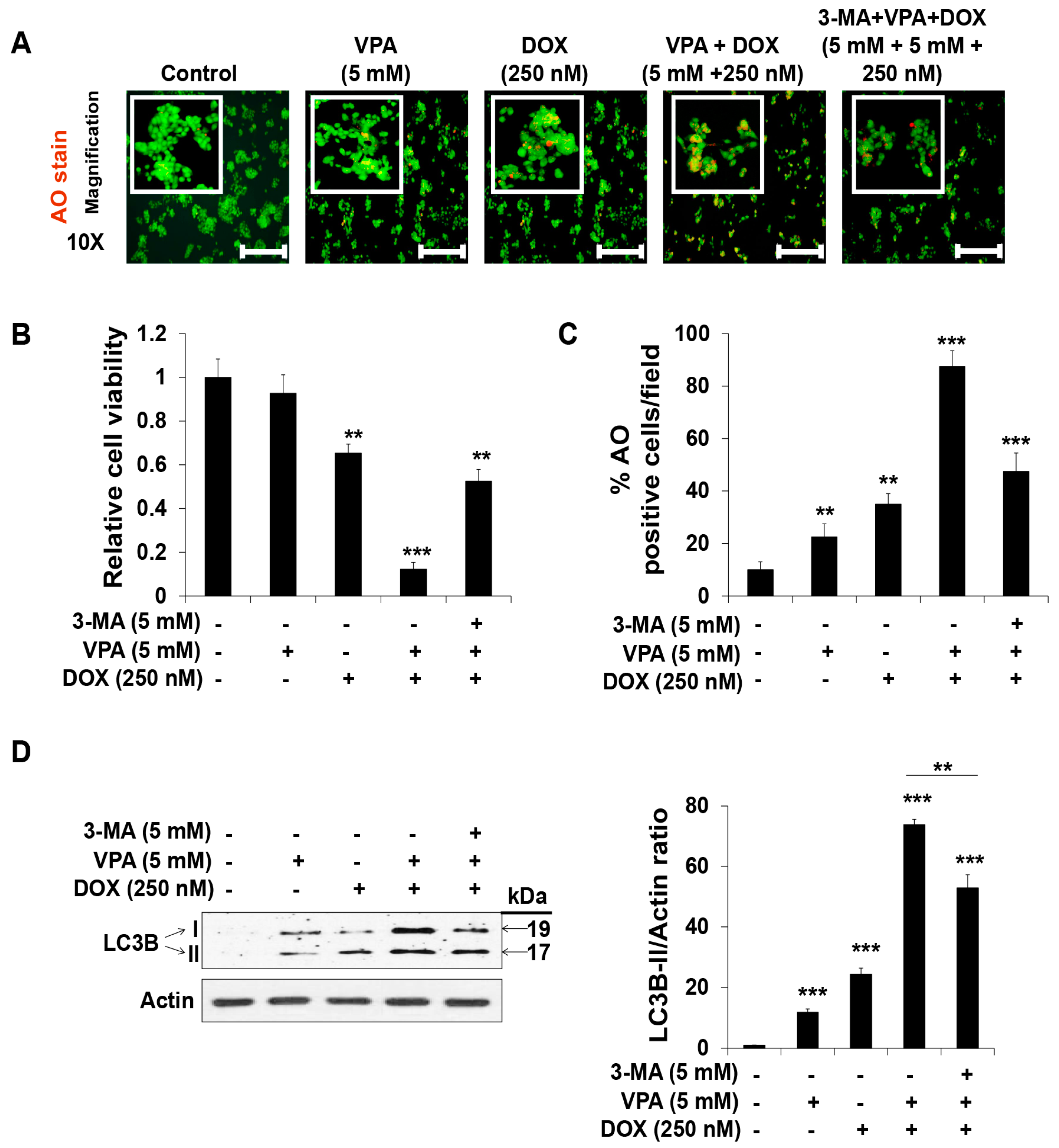
2.3. Combination Treatment of VPA and DOX Synergistically Induces ROS and Autophagy Generation in HepG2 Cells
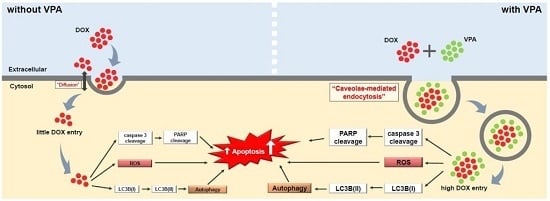
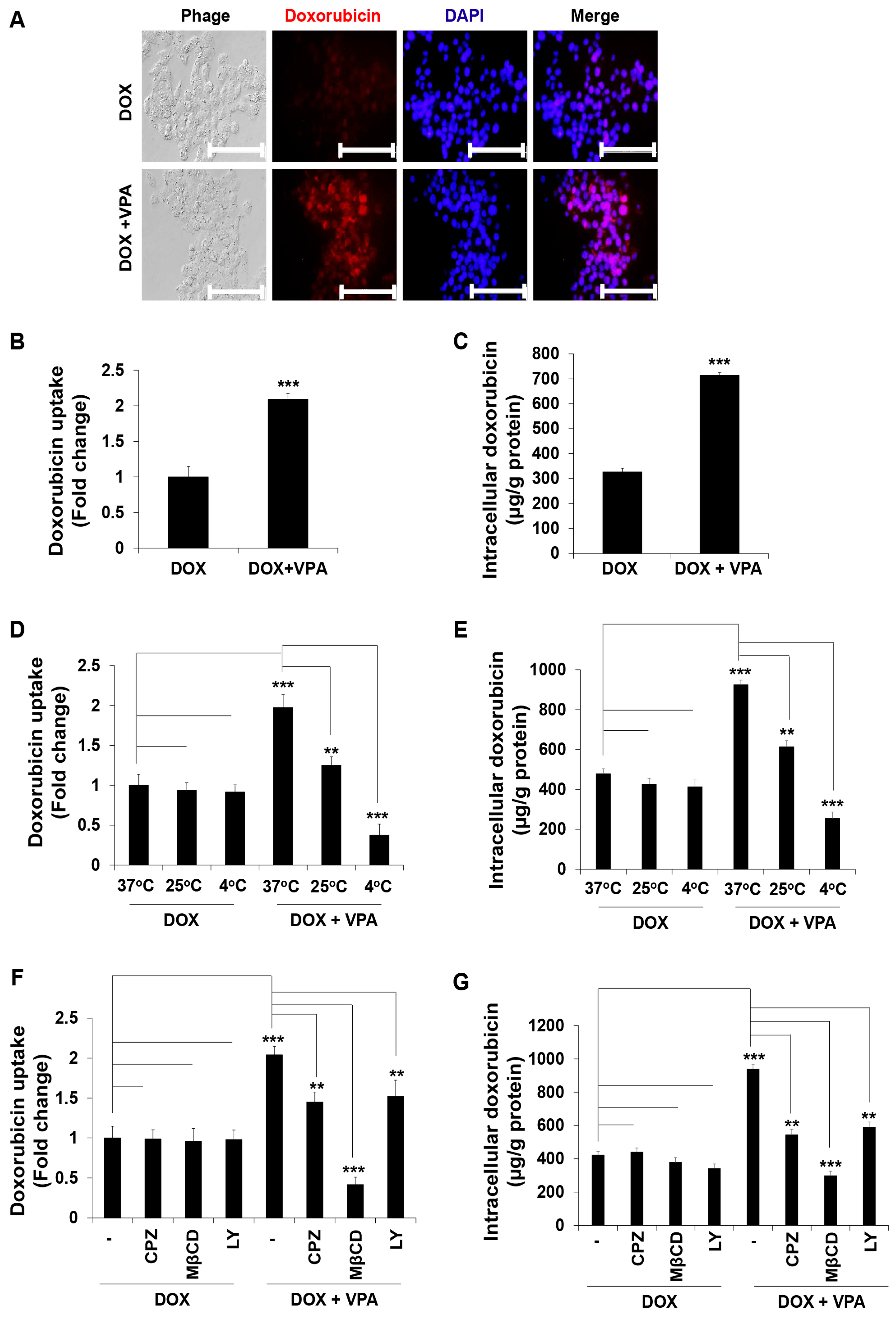
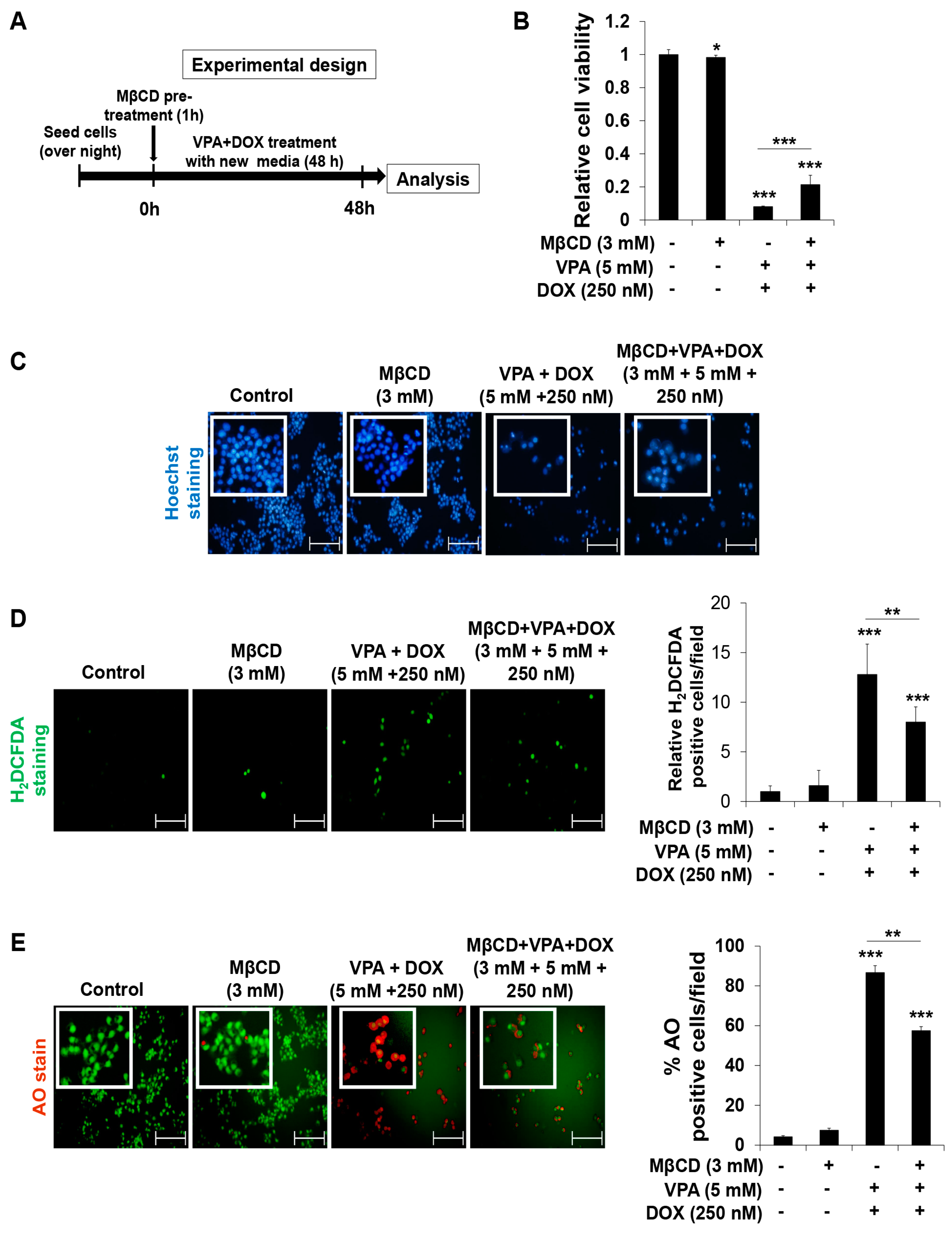
2.4. VPA Induces Internalization of DOX in HepG2 Cells through Caveolae-Mediated Endocytosis
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Cell Viability Assay
4.3. Histone Deacetylase (HDAC) Activity Assay
4.4. Hoechst Staining for Apoptotic Cell Detection
4.5. Western Blot Analysis
4.6. Reactive Oxygen Species (ROS) Generation Analysis
4.7. AO Staining for Autophagy Detection
4.8. DOX Internalization Analysis
4.9. Determination of the Endocytosis Pathways
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| VPA | valproic acid |
| DOX | doxorubicin |
| HCC | hepatocellular carcinoma |
| CDI | coefficient of drug interaction |
| PARP | poly(ADP-ribose) polymerase |
| ROS | reactive oxygen species |
| TACE | transarterial chemoembolization |
| HDAC | histone deacetylase |
| ATCC | american type culture collection |
| RPMI | roswell park memorial institute |
| DMEM | Dulbecco’s modified eagle medium |
| FBS | fetal bovine serum |
| STR | short tandem repeat |
| NAC | N-acetylcysteine |
| 3-MA | 3-methyladenine |
| CPZ | chlorpromazine |
| MβCD | methyl-β-cyclodextrin |
| LY | LY294002 |
| CD | cyclodextrin |
| AO | acridine orange |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| PBS | phosphate-buffered saline |
| PMSF | phenylmethylsulfonyl fluoride |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| TBST | tris-buffered saline tween 20 |
| HRP | horse radish peroxidase |
| ECL | enhanced chemiluminescence |
| SD | standard deviation |
| ANOVA | analysis of variance |
| HDEI | histone deacetylase inhibitor |
| ATC | anaplastic thyroid carcinoma |
| GSH | glutathione |
References
- WHO. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- Attwa, M.H.; El-Etreby, S.A. Guide for diagnosis and treatment of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 1632–1651. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Watson, M.B.; O’Kane, S.L.; Drew, P.J.; Lind, M.J.; Cawkwell, L. The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Mol. Cancer Ther. 2006, 5, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Ali, N.; Nafees, S.; Ahmad, S.T.; Arjumand, W.; Hasan, S.K.; Sultana, S. Alleviation of doxorubicin-induced nephrotoxicity and hepatotoxicity by chrysin in Wistar rats. Toxicol. Mech. Methods 2013, 23, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Mishra, S.; Suman, S.; Shukla, Y. Resveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: A mechanistic insight. Phytomedicine 2016, 23, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Phan, H.; Yang, L.X. Improved chemotherapy for hepatocellular carcinoma. Anticancer Res. 2012, 32, 1379–1386. [Google Scholar] [PubMed]
