NGF and Its Receptors in the Regulation of Inflammatory Response
Abstract
:1. Introduction
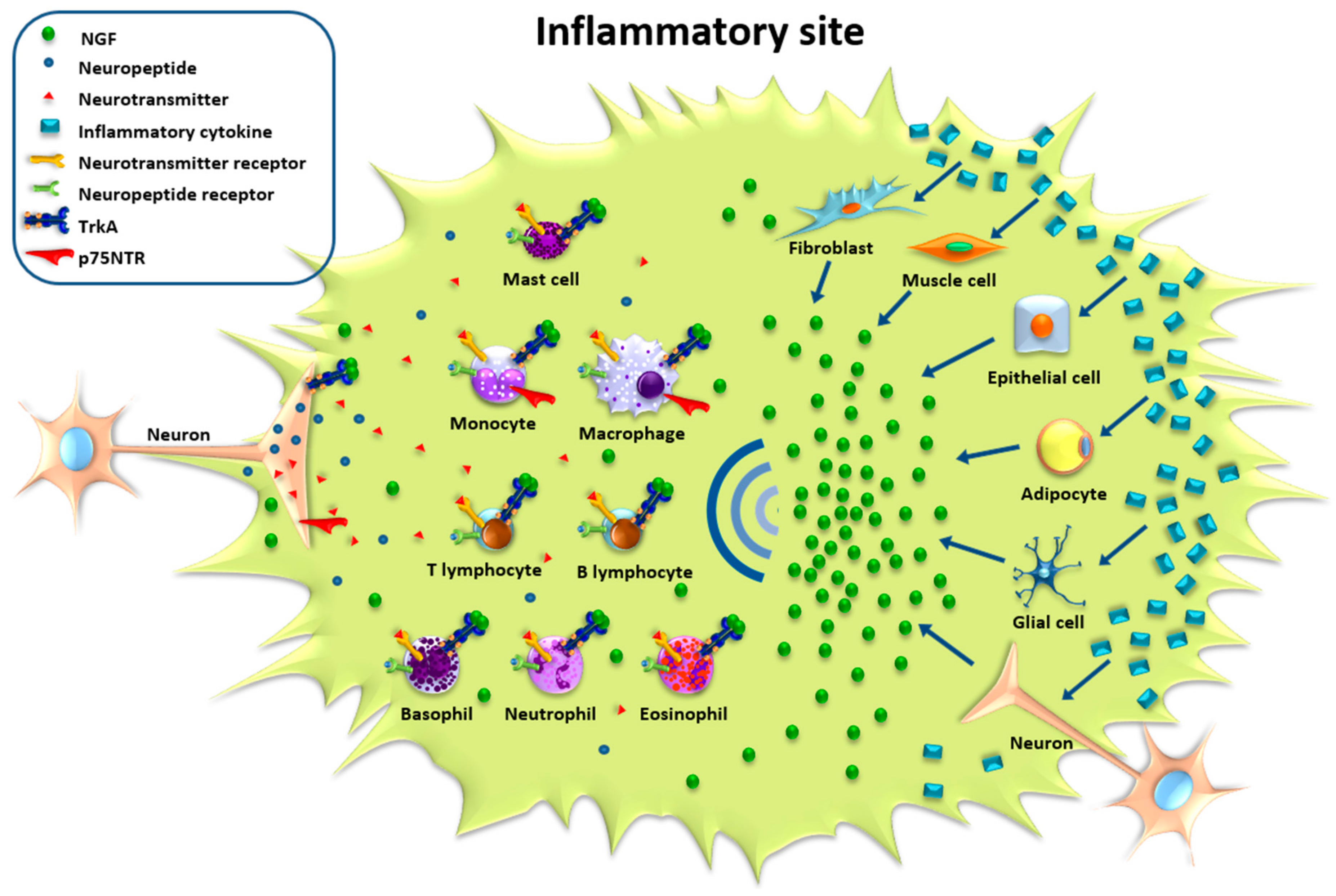
2. NGF Is Enhanced during the Inflammatory Response
3. Expression of NGF Receptors in the Immune System
3.1. Expression of NGF Receptors in Primary and Secondary Lymphoid Organs
3.2. Expression of NGF Receptors in Immune Cells
4. NGF and Its Direct and Indirect Effects on Immune Response
4.1. Indirect Action
4.2. Direct Action
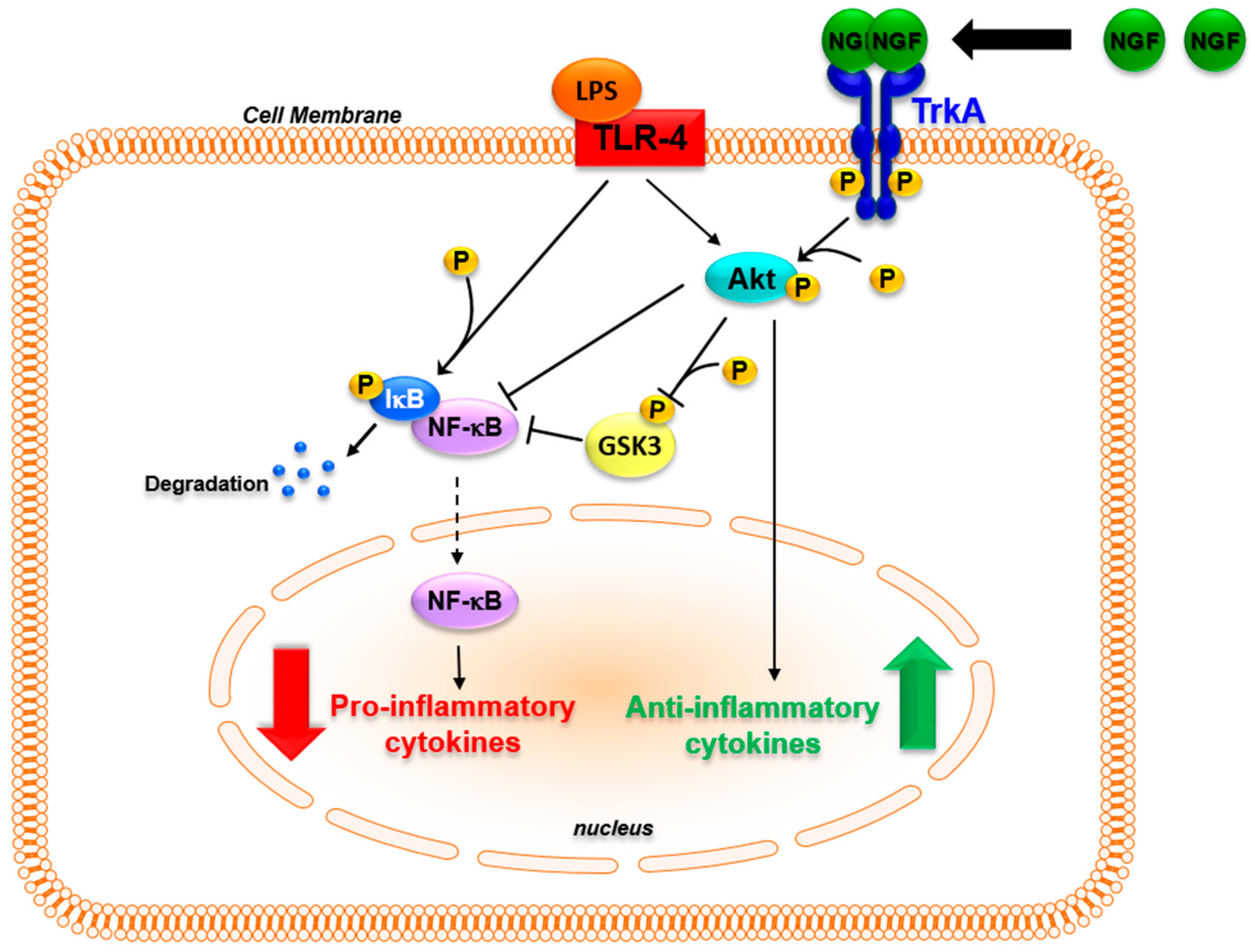
5. In Vivo Inflammatory and Anti-Inflammatory Mechanisms and the Roles of NGF and TrkA
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Levi-Montalcini, R. The nerve growth factor 35 years later. Science 1987, 237, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.; Ahn, S.; Davenport, C.M.; Blendy, J.A.; Ginty, D.D. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 1999, 286, 2358–2361. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.W.; Ginty, D.D. Long-distance retrograde neurotrophic factor signalling in neurons. Nat. Rev. Neurosci. 2013, 14, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R.; Aloe, L.; Mugnaini, E.; Oesch, F.; Thoenen, H. Nerve growth factor induces volume increase and enhances tyrosine hydroxylase synthesis in chemically axotomized sympathetic ganglia of newborn rats. Proc. Natl. Acad. Sci. USA 1975, 72, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.M.; Harmar, A.J. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature 1989, 337, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Leslie, T.A.; Emson, P.C.; Dowd, P.M.; Woolf, C.J. Nerve growth factor contributes to the up-regulation of growth-associated protein 43 and preprotachykinin A messenger RNAs in primary sensory neurons following peripheral inflammation. Neuroscience 1995, 67, 753–761. [Google Scholar] [CrossRef]
- Zigmond, R.E.; Sun, Y. Regulation of neuropeptide expression in sympathetic neurons. Paracrine and retrograde influences. Ann. N. Y. Acad. Sci. 1997, 814, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Korsching, S.; Thoenen, H. Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat: Correlation with density of sympathetic innervation. Proc. Natl. Acad. Sci. USA 1983, 80, 3513–3516. [Google Scholar] [CrossRef] [PubMed]
- Thrasivoulou, C.; Cowen, T. Regulation of rat sympathetic nerve density by target tissues and NGF in maturity and old age. Eur. J. Neurosci. 1995, 7, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, J.; McNaughton, P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005, 24, 4211–4223. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, L.G.; White, G. Effects of nerve growth factor on TTX- and capsaicin-sensitivity in adult rat sensory neurons. Brain Res. 1992, 570, 61–66. [Google Scholar] [CrossRef]
- Gould, H.J.; Gould, T.N.; England, J.D.; Paul, D.; Liu, Z.P.; Levinson, S.R. A possible role for nerve growth factor in the augmentation of sodium channels in models of chronic pain. Brain Res. 2000, 854, 19–29. [Google Scholar] [CrossRef]
- Verge, V.M.; Richardson, P.M.; Wiesenfeld-Hallin, Z.; Hökfelt, T. Differential influence of nerve growth factor on neuropeptide expression in vivo: A novel role in peptide suppression in adult sensory neurons. J. Neurosci. 1995, 15, 2081–2096. [Google Scholar] [PubMed]
- Donnerer, J.; Schuligoi, R.; Stein, C. Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: Evidence for a regulatory function of nerve growth factor in vivo. Neuroscience 1992, 49, 693–698. [Google Scholar] [CrossRef]
- Bennett, D.L.; Koltzenburg, M.; Priestley, J.V.; Shelton, D.L.; McMahon, S.B. Endogenous nerve growth factor regulates the sensitivity of nociceptors in the adult rat. Eur. J. Neurosci. 1998, 10, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Spillane, M.; Ketschek, A.; Donnelly, C.J.; Pacheco, A.; Twiss, J.L.; Gallo, G. Nerve growth factor-induced formation of axonal filopodia and collateral branches involves the intra-axonal synthesis of regulators of the actin-nucleating ARP2/3 complex. J. Neurosci. 2012, 32, 17671–17689. [Google Scholar] [CrossRef] [PubMed]
- Albers, K.M.; Wright, D.E.; Davis, B.M. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. J. Neurosci. 1994, 14, 1422–1432. [Google Scholar] [PubMed]
- Takami, S.; Getchell, M.L.; Yamagishi, M.; Albers, K.M.; Getchell, T.V. Enhanced extrinsic innervation of nasal and oral chemosensory mucosae in keratin 14-NGF transgenic mice. Cell Tissue Res. 1995, 282, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Goodness, T.P.; Albers, K.M.; Davis, F.E.; Davis, B.M. Overexpression of nerve growth factor in skin increases sensory neuron size and modulates Trk receptor expression. Eur. J. Neurosci. 1997, 9, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Schnegelsberg, B.; Sun, T.T.; Cain, G.; Bhattacharya, A.; Nunn, P.A.; Ford, A.P.; Vizzard, M.A.; Cockayne, D.A. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R534–R547. [Google Scholar] [CrossRef] [PubMed]
- Bracci-Laudiero, L.; Aloe, L.; Levi-Montalcini, R.; Buttinelli, C.; Schilter, D.; Gillessen, S.; Otten, U. Multiple sclerosis patients express increased levels of B-nerve growth factor in cerebrospinal fluid. Neurosci. Lett. 1992, 147, 9–12. [Google Scholar] [CrossRef]
- Aloe, L.; Tuveri, M.A.; Carcassi, U.; Levi-Montalcini, R. Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis Rheumatol. 1992, 35, 351–355. [Google Scholar] [CrossRef]
- Falcini, F.; Matucci Cerinic, M.; Lombardi, A.; Generini, S.; Pignone, A.; Tirassa, P.; Ermini, M.; Lepore, L.; Partsch, G.; Aloe, L. Increased circulating nerve growth factor is directly correlated with disease activity in juvenile chronic arthritis. Ann. Rheum. Dis. 1996, 55, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Bracci-Laudiero, L.; Aloe, L.; Levi-Montalcini, R.; Galeazzi, M.; Schilter, D.; Scully, J.L.; Otten, U. Increased levels of NGF in sera of systemic lupus erythematosus patients. Neuro. Report 1992, 4, 563–565. [Google Scholar]
