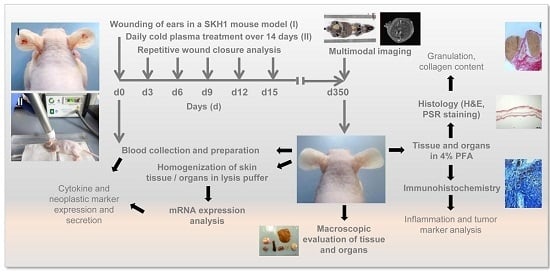
One Year Follow-Up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet
Abstract
:1. Introduction
2. Results
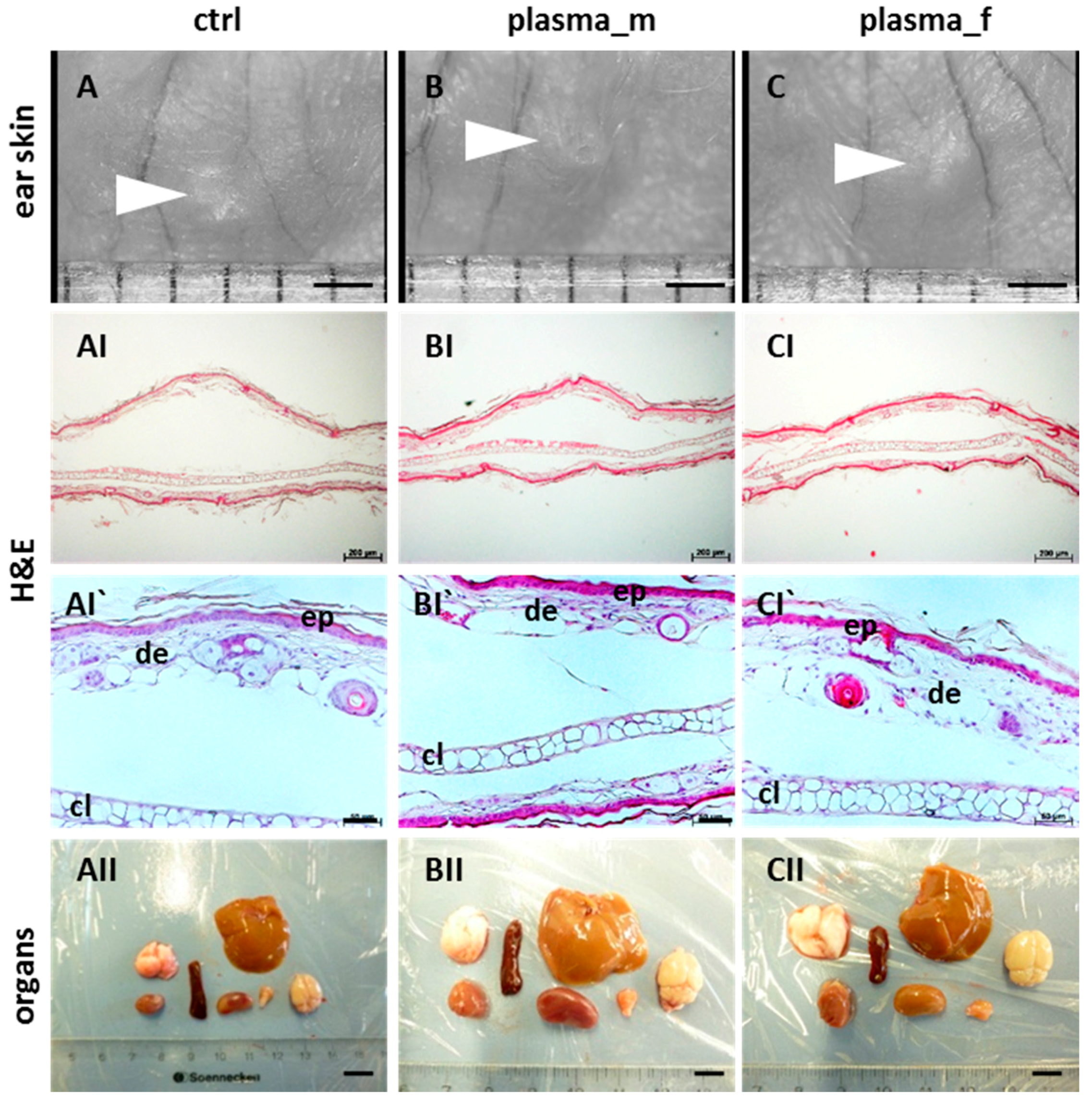
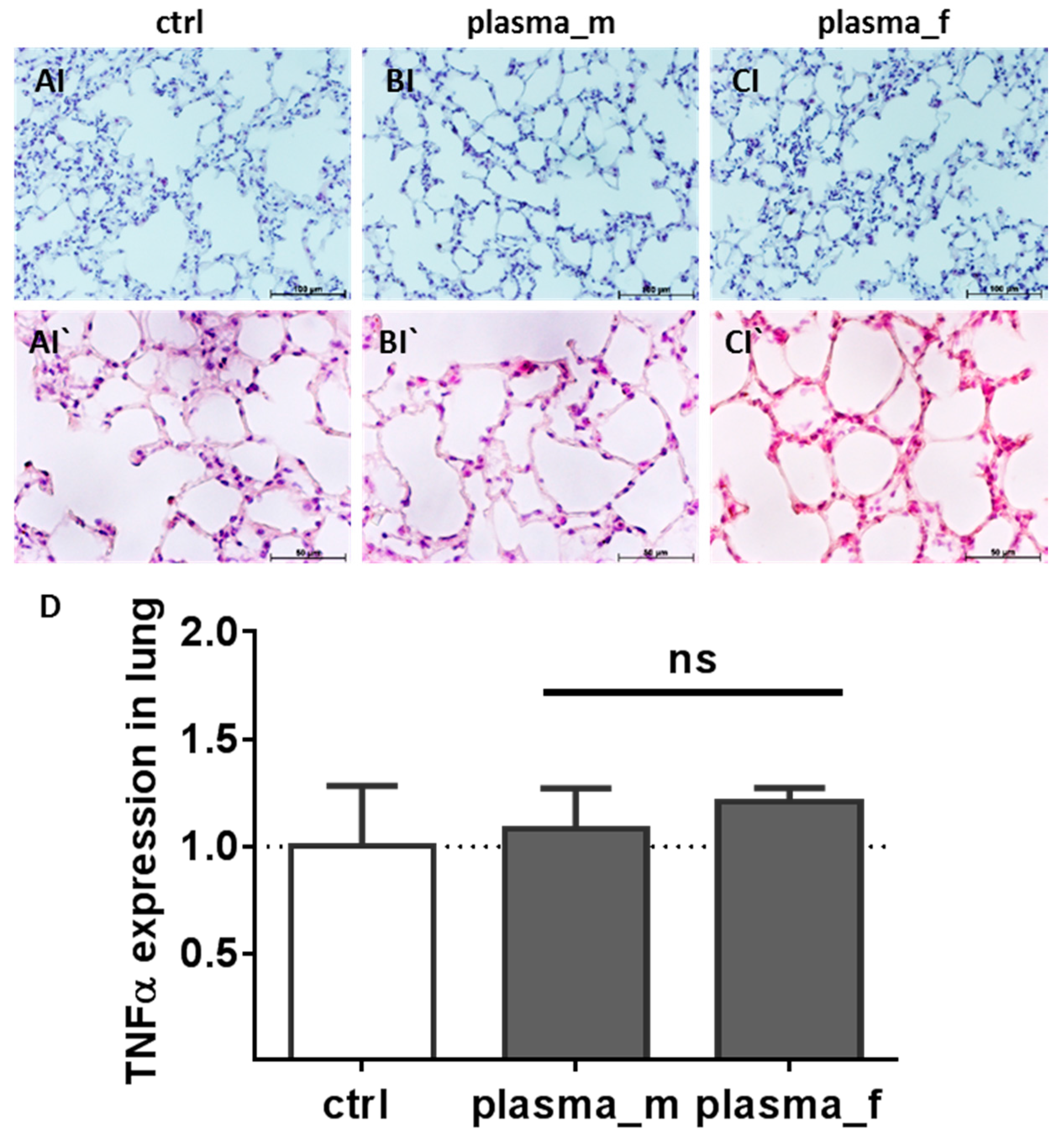
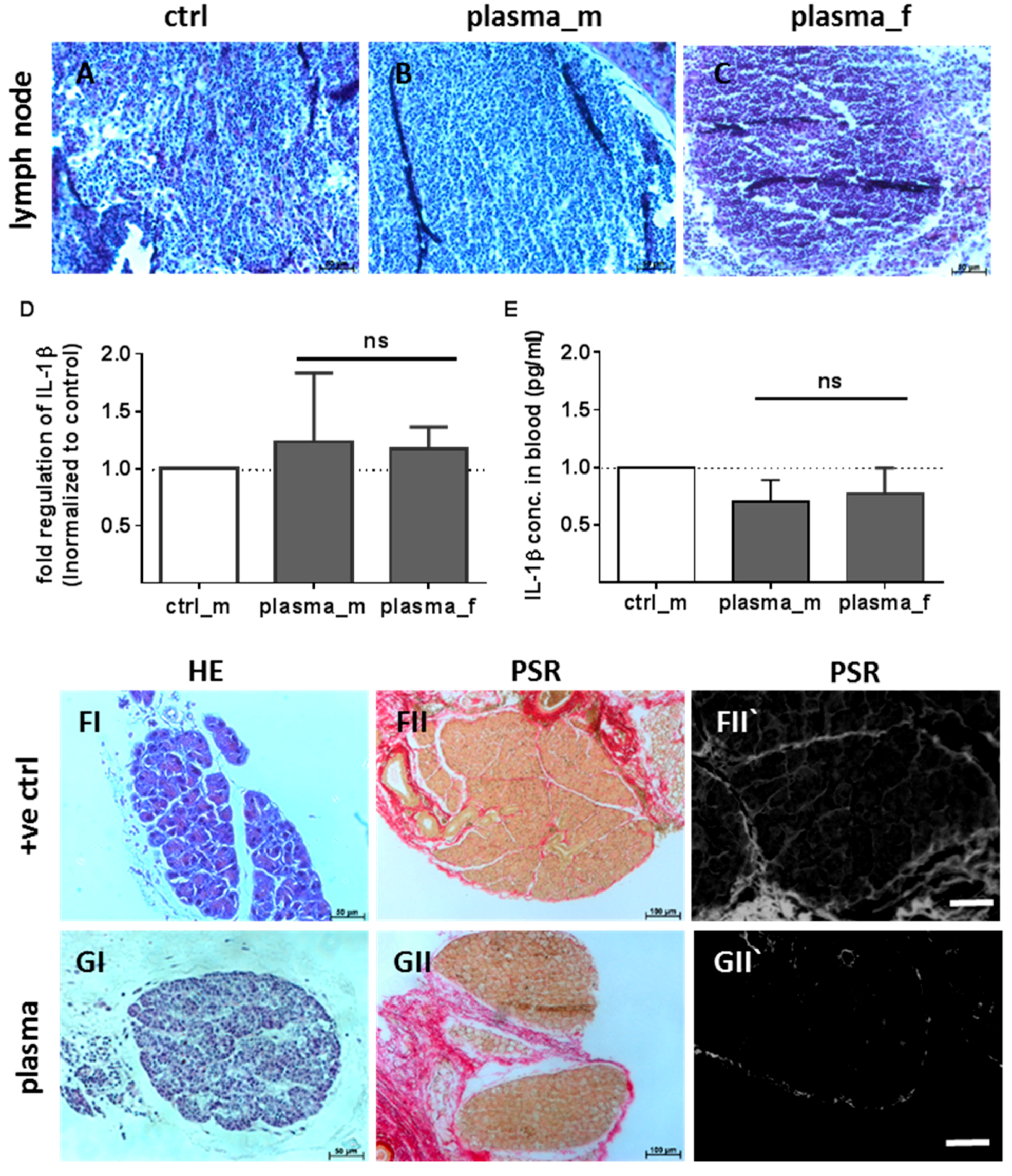
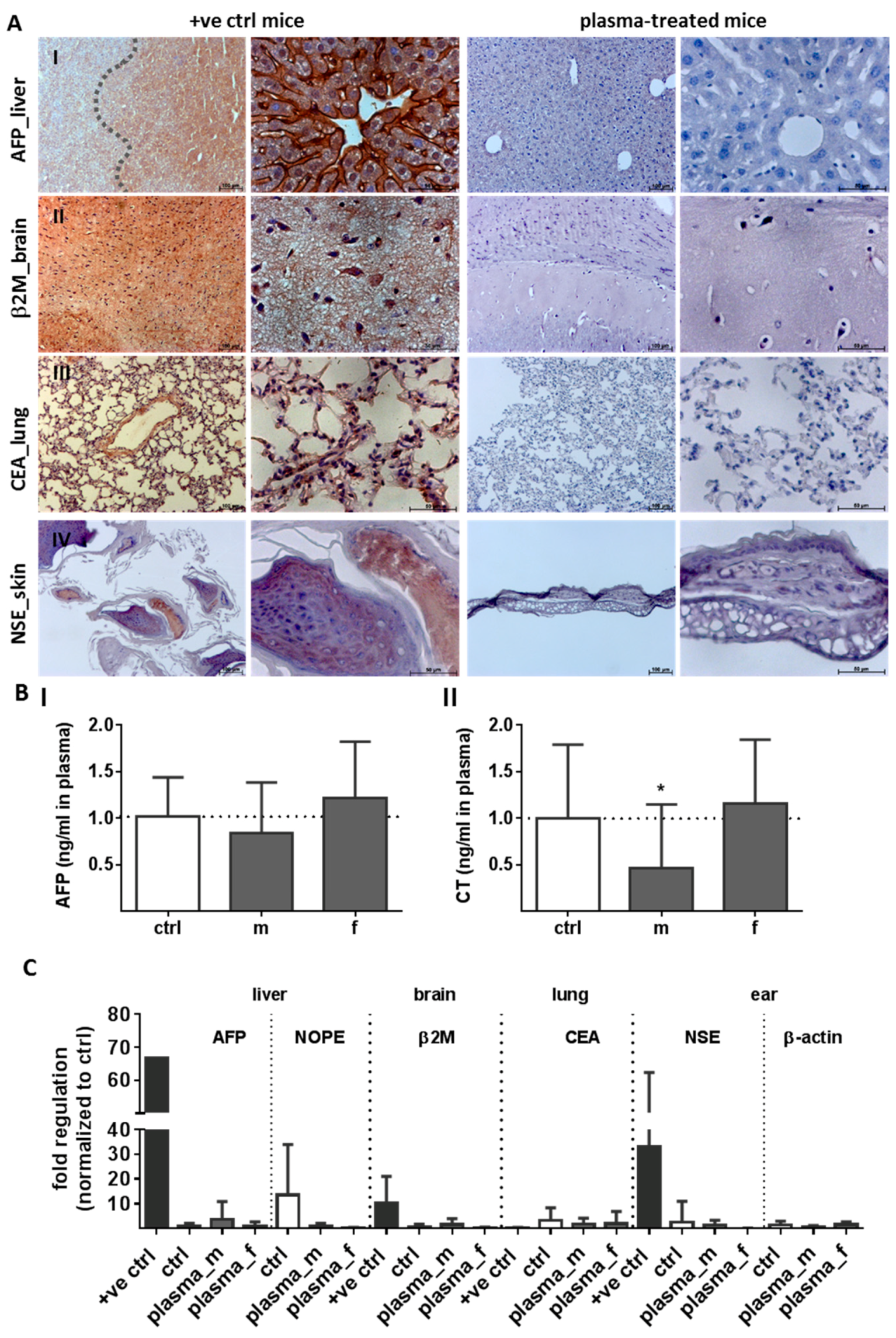
2.1. Evaluation of Histological Architecture and Inflammation Status after One Year
2.2. Evaluation of Tumor Marker (TM) Expression and Secretion
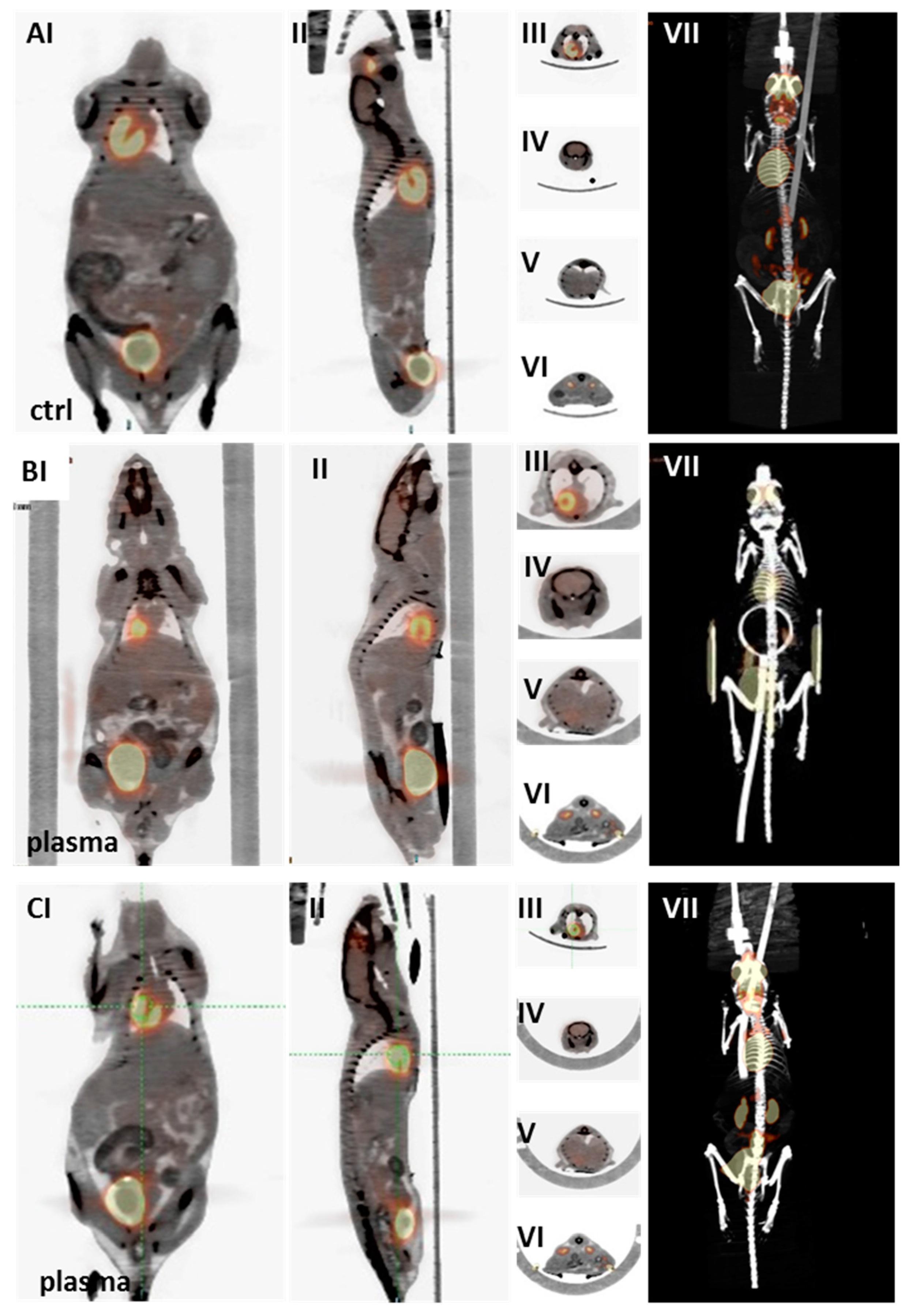
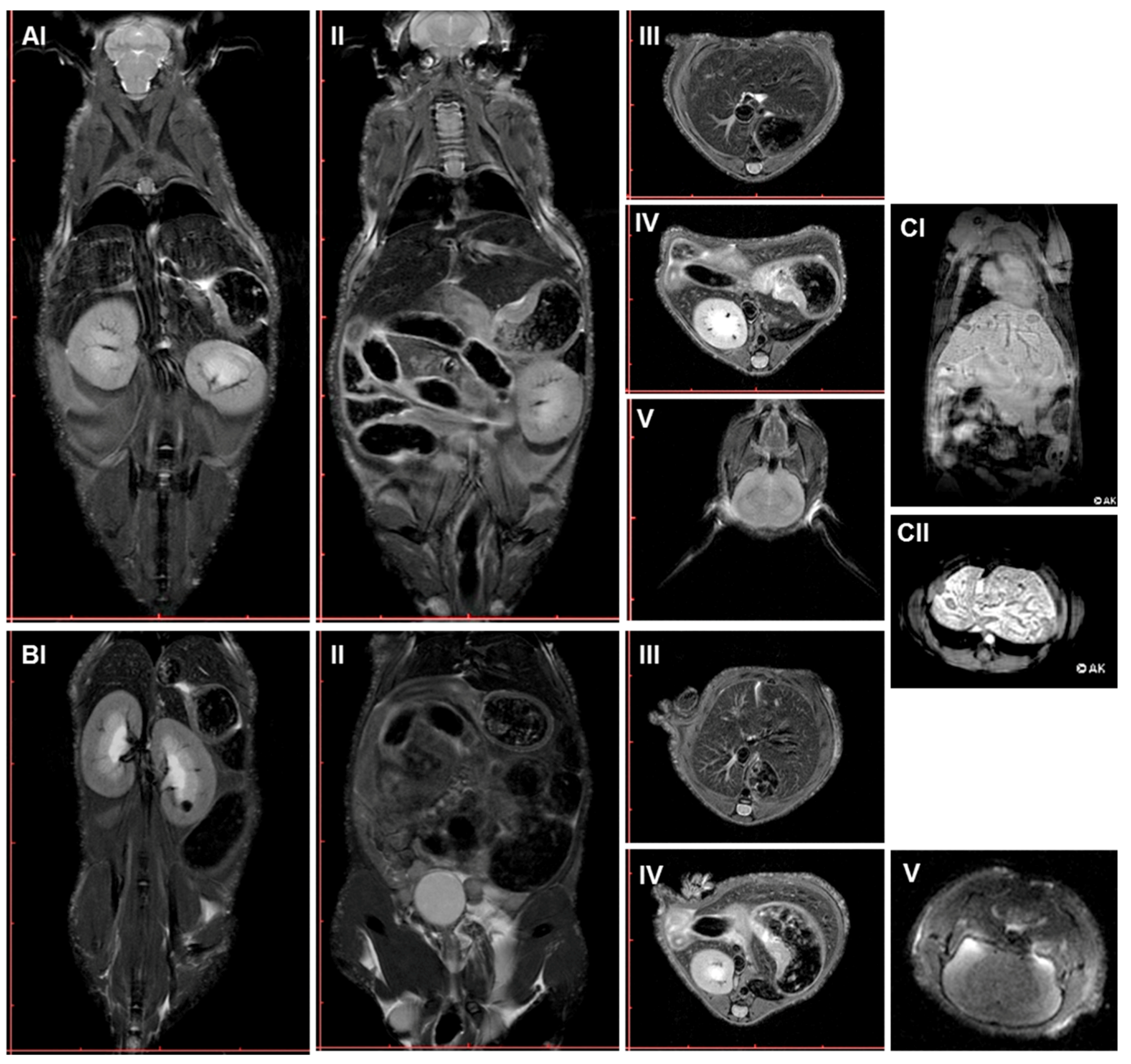
2.3. Multimodal Imaging of Mice 350 Days after Plasma Treatment
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Wounding and Exposure to Cold Physical Plasma
4.3. Wound Closure Observations and Histological Evaluation
4.4. Homogenisation of Tissues and Gene Expression Analysis
4.5. ELISA Measurements and Bead-Based Cytokine Analysis
4.6. MRI, PET/CT Scans, and Interpretation of Data
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FoV | field of view |
| MRI | magnetic resonance imaging |
| PET/CT | positron emission tomography/computed tomography |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| TE | echo time |
| TR | repetition time |
Appendix A
| Name | Gene | Sequences/# (Biomol) |
|---|---|---|
| mGAPDH_s | House keeping gene | CATGGCCTCCAAGGAGTAAG |
| mGAPDH_as | TGTGAGGGAGATGCTCAGTG | |
| mTNFα_s | Tumor necrosis factor α | TCTCATGCACCACCATCAAGGACT |
| mTNFα_as | ACCACTCTCCCTTTGCAGAACTCA | |
| mIL-1β_s | Interleukin 1β | GCAACTGTTCCTGAACTCAACT |
| mIL-1β_as | ATCTTTTGGGGTCCGTCAACT | |
| mAFP_s | α fetoprotein | CCAGGAAGTCTGTTTCACAGAAG |
| mAFP_as | CAAAAGGCTCACACCAAAGAG | |
| mβ2M | β2 microglobulin | VMPS-563 |
| mCEA1 | Carcinoembryonic antigen | VMPS-1054 |
| mCT | Calcitonin | VMPS-785 |
| mNSE | Neuron specific enolase | VMPS-1942 |
References