- Yeo, W.; Mok, T.S.; Zee, B.; Leung, T.W.; Lai, P.B.; Lau, W.Y.; Koh, J.; Mo, F.K.; Yu, S.C.; Chan, A.T.; et al. A randomized phase III study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J. Natl. Cancer Inst. 2005, 97, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.L.; Song, B.; Sun, G.P.; Ma, T.; Zhong, F.; Wei, W. Endoplasmic reticulum stress-induced resistance to doxorubicin is reversed by paeonol treatment in human hepatocellular carcinoma cells. PLoS ONE 2013, 8, e62627. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.F.; Zhang, D.M.; Wang, J.N.; Zhang, H.W.; Zheng, Z.Y.; Yu, D.C.; Li, Y.J.; Xu, J.; Chen, Y.J.; Shang, C.Z. Cabozantinib reverses multidrug resistance of human hepatoma HepG2/adr cells by modulating the function of P-glycoprotein. Liver Int. 2015, 35, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wan, B.; Liu, X.M.; Li, R.W.; Wang, Y.L.; Yu, L. MK5 is degraded in response to doxorubicin and negatively regulates doxorubicin-induced apoptosis in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2012, 427, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.U.; Yoon, J.H.; Lee, Y.J.; Lee, J.H.; Kim, B.H.; Yu, S.J.; Myung, S.J.; Kim, Y.J.; Lee, H.S. Hypoxia and retinoic acid-inducible NDRG1 expression is responsible for doxorubicin and retinoic acid resistance in hepatocellular carcinoma cells. Cancer Lett. 2010, 298, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Thakur, J.S.; Chauhan, C.G.; Diwana, V.K.; Chauhan, D.C.; Thakur, A. Extravasational side effects of cytotoxic drugs: A preventable catastrophe. Indian J. Plast. Surg. 2008, 41, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E. Pharmacological and therapeutic properties of valproate: A summary after 35 years of clinical experience. CNS Drugs 2002, 16, 695–714. [Google Scholar] [CrossRef] [PubMed]
- Carriere, C.H.; Kang, N.H.; Niles, L.P. Neuroprotection by valproic acid in an intrastriatal rotenone model of Parkinson’s disease. Neuroscience 2014, 267, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.P.; Xie, J.W.; Wang, C.Y.; Wang, T.; Wang, X.; Wang, S.L.; Teng, W.P.; Wang, Z.Y. Valproate reduces tau phosphorylation via cyclin-dependent kinase 5 and glycogen synthase kinase 3 signaling pathways. Brain Res. Bull. 2011, 85, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Penas, C.; Verdu, E.; Asensio-Pinilla, E.; Guzman-Lenis, M.S.; Herrando-Grabulosa, M.; Navarro, X.; Casas, C. Valproate reduces CHOP levels and preserves oligodendrocytes and axons after spinal cord injury. Neuroscience 2011, 178, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Zhang, L.; Tang, B.; Han, W.; Zhou, Y.; Chen, Z.; Jia, D.; Jiang, H. Sodium valproate alleviates neurodegeneration in SCA3/MJD via suppressing apoptosis and rescuing the hypoacetylation levels of histone H3 and H4. PLoS ONE 2013, 8, e54792. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.M.; Freudenmann, R.W.; Keller, F.; Connemann, B.J.; Hiemke, C.; Gahr, M.; Kratzer, W.; Fuchs, M.; Schonfeldt-Lecuona, C. Non-fatal and fatal liver failure associated with valproic acid. Pharmacopsychiatry 2013, 46, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; Aires, C.C.; Luis, P.B.; Ruiter, J.P.; IJlst, L.; Duran, M.; Wanders, R.J.; Tavares de Almeida, I. Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation: A review. J. Inherit. Metab. Dis. 2008, 31, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Nie, D.; Huang, K.; Yin, S.; Li, Y.; Xie, S.; Ma, L.; Wang, X.; Wu, Y.; Xiao, J. Synergistic/additive interaction of valproic acid with bortezomib on proliferation and apoptosis of acute myeloid leukemia cells. Leuk. Lymphoma 2012, 53, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Sidana, A.; Wang, M.; Shabbeer, S.; Chowdhury, W.H.; Netto, G.; Lupold, S.E.; Carducci, M.; Rodriguez, R. Mechanism of growth inhibition of prostate cancer xenografts by valproic acid. J. Biomed. Biotechnol. 2012, 2012, 180363. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Edwards, H.; Lograsso, S.B.; Buck, S.A.; Matherly, L.H.; Taub, J.W.; Ge, Y. Valproic acid synergistically enhances the cytotoxicity of clofarabine in pediatric acute myeloid leukemia cells. Pediatr. Blood Cancer 2012, 59, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Izumi, Y.; Asakura, K.; Fukutomi, T.; Serizawa, A.; Kawai, K.; Wakui, M.; Suematsu, M.; Nomori, H. Lovastatin and valproic acid additively attenuate cell invasion in ACC-MESO-1 cells. Biochem. Biophys. Res. Commun. 2011, 410, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, Y.; He, H.; Lou, L.; Ye, W.; Chen, Y.; Wang, J. Synergistically killing activity of aspirin and histone deacetylase inhibitor valproic acid (VPA) on hepatocellular cancer cells. Biochem. Biophys. Res. Commun. 2013, 436, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.C.; Bellodi-Privato, M.; Kubrusly, M.S.; Molan, N.A.; Tharcisio, T., Jr.; de Oliveira, E.R.; D’Albuquerque, L.A. Valproic acid inhibits human hepatocellular cancer cells growth in vitro and in vivo. J. Exp. Ther. Oncol. 2011, 9, 85–92. [Google Scholar] [PubMed]
- Tatebe, H.; Shimizu, M.; Shirakami, Y.; Sakai, H.; Yasuda, Y.; Tsurumi, H.; Moriwaki, H. Acyclic retinoid synergises with valproic acid to inhibit growth in human hepatocellular carcinoma cells. Cancer Lett. 2009, 285, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Gottlicher, M.; Minucci, S.; Zhu, P.; Kramer, O.H.; Schimpf, A.; Giavara, S.; Sleeman, J.P.; Lo Coco, F.; Nervi, C.; Pelicci, P.G.; et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001, 20, 6969–6978. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, M.; Kuno, T.; Kita, A.; Katsura, K.; Takegawa, K.; Uno, S.; Nabata, T.; Sugiura, R. Valproic acid affects membrane trafficking and cell-wall integrity in fission yeast. Genetics 2007, 175, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Muller-Taubenberger, A.; Adley, K.E.; Pawolleck, N.; Lee, V.W.Y.; Wiedemann, C.; Sihra, T.S.; Maniak, M.; Jin, T.; Williams, R.S.B. Attenuation of phospholipid signaling provides a novel mechanism for the action of valproic acid. Eukaryot. Cell 2007, 6, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Elphick, L.M.; Pawolleck, N.; Guschina, I.A.; Chaieb, L.; Eikel, D.; Nau, H.; Harwood, J.L.; Plant, N.J.; Williams, R.S.B. Conserved valproic-acid-induced lipid droplet formation in Dictyostelium and human hepatocytes identifies structurally active compounds. Dis. Model. Mech. 2012, 5, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Cowles, C.R.; Emr, S.D.; Horazdovsky, B.F. Mutations in the vps45 gene, a Sec1 homologue, result in vacuolar protein sorting defects and accumulation of membrane vesicles. J. Cell Sci. 1994, 107, 3449–3459. [Google Scholar] [PubMed]
- Bryant, N.J.; Piper, R.C.; Gerrard, S.R.; Stevens, T.H. Traffic into the prevacuolar/endosomal compartment of Saccharomyces cerevisiae: A vps45-dependent intracellular route and a vps45-independent, endocytic route. Eur. J. Cell Biol. 1998, 76, 43–52. [Google Scholar] [CrossRef]
- Selkoe, D.J. Showing transmitters the door: Synucleins accelerate vesicle release. Nat. Neurosci. 2017, 20, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Schreij, A.M.A.; Fon, E.A.; McPherson, P.S. Endocytic membrane trafficking and neurodegenerative disease. Cell Mol. Life Sci. 2016, 73, 1529–1545. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Garrity, A.G.; Xu, H. Regulation of membrane trafficking by signalling on endosomal and lysosomal membranes. J. Pphysiol. 2013, 591, 4389–4401. [Google Scholar] [CrossRef] [PubMed]
- Mazzolini, J.; Weber, R.J.; Chen, H.-S.; Khan, A.; Guggenheim, E.; Shaw, R.K.; Chipman, J.K.; Viant, M.R.; Rappoport, J.Z. Protein corona modulates uptake and toxicity of nanoceria via clathrin-mediated endocytosis. Biol. Bull. 2016, 231, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Maes, H.; Olmeda, D.; Soengas, M.S.; Agostinis, P. Vesicular trafficking mechanisms in endothelial cells as modulators of the tumor vasculature and targets of antiangiogenic therapies. FEBS J. 2016, 283, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, X.; Lv, Y.; Xin, X.; Qin, C.; Han, X.; Yang, L.; He, W.; Yin, L. Cytosolic co-delivery of miRNA-34a and docetaxel with core-shell nanocarriers via caveolae-mediated pathway for the treatment of metastatic breast cancer. Sci. Rep. 2017, 7, 46186. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Yang, P.-H.; Kao, Y.-C.; Chen, P.-Y.; Chung, C.-L.; Wang, S.-W. Pentabromophenol suppresses TGF-β signaling by accelerating degradation of type II TGF-β receptors via caveolae-mediated endocytosis. Sci. Rep. 2017, 7, 43206. [Google Scholar] [CrossRef] [PubMed]