- Aalto, K.; Korhonen, L.; Lahdenne, P.; Pelkonen, P.; Lindholm, D. Nerve growth factor in serum of children with systemic lupus erythematosus is correlated with disease activity. Cytokine 2002, 20, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Fauchais, A.L.; Boumediene, A.; Lalloue, F.; Gondran, G.; Loustaud-Ratti, V.; Vidal, E.; Jauberteau, M.O. Brain-derived neurotrophic factor and nerve growth factor correlate with T-cell activation in primary Sjogren’s syndrome. Scand. J. Rheumatol. 2009, 38, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Samad, T.A.; Jin, S.X.; Schmoll, R.; Woolf, C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002, 36, 57–68. [Google Scholar] [CrossRef]
- Mamet, J.; Lazdunski, M.; Voilley, N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J. Biol. Chem. 2003, 278, 48907–48913. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, J.R.; Freeman, K.T.; Jimenez-Andrade, J.M.; Coughlin, K.A.; Kaczmarska, M.J.; Castaneda-Corral, G.; Bloom, A.P.; Kuskowski, M.A.; Mantyh, P.W. Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful arthritic joint. Arthritis Rheumatol. 2012, 64, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Reinert, A.; Kaske, A.; Mense, S. Inflammation-induced increase in the density of neuropeptide-immunoreactive nerve endings in rat skeletal muscle. Exp. Brain Res. 1998, 121, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef] [PubMed]
- Kioussis, D.; Pachnis, V. Immune and nervous systems: More than just a superficial similarity? Immunity 2009, 31, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Madden, K.S.; Felten, D.L. Experimental basis for neuronal-immune interactions. Physiol. Rev. 1995, 75, 77–106. [Google Scholar] [PubMed]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The sympathetic nerve—An integrative interface between two supersystems: The brain and the immune system. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar] [PubMed]
- Aloe, L.; Probert, L.; Kollias, G.; Bracci-Laudiero, L.; Spillantini, M.G.; Levi-Montalcini, R. The synovium of transgenic arthritic mice expressing human tumor necrosis factor contains a high level of nerve growth factor. Growth Factors 1993, 9, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Manni, L.; Aloe, L. Role of IL-1β and TNF-α in the regulation of NGF in experimentally induced arthritis in mice. Rheumatol. Int. 1998, 18, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Bracci-Laudiero, L.; Lundeberg, T.; Stenfors, C.; Theodorsson, E.; Tirassa, P.; Aloe, L. Modification of lymphoid and brain nerve growth factor levels in systemic lupus erythematosus mice. Neurosci. Lett. 1996, 204, 13–16. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Smaldone, M.C.; Tyagi, V.; Philips, B.J.; Jackman, S.V.; Leng, W.W.; Tyagi, P. Increased nerve growth factor in neurogenic overactive bladder and interstitial cystitis patients. Can. J. Urol. 2010, 17, 4989–4994. [Google Scholar] [PubMed]
- Bonini, S.; Lambiase, A.; Bonini, S.; Angelucci, F.; Magrini, L.; Manni, L.; Aloe, L. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc. Natl. Acad. Sci. USA 1996, 93, 10955–10960. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Bonini, S.; Bonini, S.; Micera, A.; Magrini, L.; Bracci-Laudiero, L.; Aloe, L. Increased plasma levels of nerve growth factor in vernal keratoconjunctivitis and relationship to conjunctival mast cells. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2127–2132. [Google Scholar]
- Di Mola, F.F.; Friess, H.; Zhu, Z.W.; Koliopanos, A.; Bley, T.; di Sebastiano, P.; Innocenti, P.; Zimmermann, A.; Büchler, M.W. Nerve growth factor and Trk high affinity receptor (TrkA) gene expression in inflammatory bowel disease. Gut 2000, 46, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Stanzel, R.D.; Lourenssen, S.; Blennerhassett, M.G. Inflammation causes expression of NGF in epithelial cells of the rat colon. Exp. Neurol. 2008, 211, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Fantini, F.; Magnoni, C.; Bracci-Laudiero, L.; Pincelli, C.T.E. Nerve growth factor is increased in psoriatic skin. J. Investig. Dermatol. 1995, 105, 854–855. [Google Scholar] [PubMed]
- Raychaudhuri, S.P.; Jiang, W.Y.; Farber, E.M. Psoriatic keratinocytes express high levels of nerve growth factor. Acta Dermato. Venereol. 1998, 78, 84–86. [Google Scholar]
- Toyoda, M.; Nakamura, M.; Makino, T.; Hino, T.; Kagoura, M.; Morohashi, M. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br. J. Dermatol. 2002, 147, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Allchorne, A.; Safieh-Garabedian, B.; Poole, S. Cytokines, nerve growth factor and inflammatory hyperalgesia: The contribution of tumour necrosis factor α. Br. J. Pharmacol. 1997, 121, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Steiner, P.; Pfeilschifter, J.; Boeckh, C.; Radeke, H.; Otten, U. Interleukin-1β and tumor necrosis factor-α synergistically stimulate nerve growth factor synthesis in rat mesangial cells. Am. J. Physiol. 1991, 261, 792–798. [Google Scholar]
- März, P.; Heese, K.; Dimitriades-Schmutz, B.; Rose-John, S.; Otten, U. Role of interleukin-6 and soluble IL-6 receptor in region-specific induction of astrocytic differentiation and neurotrophin expression. Glia 1999, 26, 191–200. [Google Scholar] [CrossRef]
- Freund, V.; Pons, F.; Joly, V.; Mathieu, E.; Martinet, N.; Frossard, N. Upregulation of nerve growth factor expression by human airway smooth muscle cells in inflammatory conditions. Eur. Respir. J. 2002, 20, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Manni, L.; Lundeberg, T.; Fiorito, S.; Bonini, S.; Vigneti, E.; Aloe, L. Nerve growth factor release by human synovial fibroblasts prior to and following exposure to tumor necrosis factor-α, interleukin-1β and cholecystokinin-8: The possible role of NGF in the inflammatory response. Clin. Exp. Rheumatol. 2003, 21, 617–624. [Google Scholar] [PubMed]