- Weltmann, K.-D.; von Woedtke, T. Plasma medicine-current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Kramer, A.; Bekeschus, S.; Matthes, R.; Bender, C.; Stope, M.B.; Napp, M.; Lademann, O.; Lademann, J.; Weltmann, K.-D.; Schauer, F. Cold Physical Plasmas in the Field of Hygiene-Relevance, Significance, and Future Applications. Plasma Process. Polym. 2015, 12, 1410–1422. [Google Scholar] [CrossRef]
- Matthes, R.; Hubner, N.O.; Bender, C.; Koban, I.; Horn, S.; Bekeschus, S.; Weltmann, K.-D.; Kocher, T.; Kramer, A.; Assadian, O. Efficacy of different carrier gases for barrier discharge plasma generation compared to chlorhexidine on the survival of Pseudomonas aeruginosa embedded in biofilm in vitro. Skin Pharmacol. Physiol. 2014, 27, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Daeschlein, G.; Napp, M.; von Podewils, S.; Lutze, S.; Emmert, S.; Lange, A.; Klare, I.; Haase, H.; Gumbel, D.; von Woedtke, T.; Junger, M. In Vitro Susceptibility of Multidrug Resistant Skin and Wound Pathogens Against Low Temperature Atmospheric Pressure Plasma Jet (APPJ) and Dielectric Barrier Discharge Plasma (DBD). Plasma Process. Polym. 2014, 11, 175–183. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.; Schmidt, H.U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.; et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Stolz, W.; Shimizu, T.; Monetti, R.; Bunk, W.; Schmidt, H.-U.; Morfill, G.; Klämpfl, T.; Steffes, B.; Thomas, H. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clin. Plasma Med. 2013, 1, 25–30. [Google Scholar] [CrossRef]
- Klebes, M.; Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Richter, H.; Bob, A.; von Hutten, J.; Krediet, J.T.; Kramer, A.; et al. Combined antibacterial effects of tissue-tolerable plasma and a modern conventional liquid antiseptic on chronic wound treatment. J. Biophotonics 2015, 8, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Wound