- Du, J.Z.; Du, X.J.; Mao, C.Q.; Wang, J. Tailor-made dual pH-sensitive polymer-doxorubicin nanoparticles for efficient anticancer drug delivery. J. Am. Chem. Soc. 2011, 133, 17560–17563. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.C.; Dou, S.; Xiong, M.H.; Sun, T.M.; Wang, J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano 2011, 5, 3679–3692. [Google Scholar] [CrossRef] [PubMed]
- Thao, L.Q.; Lee, C.; Kim, B.; Lee, S.; Kim, T.H.; Kim, J.O.; Lee, E.S.; Oh, K.T.; Choi, H.G.; Yoo, S.D.; Youn, Y.S.; et al. Doxorubicin and paclitaxel co-bound lactosylated albumin nanoparticles having targetability to hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2017, 152, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Herzog, A.G.; Farina, E.L.; Blum, A.S. Serum valproate levels with oral contraceptive use. Epilepsia 2005, 46, 970–971. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.Q.; Li, Q.; Xu, S.P.; Shen, Y.X.; Sun, G.Y. Effect of lumiracoxib on proliferation and apoptosis of human nonsmall cell lung cancer cells in vitro. Chin. Med. J. 2008, 121, 602–607. [Google Scholar] [PubMed]
- Zhang, J.; Yi, M.; Zha, L.; Chen, S.; Li, Z.; Li, C.; Gong, M.; Deng, H.; Chu, X.; Chen, J.; et al. Sodium butyrate induces endoplasmic reticulum stress and autophagy in colorectal cells: Implications for apoptosis. PLoS ONE 2016, 11, e0147218. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Alhowyan, A.A.; Yang, Q.; Forrest, W.C.; Shnayder, Y.; Forrest, M.L. Cellular uptake and internalization of hyaluronan-based doxorubicin and cisplatin conjugates. J. Drug Target. 2014, 22, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Tejo, B.A.; Badawi, A.H.; Moore, D.; Krise, J.P.; Siahaan, T.J. Effect of modification of the physicochemical properties of ICAM-1-derived peptides on internalization and intracellular distribution in the human leukemic cell line HL-60. Mol. Pharm. 2009, 6, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.; McMahon, H.T. The structural era of endocytosis. Science 1999, 285, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ghosh, R.N.; Maxfield, F.R. Endocytosis. Physiol. Rev. 1997, 77, 759–803. [Google Scholar] [PubMed]
- Hao, X.; Wu, J.; Shan, Y.; Cai, M.; Shang, X.; Jiang, J.; Wang, H. Caveolae-mediated endocytosis of biocompatible gold nanoparticles in living Hela cells. J. Phys. Condens. Matter 2012, 24, 164207. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Raoul, J.L.; Sherman, M.; Mazzaferro, V.; Bolondi, L.; Craxi, A.; Galle, P.R.; Santoro, A.; Beaugrand, M.; Sangiovanni, A.; et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: Subanalyses of a phase III trial. J. Hepatol. 2012, 57, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.G.; Fortunati, N.; Pugliese, M.; Poli, R.; Bosco, O.; Mastrocola, R.; Aragno, M.; Boccuzzi, G. Valproic acid, a histone deacetylase inhibitor, enhances sensitivity to doxorubicin in anaplastic thyroid cancer cells. J. Endocrinol. 