- Wang, B.; Jenkins, J.R.; Trayhurn, P. Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: Integrated response to TNF-α. Am. J. Physiol. Endocrinol. Metab. 2005, 288, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Von Boyen, G.B.; Steinkamp, M.; Reinshagen, M.; Schäfer, K.H.; Adler, G.; Kirsch, J. Nerve growth factor secretion in cultured enteric glia cells is modulated by proinflammatory cytokines. J. Neuroendocrinol. 2006, 18, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Bulló, M.; Peeraully, M.R.; Trayhurn, P. Stimulation of NGF expression and secretion in 3T3-L1 adipocytes by prostaglandins PGD2, PGJ2, and δ12-PGJ2. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E62–E67. [Google Scholar] [CrossRef] [PubMed]
- Toyomoto, M.; Ohta, M.; Okumura, K.; Yano, H.; Matsumoto, K.; Inoue, S.; Hayashi, K.; Ikeda, K. Prostaglandins are powerful inducers of NGF and BDNF production in mouse astrocyte cultures. FEBS Lett. 2004, 562, 211–215. [Google Scholar] [CrossRef]
- Lipnik-Stangelj, M.; Carman-Krzan, M. Activation of histamine H1-receptor enhances neurotrophic factor secretion from cultured astrocytes. Inflamm. Res. 2004, 53, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Watanabe, S. Histamine enhances the production of nerve growth factor in human keratinocytes. J. Investig. Dermatol. 2003, 121, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Iannone, F.; de Bari, C.; Dell’Accio, F.; Covelli, M.; Patella, V.; Lo Bianco, G.; Lapadula, G. Increased expression of nerve growth factor (NGF) and high affinity NGF receptor (p140 TrkA) in human osteoarthritic chondrocytes. Rheumatology 2002, 41, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Rihl, M.; Kruithof, E.; Barthel, C.; de Keyser, F.; Veys, E.M.; Zeidler, H.; Yu, D.T.; Kuipers, J.G.; Baeten, D. Involvement of neurotrophins and their receptors in spondyloarthritis synovitis: Relation to inflammation and response to treatment. Ann. Rheum. Dis. 2005, 64, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Barthel, C.; Yeremenko, N.; Jacobs, R.; Schmidt, R.E.; Bernateck, M.; Zeidler, H.; Tak, P.P.; Baeten, D.; Rihl, M. Nerve growth factor and receptor expression in rheumatoid arthritis and spondyloarthritis. Arthritis Res. Ther. 2009, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Townley, S.L.; Grimbaldeston, M.A.; Ferguson, I.; Rush, R.A.; Zhang, S.H.; Zhou, X.F.; Conner, J.M.; Finlay-Jones, J.J.; Hart, P.H. Nerve growth factor, neuropeptides, and mast cells in ultraviolet-B-induced systemic suppression of contact hypersensitivity responses in mice. J. Investig. Dermatol. 2002, 118, 396–401. [Google Scholar] [CrossRef] [PubMed]
- García-Suárez, O.; Germanà, A.; Hannestad, J.; Ciriaco, E.; Laurà, R.; Naves, J.; Esteban, I.; Silos-Santiago, I.; Vega, J.A. TrkA is necessary for the normal development of the murine thymus. J. Neuroimmunol. 2000, 108, 11–21. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.; García-Suárez, O.; Esteban, I.; Germanà, A.; Fariñas, I.; Naves, F.J.; Vega, J.A. p75NTR in the spleen: Age-dependent changes, effect of NGF and 4-methylcatechol treatment, and structural changes in p75NTR-deficient mice. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 270, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Indo, Y.; Tsuruta, M.; Hayashida, Y.; Karim, M.A.; Ohta, K.; Kawano, T.; Mitsubuchi, H.; Tonoki, H.; Awaya, Y.; Matsuda, I. Mutations in the TrkA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat. Genet. 1996, 13, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Melamed, I.; Levy, J.; Parvari, R.; Gelfand, E.W. A novel lymphocyte signaling defect: Trk A mutation in the syndrome of congenital insensitivity to pain and anhidrosis (CIPA). J. Clin. Immunol. 2004, 24, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Tsuboi, Y.; Kurosawa, H.; Sugita, K.; Eguchi, M. Anti-apoptotic effect of nerve growth factor is lost in congenital insensitivity to pain with anhidrosis (CIPA) B lymphocytes. J. Clin. Immunol. 2004, 24, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Lempereur, L.; Anastasi, M.; Parano, E.; Pavone, P. Congenital insensitivity to pain with Anhidrosis (NTRK1 mutation) and early onset renal disease: Clinical report on three sibs with a 25-year follow-up in one of them. Neuropediatrics 2005, 36, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Hehlgans, T.; Pfeffer, K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: Players, rules and the games. Immunology 2005, 115, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M. Recent advances in understanding neurotrophin signaling. F1000 Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Deinhardt, K.; Chao, M.V. Trk receptors. Handb. Exp. Pharmacol. 2014, 220, 103–119. [Google Scholar] [PubMed]
- Shu, Y.H.; Lu, X.M.; Wei, J.X.; Xiao, L.; Wang, Y.T. Update on the role of p75NTR in neurological disorders: A novel therapeutic target. Biomed. Pharmacother. 2015, 76, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kurd, N.; Robey, E.A. T-cell selection in the thymus: A spatial and temporal perspective. Immunol. Rev. 2016, 271, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Johnson, E.M., Jr. An immunohistochemical study of the nerve growth factor receptor in developing rats. J. Neurosci. 1988, 8, 3481–3498. [Google Scholar] [PubMed]
- Parrens, M.; Labouyrie, E.; Groppi, A.; Dubus, P.; Carles, D.; Velly, J.F.; de Mascarel, A.; Merlio, J.P. Expression of NGF receptors in normal and pathological human thymus. J. Neuroimmunol. 1998, 85, 11–21. [Google Scholar] [CrossRef]
- Marinova, T.T.; Velikova, K.K.; Petrov, D.B.; Kutev, N.S.; Stankulov, I.S.; Chaldakov, G.N.; Triaca, V.; Manni, L.; Aloe, L. Structural and ultrastructural localization of NGF and NGF receptors in the thymus of subjects affected by myasthenia gravis. Autoimmunity 2004, 37, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Hannestad, J.; García-Suárez, O.; Huerta, J.J.; Esteban, I.; Naves, F.J.; Vega, J.A. TrkA neutrophin receptor protein in the rat and human thymus. Anat. Rec. 1997, 249, 373–379. [Google Scholar] [CrossRef]