healing essentials: Let there be oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Khanna, S.; Nallu, K.; Hunt, T.K.; Sen, C.K. Dermal wound healing is subject to redox control. Mol. Ther. 2006, 13, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Kranke, P.; Bennett, M.H.; James, M.M.; Schnabel, A.; Debus, S.E. Hyperbaric Oxygen Therapy for Chronic Wounds. Cochrane Database Syst Rev. 2012, 18, CD004123. [Google Scholar]
- Sen, C.K. The general case for redox control of wound repair. In Wound Repair and Regeneration: Official Publication of the Wound Healing Society [and] the European Tissue Repair Society; European Tissue Repair Society: St. Louis, MO, USA, 2003; Volume 11, pp. 431–438. [Google Scholar]
- Sunkari, V.G.; Lind, F.; Botusan, I.R.; Kashif, A.; Liu, Z.J.; Ylä-Herttuala, S.; Brismar, K.; Velazquez, O.; Catrina, S.B. Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice. Wound Repair Regen. 2015, 23, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Barbul, A. Understanding the role of immune regulation in wound healing. Am. J. Surg. 2004, 187, 11S–16S. [Google Scholar] [CrossRef]
- Isbary, G.; Shimizu, T.; Zimmermann, J.L.; Thomas, H.M.; Morfill, G.E.; Stolz, W. Cold atmospheric plasma for local infection control and subsequent pain reduction in a patient with chronic post-operative ear infection. New Microbes New Infect. 2013, 1, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Vandersee, S.; Terhorst, D.; Humme, D.; Beyer, M. Treatment of indolent primary cutaneous B-cell lymphomas with subcutaneous interferon-alfa. J. Am. Acad. Dermatol. 2014, 70, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Jablonowski, H.; von Woedtke, T. Research on plasma medicine-relevant plasma–liquid interaction: What happened in the past five years? Clin. Plasma Med. 2015, 3, 42–52. [Google Scholar] [CrossRef]