2006, 191, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yoo, Y.H.; Kang, D.Y.; Suh, H.; Park, M.K.; Park, K.J.; Kim, S.H. Efficacy on anaplastic thyroid carcinoma of valproic acid alone or in combination with doxorubicin, a synthetic chenodeoxycholic acid derivative, or lactacystin. Int. J. Oncol. 2009, 34, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.H.; Kang, D.Y.; Park, K.J.; Choi, H.J.; Lee, H.S.; Yee, S.B.; Yoo, Y.H. Doxorubicin induces apoptosis with profile of large-scale DNA fragmentation and without DNA ladder in anaplastic thyroid carcinoma cells via histone hyperacetylation. Int. J. Oncol. 2005, 27, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.S.; Yu, S.J.; Yoon, J.H.; Lee, S.H.; Lee, S.M.; Lee, J.H.; Kim, Y.J.; Lee, H.S.; Kim, C.Y. Synergistic anti-tumor efficacy of doxorubicin and flavopiridol in an in vivo hepatocellular carcinoma model. J. Cancer Res. Clin. Oncol. 2015, 141, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jing, Y.; Ouyang, S.; Liu, B.; Zhu, T.; Niu, H.; Tian, Y. Inhibitory effect of valproic acid on bladder cancer in combination with chemotherapeutic agents in vitro and in vivo. Oncol. Lett. 2013, 6, 1492–1498. [Google Scholar] [PubMed]
- Eot-Houllier, G.; Fulcrand, G.; Magnaghi-Jaulin, L.; Jaulin, C. Histone deacetylase inhibitors and genomic instability. Cancer Lett. 2009, 274, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Han, B.R.; You, B.R.; Park, W.H. Valproic acid inhibits the growth of Hela cervical cancer cells via caspase-dependent apoptosis. Oncol. Rep. 2013, 30, 2999–3005. [Google Scholar] [PubMed]
- Ji, M.M.; Wang, L.; Zhan, Q.; Xue, W.; Zhao, Y.; Zhao, X.; Xu, P.P.; Shen, Y.; Liu, H.; Janin, A.; et al. Induction of autophagy by valproic acid enhanced lymphoma cell chemosensitivity through HDAC-independent and IP3-mediated PRKAA activation. Autophagy 2015, 11, 2160–2171. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, S.H.; Kim, H.; Ji, S.T.; Jang, W.B.; Kim, J.H.; Baek, S.H.; Kwon, S.M. Doxorubicin regulates autophagy signals via accumulation of cytosolic Ca2+ in human cardiac progenitor cells. Int. J. Mol. Sci. 2016, 17, 1680. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, J.S.; Sowa, Y.; Xu, W.S.; Shao, Y.; Dokmanovic, M.; Perez, G.; Ngo, L.; Holmgren, A.; Jiang, X.; Marks, P.A. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc. Natl. Acad. Sci. USA 2005, 102, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zheng, Y.; Jiang, W.; Huang, Z.; Wang, M.; Rodriguez, R.; Jin, X. Valproic acid induces autophagy by suppressing the Akt/mTOR pathway in human prostate cancer cells. Oncol. Lett. 2016, 12, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Paglin, S.; Hollister, T.; Delohery, T.; Hackett, N.; McMahill, M.; Sphicas, E.; Domingo, D.; Yahalom, J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001, 61, 439–444. [Google Scholar] [PubMed]