- Dubus, P.; Parrens, M.; El-Mokhtari, Y.; Ferrer, J.; Groppi, A.; Merlio, J.P. Identification of novel TrkA variants with deletions in leucine-rich motifs of the extracellular domain. J. Neuroimmunol. 2000, 107, 42–49. [Google Scholar] [CrossRef]
- Laurenzi, M.A.; Barbany, G.; Timmusk, T.; Lindgren, J.A.; Persson, H. Expression of mRNA encoding neurotrophins and neurotrophin receptors in rat thymus, spleen tissue and immunocompetent cells. Regulation of neurotrophin-4 mRNA expression by mitogens and leukotriene B4. Eur. J. Biochem. 1994, 223, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Lomen-Hoerth, C.; Shooter, E.M. Widespread neurotrophin receptor expression in the immune system and other nonneuronal rat tissues. J. Neurochem. 1995, 64, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Labouyrie, E.; Parrens, M.; de Mascarel, A.; Bloch, B.; Merlio, J.P. Distribution of NGF receptors in normal and pathologic human lymphoid tissues. J. Neuroimmunol. 1997, 77, 161–173. [Google Scholar] [CrossRef]
- Garcia-Suárez, O.; Germanà, A.; Hannestad, J.; Ciriaco, E.; Silos-Santiago, I.; Germanà, G.; Vega, J.A. Involvement of the NGF receptors (TrkA and p75LNGFR) in the development and maintenance of the thymus. Ital. J. Anat. Embryol. 2001, 106, 279–285. [Google Scholar] [PubMed]
- Aloe, L.; Micera, A.; Bracci-Laudiero, L.; Vigneti, E.; Turrini, P. Presence of nerve growth factor in the thymus of prenatal, postnatal and pregnant rats. Thymus 1997, 24, 221–231. [Google Scholar] [CrossRef] [PubMed]
- García-Suárez, O.; Germanà, A.; Hannestad, J.; Pérez-Pérez, M.; Esteban, I.; Naves, F.J.; Vega, J.A. Changes in the expression of the nerve growth factor receptors TrkA and p75LNGR in the rat thymus with ageing and increased nerve growth factor plasma levels. Cell Tissue Res. 2000, 301, 225–234. [Google Scholar] [PubMed]
- Parrens, M.; Dubus, P.; Groppi, A.; Velly, J.F.; Labouyrie, E.; de Mascarel, A.; Merlio, J.P. Differential expression of NGF receptors in human thymic epithelial tumors. Pathol. Res. Pract. 1999, 195, 549–553. [Google Scholar] [CrossRef]
- Cattoretti, G.; Schirò, R.; Orazi, A.; Soligo, D.; Colombo, M.P. Bone marrow stroma in humans: Anti-nerve growth factor receptor antibodies selectively stain reticular cells in vivo and in vitro. Blood 1993, 81, 1726–1738. [Google Scholar] [PubMed]
- Chevalier, S.; Praloran, V.; Smith, C.; MacGrogan, D.; Ip, N.Y.; Yancopoulos, G.D.; Brachet, P.; Pouplard, A.; Gascan, H. Expression and functionality of the TrkA proto-oncogene product/NGF receptor in undifferentiated hematopoietic cells. Blood 1994, 83, 1479–1485. [Google Scholar] [PubMed]
- Bracci-Laudiero, L.; Celestino, D.; Starace, G.; Antonelli, A.; Lambiase, A.; Procoli, A.; Rumi, C.; Lai, M.; Picardi, A.; Ballatore, G.; et al. CD34-positive cells in human umbilical cord blood express nerve growth factor and its specific receptor TrkA. J. Neuroimmunol. 2001, 136, 130–139. [Google Scholar] [CrossRef]
- Paczkowska, E.; Piecyk, K.; Luczkowska, K.; Kotowski, M.; Roginska, D.; Pius-Sadowska, E.; Oronowicz, K.; Ostrowski, M.; Machalinski, B. Expression of neurotrophins and their receptors in human CD34+ bone marrow cells. J. Physiol. Pharmacol. 2016, 67, 151–159. [Google Scholar] [PubMed]
- Ernfors, P.; Hallböök, F.; Ebendal, T.; Shooter, E.M.; Radeke, M.J.; Misko, T.P.; Persson, H. Developmental and regional expression of β-nerve growth factor receptor mRNA in the chick and rat. Neuron 1988, 1, 983–996. [Google Scholar] [CrossRef]
- Ciriaco, E.; Dall’Aglio, C.; Hannestad, J.; Huerta, J.J.; Laurà, R.; Germanà, G.; Vega, J.A. Localization of TrK neurotrophin receptor-like proteins in avian primary lymphoid organs: Thymus and bursa of Fabricius. J. Neuroimmunol. 1996, 69, 73–83. [Google Scholar] [CrossRef]
- Ciriaco, E.; García-Suárez, O.; Ricci, A.; Abbate, F.; Piedimonte, G.; Vega, J.A. Trk-like proteins during the post-hatching growth of the avian bursa of Fabricius. Vet. Immunol. Immunopathol. 1997, 55, 313–320. [Google Scholar] [CrossRef]
- Bracci-Laudiero, L.; Vigneti, E.; Aloe, L. In vivo and in vitro effect of NGF on bursa of Fabricius cells during chick embryo development. Int. J. Neurosci. 1991, 59, 189–198. [Google Scholar] [CrossRef]
- Bracci-Laudiero, L.; Vigneti, E.; Iannicola, C.; Aloe, L. NGF retards apoptosis in chick embryo bursal cell in vitro. Differentiation 1993, 53, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Pittet, M.J. The spleen in local and systemic regulation of immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Bronzetti, E.; Artico, M.; Pompili, E.; Felici, L.M.; Stringaro, A.; Bosco, S.; Magliulo, G.; Colone, M.; Arancia, G.; Vitale, M.; et al. Neurotrophins and neurotransmitters in human palatine tonsils: An immunohistochemical and RT-PCR analysis. Int. J. Mol. Med. 2006, 18, 49–58. [Google Scholar] [CrossRef] [PubMed]
- García-Suárez, O.; Hannestad, J.; Esteban, I.; Martínez del Valle, M.; Naves, F.J.; Vega, J.A. Neurotrophin receptor-like protein immunoreactivity in human lymph nodes. Anat. Rec. 1997, 249, 226–232. [Google Scholar] [CrossRef]
- Pezzati, P.; Stanisz, A.M.; Marshall, J.S.; Bienenstock, J.; Stead, R.H. Expression of nerve growth factor receptor immunoreactivity on follicular dendritic cells from human mucosa associated lymphoid tissues. Immunology 1992, 76, 485–490. [Google Scholar] [PubMed]
- Carlson, S.L.; Albers, K.M.; Beiting, D.J.; Parish, M.; Conner, J.M.; Davis, B.M. NGF modulates sympathetic innervation of lymphoid tissues. J. Neurosci. 1995, 15, 5892–5899. [Google Scholar] [PubMed]