- Reuter, S.; Tresp, H.; Wende, K.; Hammer, M.U.; Winter, J.; Masur, K.; Schmidt-Bleker, A.; Weltmann, K.-D. From RONS to ROS: Tailoring Plasma Jet Treatment of Skin Cells. IEEE Trans. Plasma Sci. 2012, 40, 2986–2993. [Google Scholar] [CrossRef]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Hanschmann, E.M.; Godoy, J.R.; Berndt, C.; Hudemann, C.; Lillig, C.H. Thioredoxins, glutaredoxins, and peroxiredoxins—Molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxid. Redox Signal. 2013, 19, 1539–1605. [Google Scholar] [CrossRef] [PubMed]
- Castro Fernandes, D.; Bonatto, D.; Laurindo, F.M. The Evolving Concept of Oxidative Stress. In Studies on Cardiovascular Disorders; Sauer, H., Shah, A.M., Laurindo, F.R.M., Eds.; Humana Press: New York, NY, USA, 2010; pp. 1–41. [Google Scholar]
- Schmidt, A.; Rödder, K.; Hasse, S.; Masur, K.; Toups, L.; Lillig, C.H.; von Woedtke, T.; Wende, K.; Bekeschus, S. Redox-regulation of activator protein 1 family members in blood cancer cell lines exposed to cold physical plasma-treated medium. Plasma Process. Polym. 2016, 13, 1179–1188. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Milisav, I. The neglected significance of “antioxidative stress”. Oxid. Med. Cell. Longev. 2012, 2012, 480895. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.-S. Atmospheric-Pressure Plasma Jet Induces Apoptosis Involving Mitochondria via Generation of Free Radicals. PLoS ONE 2011, 6, e28154. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Rödder, K.; Schmidt, A.; Stope, M.B.; von Woedtke, T.; Miller, V.; Fridman, A.; Weltmann, K.-D.; Masur, K.; Metelmann, H.-R.; et al. Cold physical plasma selects for specific T helper cell subsets with distinct cells surface markers in a caspase-dependent and NF-κB-independent manner. Plasma Process. Polym. 2016, 13, 1144–1150. [Google Scholar] [CrossRef]