- Graziani, G.; Tentori, L.; Portarena, I.; Vergati, M.; Navarra, P. Valproic acid increases the stimulatory effect of estrogens on proliferation of human endometrial adenocarcinoma cells. Endocrinology 2003, 144, 2822–2828. [Google Scholar] [CrossRef] [PubMed]
- Takai, N.; Desmond, J.C.; Kumagai, T.; Gui, D.; Said, J.W.; Whittaker, S.; Miyakawa, I.; Koeffler, H.P. Histone deacetylase inhibitors have a profound antigrowth activity in endometrial cancer cells. Clin. Cancer Res. 2004, 10, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.G.; Pugliese, M.; Gallo, M.; Brignardello, E.; Milla, P.; Orlandi, F.; Limone, P.P.; Arvat, E.; Boccuzzi, G.; Piovesan, A. Valproic acid, a histone deacetylase inhibitor, in combination with paclitaxel for anaplastic thyroid cancer: Results of a multicenter randomized controlled phase II/III trial. Int. J. Endocrinol. 2016, 2016, 2930414. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinska, P.; Tauboll, E.; Grzyb, E.; Fiedor, E.; Ptak, A.; Gregoraszczuk, E.L. Valproic acid as a promising co-treatment with paclitaxel and doxorubicin in different ovarian carcinoma cell lines. Int. J. Gynecol. Cancer 2016, 26, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Tarasenko, N.; Cutts, S.M.; Phillips, D.R.; Berkovitch-Luria, G.; Bardugo-Nissim, E.; Weitman, M.; Nudelman, A.; Rephaeli, A. A novel valproic acid prodrug as an anticancer agent that enhances doxorubicin anticancer activity and protects normal cells against its toxicity in vitro and in vivo. Biochem. Pharmacol. 2014, 88, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Ververis, K.; Rodd, A.L.; Tang, M.M.; El-Osta, A.; Karagiannis, T.C. Histone deacetylase inhibitors augment doxorubicin-induced DNA damage in cardiomyocytes. Cell Mol. Life Sci. 2011, 68, 4101–4114. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Li, K.; Liao, K. Macropinocytosis contributes to the macrophage foam cell formation in RAW264.7 cells. Acta Biochim. Biophys. Sin. 2009, 41, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Rauniyar, N.; Subramanian, K.; Lavallée-Adam, M.; Martínez-Bartolomé, S.; Balch, W.E.; Yates, J.R. Quantitative proteomics of human fibroblasts with I1061T mutation in Niemann-Pick C1 (NPC1) protein provides insights into the disease pathogenesis. Mol. Cell. Proteom. 2015, 14, 1734–1749. [Google Scholar] [CrossRef] [PubMed]