- Lucas, D.; Scheiermann, C.; Chow, A.; Kunisaki, Y.; Bruns, I.; Barrick, C.; Tessarollo, L.; Frenette, P.S. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat. Med. 2013, 19, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, F.; Rellick, S.L.; Piedimonte, G.; Akers, S.M.; O’Leary, H.A.; Martin, K.; Craig, M.D.; Gibson, L.F. Neurotrophins regulate bone marrow stromal cell IL-6 expression through the MAPK pathway. PLoS ONE 2010, 5, e9690. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Na, Y.J.; Jung, P.K.; Kim, M.N.; Kim, S.M.; Chung, J.S.; Kim, B.S.; Kim, J.B.; Moon, J.O.; Yoon, S. Nerve growth factor stimulates proliferation, adhesion and thymopoietic cytokine expression in mouse thymic epithelial cells in vitro. Regul. Pept. 2008, 147, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Auffray, I.; Chevalier, S.; Froger, J.; Izac, B.; Vainchenker, W.; Gascan, H.; Coulombel, L. Nerve growth factor is involved in the supportive effect by bone marrow derived stromal cells of the factor-dependent human cell line UT-7. Blood 1996, 88, 1608–1618. [Google Scholar] [PubMed]
- Matsuda, H.; Coughlin, M.D.; Bienenstock, J.; Denburg, J.A. Nerve growth factor promotes human hemapoietic colony growth and differentiation. Proc. Natl. Acad. Sci. USA 1988, 85, 6508–6512. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Wong, D.; Dolovich, J.; Bienenstock, J.; Marshall, J.; Denburg, J.A. Synergistic effects of nerve growth factor and granulocytes-macrophage colony-stimulating factor on human basophilic cell differentiation. Blood 1991, 77, 971–979. [Google Scholar] [PubMed]
- Welker, P.; Grabbe, J.; Gibbs, B.; Zuberbier, T.; Henz, B.M. Nerve growth factor-β induces mast-cell marker expression during in vitro culture of human umbilical cord blood cells. Immunology 2000, 99, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Bracci-Laudiero, L.; Aloe, L. Altered plasma nerve growth factor-like immunoreactivity and nerve growth factor-receptor expression in human old age. Gerontology 2003, 49, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Ehrhard, P.B.; Erb, P.; Graumann, U.; Otten, U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc. Natl. Acad. Sci. USA 1993, 90, 10984–10988. [Google Scholar] [CrossRef] [PubMed]
- Torcia, M.; Bracci-Laudiero, L.; Lucibello, M.; Nencioni, L.; Labardi, D.; Rubartelli, A.; Cozzolino, F.; Aloe, L.; Garaci, E. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell 1996, 85, 345–356. [Google Scholar] [CrossRef]
- Ehrhard, P.B.; Ganter, U.; Bauer, J.; Otten, U. Expression of functional Trk protooncogene in human monocytes. Proc. Natl. Acad. Sci. USA 1993, 90, 5423–5427. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Forsberg-Nilsson, K.; Xiang, Z.; Hallböök, F.; Nilsson, K.; Metcalfe, D.D. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur. J. Immunol. 1997, 27, 2295–2301. [Google Scholar] [CrossRef] [PubMed]
- Burgi, B.; Otten, U.H.; Ochensberger, B.; Rihs, S.; Heese, K.; Ehrhard, P.B.; Ibanez, C.F.; Dahinden, C.A. Basophil priming by neurotrophic factors. Activation through the Trk receptor. J. Immunol. 1996, 157, 5582–5588. [Google Scholar]
- Noga, O.; Englmann, C.; Hanf, G.; Grützkau, A.; Guhl, S.; Kunkel, G. Activation of the specific neurotrophin receptors TrkA, TrkB and TrkC influences the function of eosinophils. Clin. Exp. Allergy 2002, 32, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Ikari, S.; Matsuno, T.; Morimune, Y.; Nagahama, M.; Sakurai, J. Signal transduction mechanism involved in Clostridium perfringens α-toxin-induced superoxide anion generation in rabbit neutrophils. Infect. Immun. 2006, 74, 2876–2886. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Bracci-Laudiero, L.; Bonini, Se.; Bonini, St.; Starace, G.; D’Elios, M.M.; de Carli, M.; Aloe, L. Human CD4+ T-clones produce and release nerve growth factor and express TrkA. J. Allergy Clin. Immunol. 1997, 100, 408–414. [Google Scholar] [CrossRef]
- Caroleo, M.C.; Costa, N.; Bracci-Laudiero, L.; Aloe, L. Human monocyte/macrophages activate by exposure to LPS overexpress NGF and NGF receptors. J. Neuroimmunol. 2001, 113, 193–201. [Google Scholar] [CrossRef]
- Fischer, T.C.; Lauenstein, H.D.; Serowka, F.; Pilzner, C.; Groneberg, D.A.; Welker, P. Pan-neurotrophin receptor p75NTR expression is strongly induced in lesional atopic mast cells. Clin. Exp. Allergy 2008, 38, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.L.; Bailey, S.; Matusica, D.; Nicholson, I.; Muyderman, H.; Pagadala, P.C.; Neet, K.E.; Zola, H.; Macardle, P.; Rush, R.A. ProNGF mediates death of Natural Killer cells through activation of the p75NTR-sortilin complex. J. Neuroimmunol. 2010, 226, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, G.; Minnone, G.; Strippoli, R.; de Pasquale, L.; Petrini, S.; Caiello, I.; Manni, L.; de Benedetti, F.; Bracci-Laudiero, L. Nerve growth factor downregulates inflammatory response in human monocytes through TrkA. J. Immunol. 2014, 192, 3345–3354. [Google Scholar] [CrossRef] [PubMed]
- La Sala, A.; Corinti, S.; Federici, M.; Saragovi, H.U.; Girolomoni, G. Ligand activation of nerve growth factor receptor TrkA protects monocytes from apoptosis. J. Leukoc. Biol. 2000, 68, 104–110. [Google Scholar] [PubMed]
- Pavlov, V.A.; Tracey, K.J. Neural circuitry and immunity. Immunol. Res. 2015, 63, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H. Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol. Sci. 2004, 25, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H. Critical roles of acetylcholine and the muscarinic and nicotinic acetylcholine receptors in the regulation of immune function. Life Sci. 2012, 91, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.L.; Lorton, D. Autonomic regulation of cellular immune function. Auton. Neurosci. 2001, 182, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Sanders, V.M. The β2-adrenergic receptor on T- and B-lymphocytes: Do we understand it yet? Brain Behav. Immun. 2012, 26, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Pongratz, G.; Straub, R.H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 2014, 16, 504. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Talbot, S.; Abdulnour, R.E.; Burkett, P.R.; Lee, S.; Cronin, S.J.; Pascal, M.A.; Laedermann, C.; Foster, S.L.; Tran, J.V.; Lai, N.; et al. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron 2015, 87, 341–354. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.J.; Undem, B.J. Inflammation-induced plasticity of the afferent innervation of the airways. Environ. Health Perspect. 2001, 109, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Lourenssen, S.; Wells, R.W.; Blennerhassett, M.G. Differential responses of intrinsic and extrinsic innervation of smooth muscle cells in rat colitis. Exp. Neurol. 2005, 195, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; McGregor, G.P.; Saria, A.; Philippin, B.; Kummer, W. Induction of tachykinin gene and peptide expression in guinea pig nodose primary afferent neurons by allergic airway inflammation. J. Clin. Investig. 1996, 98, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Galeazza, M.T.; Garry, M.G.; Yost, H.J.; Strait, K.A.; Hargreaves, K.M.; Seybold, V.S. Plasticity in the synthesis and storage of substance P and calcitonin gene-related peptide in primary afferent neurons during peripheral inflammation. Neuroscience 1995, 66, 443–458. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. Neural regulation of immunity: Molecular mechanisms and clinical translation. Nat. Neurosci. 2017, 20, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomska, G.; Mietelska-Porowska, A.; Mazurkiewicz, M. The cholinergic system, nerve growth factor and the cytoskeleton. Behav. Brain Res. 2011, 221, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Hazari, M.S.; Pan, J.H.; Myers, A.C. Nerve growth factor acutely potentiates synaptic transmission in vitro and induces dendritic growth in vivo on adult neurons in airway parasympathetic ganglia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Weigand, L.A.; Kwong, K.; Myers, A.C. The effects of nerve growth factor on nicotinic synaptic transmission in mouse airway parasympathetic neurons. Am. J. Respir. Cell Mol. Biol. 2015, 53, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Levi-Montalcini, R. Mast cells increase in tissues of neonatal rats injected with the nerve growth factor. Brain Res. 1977, 133, 358–366. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Dahinden, C.A. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood 1992, 79, 2662–2669. [Google Scholar] [PubMed]
- Miura, K.; Saini, S.S.; Gauvreau, G.; MacGlashan, D.W., Jr. Differences in functional consequences and signal transduction induced by IL-3, IL-5, and nerve growth factor in human basophils. J. Immunol. 2001, 167, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Takafuji, S.; Bischoff, S.C.; de Weck, A.L.; Dahinden, C.A. Opposing effects of tumor necrosis factor-a and nerve growth factor upon leukotriene C4 production by human eosinophils triggered with N-formyl-methionyl-leuyl-phenylalanine. Eur. J. Immunol. 1992, 22, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Sin, A.Z.; Roche, E.M.; Togias, A.; Lichtenstein, L.M.; Schroeder, J.T. Nerve growth factor or IL-3 induces more IL-13 production from basophils of allergic subjects than from basophils of nonallergic subjects. J. Allergy Clin. Immunol. 2001, 108, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, B.F.; Zillikens, D.; Grabbe, J. Nerve growth factor influences IgE-mediated human basophil activation: Functional properties and intracellular mechanisms compared with IL-3. Int. Immunopharmacol. 2005, 5, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Welker, P.; Grabbe, J.; Grützkau, A.; Henz, B.M. Effects of nerve growth factor (NGF) and other fibroblast-derived growth factors on immature human mast cells (HMC-1). Immunology 1998, 94, 310–317. [Google Scholar] [PubMed]
- Murakami, M.; Tada, K.; Nakajima, K.; Kudo, I. Cyclooxygenase-2-dependent delayed prostaglandin D2 generation is initiated by nerve growth factor in rat peritoneal mast cells: Its augmentation by extracellular type II secretory phospholipase A2. J. Immunol. 1997, 159, 439–446. [Google Scholar] [PubMed]
- Marshall, J.S.; Gomi, K.; Blennerhassett, M.G.; Bienenstock, J. Nerve growth factor modifies the expression of inflammatory cytokines by mast cells via a prostanoid-dependent mechanism. J. Immunol. 1999, 162, 4271–4276. [Google Scholar] [PubMed]
- Mazurek, N.; Weskamp, G.; Erne, P.; Otten, U. Nerve growth factor induces mast cell degranulation without changing intracellular calcium levels. FEBS Lett. 1986, 198, 315–320. [Google Scholar] [CrossRef]
- Horigome, K.; Pryor, J.C.; Bullock, E.D.; Johnson, E.M., Jr. Mediator release from mast cells by nerve growth factor. Neurotrophin specificity and receptor mediation. J. Biol. Chem. 1993, 268, 14881–14887. [Google Scholar] [PubMed]
- Sawada, J.; Itakura, A.; Tanaka, A.; Furusaka, T.; Matsuda, H. Nerve growth factor functions as a chemoattractant for mast cells through both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling pathways. Blood 2000, 95, 2052–2058. [Google Scholar] [PubMed]
- Kawamoto, K.; Okada, T.; Kannan, Y.; Ushio, H.; Matsumoto, M.; Matsuda, H. Nerve growth factor prevents apoptosis of rat peritoneal mast cells through the Trk proto-oncogene receptor. Blood 1995, 86, 4638–4644. [Google Scholar] [PubMed]
- Bullock, E.D.; Johnson, E.M. Nerve growth factor induces the expression of certain cytokines genes and Bcl2 in mast cells: Potential role in survival promotion. J. Biol. Chem. 1996, 271, 27500–27508. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Watanabe, N.; Ohtomo, H.; Matsuda, H. Nerve growth factor enhances survival and cytotoxic activity of human eosinophils. Br. J. Haematol. 1996, 93, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Kannan, Y.; Usami, K.; Okada, M.; Shimizu, S.; Matsuda, H. Nerve growth factor suppresses apoptosis of murine neutrophils. Biochem. Biophys. Res. Commun. 1992, 186, 1050–1056. [Google Scholar] [CrossRef]