- Weiss, M.; Gumbel, D.; Hanschmann, E.M.; Mandelkow, R.; Gelbrich, N.; Zimmermann, U.; Walther, R.; Ekkernkamp, A.; Sckell, A.; Kramer, A.; et al. Cold Atmospheric Plasma Treatment Induces Anti-Proliferative Effects in Prostate Cancer Cells by Redox and Apoptotic Signaling Pathways. PLoS ONE 2015, 10, e0130350. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Wende, K.; Bekeschus, S.; Schmidt, A.; Jatsch, L.; Hasse, S.; Weltmann, K.-D.; Masur, K.; von Woedtke, T. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 798–799, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kluge, S.; Bekeschus, S.; Bender, C.; Benkhai, H.; Sckell, A.; Below, H.; Stope, M.B.; Kramer, A. Investigating the Mutagenicity of a Cold Argon-Plasma Jet in an HET-MN Model. PLoS ONE 2016, 11, e0160667. [Google Scholar] [CrossRef] [PubMed]
- Boxhammer, V.; Li, Y.F.; Koritzer, J.; Shimizu, T.; Maisch, T.; Thomas, H.M.; Schlegel, J.; Morfill, G.E.; Zimmermann, J.L. Investigation of the mutagenic potential of cold atmospheric plasma at bactericidal dosages. Mutat. Res. 2013, 753, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.-R.; Vu, T.T.; Do, H.T.; Le, T.N.B.; Hoang, T.H.A.; Phi, T.T.T.; Luong, T.M.L.; Doan, V.T.; Nguyen, T.T.H.; Nguyen, T.H.M.; et al. Podmelle. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment: A clinical long term observation. Clin. Plasma Med. 2013, 1, 30–35. [Google Scholar] [CrossRef]
- Isbary, G.; Zimmermann, J.L.; Shimizu, T.; Li, Y.-F.; Morfill, G.E.; Thomas, H.M.; Steffes, B.; Heinlin, J.; Karrer, S.; Stolz, W.; et al. Non-thermal plasma—More than five years of clinical experience. Clin. Plasma Med. 2013, 1, 19–23. [Google Scholar] [CrossRef]