- Rodal, S.K.; Skretting, G.; Garred, O.; Vilhardt, F.; van Deurs, B.; Sandvig, K. Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 1999, 10, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, H.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Genetic variations and treatments that affect the lifespan of the NPC1 mouse. J. Lipid Res. 2008, 49, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Kim, B.; Gurunathan, S.; Choi, H.Y.; Yang, G.; Saha, S.K.; Han, D.; Han, J.; Kim, K.; Kim, J.H.; et al. Biologically synthesized silver nanoparticles induce neuronal differentiation of SH-SY5Y cells via modulation of reactive oxygen species, phosphatases, and kinase signaling pathways. Biotechnol. J. 2014, 9, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Yasuo, M.; Bogaard, H.J.; Kraskauskas, D.; Natarajan, R.; Voelkel, N.F. Inhibition of histone deacetylase causes emphysema. Am. J. Physiol. Lung C 2011, 300, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Pitchaimani, A.; Renganathan, A.; Cinthaikinian, S.; Premkumar, K. Photochemotherapeutic effects of UV-C on acridine orange in human breast cancer cells: Potential application in anticancer therapy. RSC Adv. 2014, 4, 22123–22128. [Google Scholar] [CrossRef]
- Saha, S.K.; Choi, H.Y.; Kim, B.W.; Dayem, A.A.; Yang, G.M.; Kim, K.S.; Yin, Y.F.; Cho, S.G. KRT19 directly interacts with β-catenin/RAC1 complex to regulate NUMB-dependent NOTCH signaling pathway and breast cancer properties. Oncogene 2017, 36, 332–349. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.R.; Gupton, J.T.; Kellogg, G.E.; Yeudall, W.A.; Cabot, M.C.; Newsham, I.F.; Gewirtz, D.A. Autophagic cell death, polyploidy and senescence induced in breast tumor cells by the substituted pyrrole JG-03-14, a novel microtubule poison. Biochem. Pharmacol. 2007, 74, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sun, W.; Qian, C.; Wang, C.; Bomba, H.N.; Gu, Z. Anticancer platelet-mimicking nanovehicles. Adv. Mater. 2015, 27, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
| Order | Doxorubicin (nM) | Valproic Acid (mM) | CDI |
|---|---|---|---|
| 1 | 10 | 5 | 0.86 |
| 2 | 50 | 5 | 0.84 |
| 3 | 100 | 5 | 0.77 |
| 4 | 250 | 5 | 0.16 |
| 5 | 500 | 5 | 0.29 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, S.K.; Yin, Y.; Kim, K.; Yang, G.-M.; Dayem, A.A.; Choi, H.Y.; Cho, S.-G. Valproic Acid Induces Endocytosis-Mediated Doxorubicin Internalization and Shows Synergistic Cytotoxic Effects in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2017, 18, 1048. https://doi.org/10.3390/ijms18051048
Saha SK, Yin Y, Kim K, Yang G-M, Dayem AA, Choi HY, Cho S-G. Valproic Acid Induces Endocytosis-Mediated Doxorubicin Internalization and Shows Synergistic Cytotoxic Effects in Hepatocellular Carcinoma Cells. International Journal of Molecular Sciences. 2017; 18(5):1048. https://doi.org/10.3390/ijms18051048
Chicago/Turabian StyleSaha, Subbroto Kumar, Yingfu Yin, Kyeongseok Kim, Gwang-Mo Yang, Ahmed Abdal Dayem, Hye Yeon Choi, and Ssang-Goo Cho. 2017. "Valproic Acid Induces Endocytosis-Mediated Doxorubicin Internalization and Shows Synergistic Cytotoxic Effects in Hepatocellular Carcinoma Cells" International Journal of Molecular Sciences 18, no. 5: 1048. https://doi.org/10.3390/ijms18051048