- Kannan, Y.; Ushio, H.; Koyama, H.; Okada, M.; Oikawa, M.; Yoshihara, T.; Kaneko, M.; Matsuda, H. Nerve growth factor enhances survival, phagocytosis, and superoxide production of murine neutrophils. Blood 1991, 77, 1320–1325. [Google Scholar] [PubMed]
- Hepburn, L.; Prajsnar, T.K.; Klapholz, C.; Moreno, P.; Loynes, C.A.; Ogryzko, N.V.; Brown, K.; Schiebler, M.; Hegyi, K.; Antrobus, R.; et al. Innate immunity. A Spaetzle-like role for nerve growth factor β in vertebrate immunity to Staphylococcus aureus. Science 2014, 346, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, L.W.; Stach, R.W.; Hashim, G.A.; Marchetti, D.; Perez-Polo, J.R. Receptors for nerve growth factor on rat spleen mononuclear cells. J. Neurosci. Res. 1987, 17, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Brodie, C.; Gelfand, E.W. Functional nerve growth factor receptors on human B lymphocytes. Interaction with IL-2. J. Immunol. 1992, 148, 3492–3497. [Google Scholar] [PubMed]
- Otten, U.; Ehrhard, P.; Peck, R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc. Natl. Acad. Sci. USA 1989, 86, 10059–10063. [Google Scholar] [CrossRef] [PubMed]
- Kimata, H.; Yoshida, A.; Ishioka, C.; Kusunoki, T.; Hosoi, S.; Mikawa, H. Nerve growth factor specifically induces human IgG4 production. Eur. J. Immunol. 1991, 21, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Brodie, C.; Gelfand, E.W. Regulation of immunoglobulin production by nerve growth factor: Comparison with anti-CD40. J. Neuroimmunol. 1994, 52, 87–96. [Google Scholar] [CrossRef]
- Brodie, C.; Oshiba, A.; Renz, H.; Bradley, K.; Gelfand, E.W. Nerve growth-factor and anti-CD40 provide opposite signals for the production of IgE in interleukin-4-treated lymphocytes. Eur. J. Immunol. 1996, 26, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Abram, M.; Wegmann, M.; Fokuhl, V.; Sonar, S.; Luger, E.O.; Kerzel, S.; Radbruch, A.; Renz, H.; Zemlin, M. Nerve growth factor and neurotrophin-3 mediate survival of pulmonary plasma cells during the allergic airway inflammation. J. Immunol. 2009, 182, 4705–4712. [Google Scholar] [CrossRef] [PubMed]
- Bayas, A.; Kruse, N.; Moriabadi, N.F.; Weber, F.; Hummel, V.; Wohleben, G.; Gold, R.; Toyka, K.V.; Rieckmann, P. Modulation of cytokine mRNA expression by brain-derived neurotrophic factor and nerve growth factor in human immune cells. Neurosci. Lett. 2003, 335, 155–158. [Google Scholar] [CrossRef]
- Kobayashi, H.; Mizisin, A.P. Nerve growth factor and neurotrophin-3 promote chemotaxis of mouse macrophages in vitro. Neurosci. Lett. 2001, 305, 157–160. [Google Scholar] [CrossRef]
- Samah, B.; Porcheray, F.; Gras, G. Neurotrophins modulate monocyte chemotaxis without affecting macrophage function. Clin. Exp. Immunol. 2008, 151, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Susaki, Y.; Shimizu, S.; Katakura, K.; Watanabe, N.; Kawamoto, K.; Matsumoto, M.; Tsudzuki, M.; Furusaka, T.; Kitamura, Y.; Matsuda, H. Functional properties of murine macrophages promoted by nerve growth factor. Blood 1996, 88, 4630–4637. [Google Scholar] [PubMed]
- Barouch, R.; Kazimirsky, G.; Appel, E.; Brodie, C. Nerve growth factor regulates TNF-α production in mouse macrophages via MAPkinase activation. J. Leukoc. Biol. 2001, 69, 1019–1026. [Google Scholar] [PubMed]
- Noga, O.; Peiser, M.; Altenähr, M.; Knieling, H.; Wanner, R.; Hanf, G.; Grosse, R.; Suttorp, N. Differential activation of dendritic cells by nerve growth factor and brain-derived neurotrophic factor. Clin. Exp. Allergy 2007, 37, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Bracci-Laudiero, L.; Aloe, L.; Stenfors, C.; Tirassa, P.; Theodorsson, E.; Lundberg, T. Nerve growth factor stimulates production of neuropeptide Y in human lymphocytes. NeuroReport 1996, 7, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Bracci-Laudiero, L.; Aloe, L.; Caroleo, M.C.; Buanne, P.; Costa, N.; Starace, G.; Lundeberg, T. Endogenous NGF regulates CGRP expression in human monocytes, and affects HLA-DR and CD86 expression and IL-10 production. Blood 2005, 106, 3507–3514. [Google Scholar] [CrossRef] [PubMed]
- Bracci-Laudiero, L.; Aloe, L.; Buanne, P.; Finn, A.; Stenfors, C.; Vigneti, E.; Theodorsson, E.; Lundeberg, T. NGF modulates CGRP synthesis in human B-lymphocytes: A possible anti-inflammatory action of NGF? J. Neuroimmunol. 2002, 123, 58–65. [Google Scholar] [CrossRef]
- Beigelman, A.; Levy, J.; Hadad, N.; Pinsk, V.; Haim, A.; Fruchtman, Y.; Levy, R. Abnormal neutrophil chemotactic activity in children with congenital insensitivity to pain with anhidrosis (CIPA): The role of nerve growth factor. Clin. Immunol. 2009, 130, 365–372. [Google Scholar]
- Safieh-Garabedian, B.; Poole, S.; Allchorne, A.; Winter, J.; Woolf, C.J. Contribution of interleukin-1β to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br. J. Pharmacol. 1995, 7, 1265–1275. [Google Scholar] [CrossRef]
- Aloe, L.; Tuveri, M.A.; Levi-Montalcini, R. Studies on carrageenan-induced arthritis in adult rats: Presence of nerve growth factor and role of sympathetic innervation. Rheumatol. Int. 1992, 12, 213–216. [Google Scholar] [CrossRef] [PubMed]
- De Vries, A.; Dessing, M.C.; Engels, F.; Henricks, P.A.; Nijkamp, F.P. Nerve growth factor induces a neurokinin-1 receptor-mediated airway hyperresponsiveness in guinea pigs. Am. J. Respir. Crit. Care Med. 1999, 159, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Rukwied, R.; Mayer, A.; Kluschina, O.; Obreja, O.; Schley, M.; Schmelz, M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain 2010, 148, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Villoslada, P.; Hauser, S.L.; Bartke, I.; Unger, J.; Heald, N.; Rosenberg, D.; Cheung, S.W.; Mobley, W.C.; Fisher, S.; Genain, C.P. Human nerve growth factor protects common marmosets against autoimmune encephalomyelitis by switching the balance of T helper cell type 1 and 2 cytokines within the central nervous system. J. Exp. Med. 2000, 191, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Flügel, A.; Matsumuro, K.; Neumann, H.; Klinkert, W.E.; Birnbacher, R.; Lassmann, H.; Otten, U.; Wekerle, H. Anti-inflammatory activity of nerve growth factor in experimental autoimmune encephalomyelitis: Inhibition of monocyte transendothelial migration. Eur. J. Immunol. 2001, 31, 11–22. [Google Scholar] [CrossRef]