- Fahmy, S.R. Anti-fibrotic effect of Holothuria arenicola extract against bile duct ligation in rats. BMC Complement. Altern. Med. 2015, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, H.; Mi, Y.; Wada, R.; Kono, M.; Yamashita, T.; Liu, Y.; Werth, N.; Sandhoff, R.; Sandhoff, K.; Proia, R.L. Systemic inflammation in glucocerebrosidase-deficient mice with minimal glucosylceramide storage. J. Clin. Investig. 2002, 109, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, A.; Bulte, C.; Kalaichelvan, A.; Cheng, M.; Krishnamachary, B.; Bhujwalla, Z.M.; Jiang, L.; Glunde, K. Metastatic breast cancer cells in lymph nodes increase nodal collagen density. Sci. Rep. 2015, 5, 10002. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Siebert, H.; Hofmann, U.; Frantz, S. Determination of collagen content within picrosirius red stained paraffin-embedded tissue sections using fluorescence microscopy. MethodsX 2015, 2, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Oda, O.; Inagi, R.; Iida, Y.; Araki, N.; Yamada, N.; Horiuchi, S.; Taniguchi, N.; Maeda, K.; Kinoshita, T. beta 2-Microglobulin modified with advanced glycation end products is a major component of hemodialysis-associated amyloidosis. J. Clin. Investig. 1993, 92, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Aarons, C.B.; Bajenova, O.; Andrews, C.; Heydrick, S.; Bushell, K.N.; Reed, K.L.; Thomas, P.; Becker, J.M.; Stucchi, A.F. Carcinoembryonic antigen-stimulated THP-1 macrophages activate endothelial cells and increase cell-cell adhesion of colorectal cancer cells. Clin. Exp. Metastasis 2007, 24, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Baudin, E.; Gigliotti, A.; Ducreux, M.; Ropers, J.; Comoy, E.; Sabourin, J.C.; Bidart, J.M.; Cailleux, A.F.; Bonacci, R.; Ruffie, P.; et al. Neuron-specific enolase and chromogranin A as markers of neuroendocrine tumours. Br. J. Cancer 1998, 78, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Fingas, C.D.; Altinbas, A.; Schlattjan, M.; Beilfuss, A.; Sowa, J.P.; Sydor, S.; Bechmann, L.P.; Ertle, J.; Akkiz, H.; Herzer, K.; et