- Arredondo, L.R.; Deng, C.; Ratts, R.B.; Lovett-Racke, A.E.; Holtzman, D.M.; Racke, M.K. Role of nerve growth factor in experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2001, 31, 625–633. [Google Scholar] [CrossRef]
- Micera, A.; Properzi, F.; Triaca, V.; Aloe, L. Nerve growth factor antibody exacerbates neuropathological signs of experimental allergic encephalomyelitis in adult lewis rats. J. Neuroimmunol. 2000, 104, 116–123. [Google Scholar] [CrossRef]
- Barada, K.A.; Mourad, F.H.; Sawah, S.I.; Khoury, C.; Safieh-Garabedian, B.; Nassar, C.F.; Tawil, A.; Jurjus, A.; Saadé, N.E. Up-regulation of nerve growth factor and interleukin-10 in inflamed and non inflamed intestinal segments in rats with experimental colitis. Cytokine 2007, 37, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Reinshagen, M.; Rohm, H.; Steinkamp, M.; Lieb, K.; Geerling, I.; Von Herbay, A.; Flämig, G.; Eysselein, V.E.; Adler, G. Protective role of neurotrophins in experimental inflammation of the rat gut. Gastroenterology 2000, 119, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ulevitch, R.J. Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 2005, 6, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Xu, D.; Brint, E.K.; O’Neill, L.A. Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005, 5, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Signaling to NF-κB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Michalek, S.M.; Jope, R.S. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol. 2010, 31, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Koyasu, S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003, 24, 358–363. [Google Scholar] [CrossRef]
| NGF Receptor Distribution in Lymphoid Organs | |||
|---|---|---|---|
| Tissue | TrkA | p75NTR | |
| Primary Lymphoid Organs | Thymus | thymocytes [77,78,79] | interdigitating reticular cells of the medulla [79]; |
| epithelial subcapsular and medullar cells [73,75,80,81] | periarteriolar macrophages [79,80,81,82]; | ||
| peripheral epithelial cells of Hassal’s bodies [79] | endothelial sinusal cells and nerve endings [72,79,80,81,82]; | ||
| interdigitating reticular cells of the medulla [79] | - | ||
| Bone marrow | stromal cells with dendritic features [77,84] | stromal cells with dendritic features [77,84]; | |
| CD34 positive hemopoietic stem cells [85,86,87] | CD34 positive hemopoietic stem cells [85,86,87]; | ||
| Bursa of fabricius | epithelial cells of the follicle [90,91] | bursa of Fabricius of chick embryo [88]; | |
| interfollicular epithelium [90,91] | |||
| blood vessels [90,91] | |||
| Secondary Lymphoid Organs | Spleen | stroma of the spleen and splenocytes [78] | stroma of the spleen and splenocytes [78]; |
| spleen mononuclear immunocompetent cells [77] | spleen mononuclear immunocompetent cells [77]; | ||
| Lymph nodes and mucosa-associated lymphoid tissues | follicular dendritic cells [79,94,95] | follicular dendritic cells [79,94,95]; | |
| blood vessel walls [79,94,95] | blood vessel walls [79,94,95]; | ||
| cryptic tonsillar epithelium [79,94,95] | cryptic tonsillar epithelium [79,94,95]; | ||
| monocyte-derived cells [79,94,95] | monocyte-derived cells [79,94,95]; | ||
| interdigitated reticular cells [79,94,95] | interdigitated reticular cells [79,94,95]. | ||
| Effect of NGF Stimulation | |
|---|---|
| Activated basophils | ↑ leukotriene and cytokine synthesis [110,138,139,140] |
| ↑ histamine release [141] | |
| ↑ response to IgE [141,142] | |
| Immature mast cells | ↑ tryptase and IgE receptors [143] |
| Mature mast cells | ↑ cyclooxygenase2 (COX2) and prostaglandin D2 [144] |
| ↑ IL-6 induction [145] | |
| ↑ histamine release [146,147] | |
| ↑ chemotaxis [148] | |
| ↑ survival (by suppressing apoptosis) [149,150] | |
| Eosinophils | ↓ suppression of leukotriene formation [140] |
| ↑ IL-4 production [111] | |
| ↑ peroxidase release and cytotoxic activity [151] | |
| ↑ survival (by suppressing apoptosis) [151] | |
| Neutrophils | ↑ survival (by suppressing apoptosis) [152] |
| ↑ superoxide production and phagocytosis [153,154] | |
| B-cells | ↑ proliferative response [155,156] |
| ↑ IL-2 receptors [156] | |
| influences the production of IgM and IgG [157,158,159,160] | |
| ↑ survival of memory B-cells [107] | |
| ↑differentiation of B-cells into immunoglobulin-secreting plasma cells [157] | |
| influences plasma cell survival [161] | |
| T-cells | ↑ proliferative response [155] |
| ↑ cytokine expression [162] | |
| Monocytes/macrophages | protection from apoptosis, by inducing the anti-apoptotic proteins Bcl-2, Bcl-xl and Bfl-1 [118] |
| ↑ CXCR4 expression and chemotactic response [163,164] | |
| ↑ phagocytosis, enhanced parasite-killing activity and IL-1β [165] | |
| ↑ TNF-α, IL-8 secretion [154,166] | |
| Dendritic cells | ↑ maturation of dendritic cells and secretion of inflammatory cytokines [167] |
| ↑ IL-6 release in allergic patients; | |
| ↑ IL-10 release in healthy controls [167] | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int. J. Mol. Sci. 2017, 18, 1028. https://doi.org/10.3390/ijms18051028
Minnone G, De Benedetti F, Bracci-Laudiero L. NGF and Its Receptors in the Regulation of Inflammatory Response. International Journal of Molecular Sciences. 2017; 18(5):1028. https://doi.org/10.3390/ijms18051028
Chicago/Turabian StyleMinnone, Gaetana, Fabrizio De Benedetti, and Luisa Bracci-Laudiero. 2017. "NGF and Its Receptors in the Regulation of Inflammatory Response" International Journal of Molecular Sciences 18, no. 5: 1028. https://doi.org/10.3390/ijms18051028