al. Expression of apoptosis- and vitamin D pathway-related genes in hepatocellular carcinoma. Digestion 2013, 87, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, J.U.; Quasdorff, M.; Varnholt, H.; Curth, H.M.; Mesghenna, S.; Protzer, U.; Goeser, T.; Nierhoff, D. Neighbor of Punc E11, a novel oncofetal marker for hepatocellular carcinoma. Int. J. Cancer. 2011, 128, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Rifkin, D.B.; Crowe, R.M.; Pollack, R. Tumor promoters induce changes in the chick embryo fibroblast cytoskeleton. Cell 1979, 18, 361–368. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Weltmann, K.-D.; Kindel, E.; Brandenburg, R.; Meyer, C.; Bussiahn, R.; Wilke, C.; von Woedtke, T. Atmospheric Pressure Plasma Jet for Medical Therapy: Plasma Parameters and Risk Estimation. Contrib. Plasma Phys. 2009, 49, 631–640. [Google Scholar] [CrossRef]
- Schmidt, A.; von Woedtke, T.; Bekeschus, S. Periodic exposure of keratinocytes to cold physical plasma—An in vitro model for redox-related diseases of the skin. Oxid. Med. Cell. Longev. 2016, 2016, 9816072. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Wende, K.; Bekeschus, S.; Bundscherer, L.; Barton, A.; Ottmuller, K.; Weltmann, K.D.; Masur, K. Non-thermal plasma treatment is associated with changes in transcriptome of human epithelial skin cells. Free Radic. Res. 2013, 47, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Dietrich, S.; Steuer, A.; Weltmann, K.-D.; von Woedtke, T.; Masur, K.; Wende, K. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J. Biol. Chem. 2015, 290, 6731–6750. [Google Scholar] [CrossRef] [PubMed]
- Bundscherer, L.; Wende, K.; Ottmuller, K.; Barton, A.; Schmidt, A.; Bekeschus, S.; Hasse, S.; Weltmann, K.-D.; Masur, K.; Lindequist, U. Impact of non-thermal plasma treatment on MAPK signaling pathways of human immune cell lines. Immunobiology 2013, 218, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and cancer: Have we moved forward? Biochem. J. 2007, 401, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The Plasma Jet kINPen—A Powerful Tool for Wound Healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Alborova, A.; Humme, D.; Patzelt, A.; Kramer, A.; Weltmann, K.D.; Hartmann, B.; Ottomann, C.; Fluhr, J.W.; et al. Risk assessment of the application of a plasma jet in dermatology. J. Biomed. Opt. 2009, 14, 054025. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Lademann, J.; Bender, C.; Sckell, A.; Hartmann, B.; Münch, S.; Hinz, P.; Ekkernkamp, A.; Matthes, R.; Koban, I.; et al. Suitability of tissue tolerable plasmas (TTP) for the management of chronic wounds. Clin. Plasma Med. 2013, 1, 11–18. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; von Woedtke, T.; Bussiahn, R.; Weltmann, K.-D.; Rieck, M.; Khalili, R.; Podmelle, F.; Waite, P.D. Experimental recovery of CO2-laser skin lesions by plasma stimulation. Am. J. Cosmet. Surg. 2012, 29, 52–56. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Metelmann, H.-R.; Weltmann, K.-D. Clinical Plasma Medicine: State and Perspectives of in Vivo Application of Cold Atmospheric Plasma. Contrib. Plasma Phys. 2014, 54, 104–117. [Google Scholar] [CrossRef]
- Hasse, S.; Tran, T.D.; Hahn, O.; Kindler, S.; Metelmann, H.R.; von Woedtke, T.; Masur, K. Induction of proliferation of basal epidermal keratinocytes by cold atmospheric-pressure plasma. Clin. Exp. Dermatol. 2016, 41, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Von Woedtke, T.; Metelmann, H.-R.; Weltmann, K.-D. Editorial. Clin. Plasma Med. 2013, 1, 1–2. [Google Scholar] [CrossRef]
- Basuyau, J.P.; Mallet, E.; Leroy, M.; Brunelle, P. Reference intervals for serum calcitonin in men, women, and children. Clin. Chem. 2004, 50, 1828–1830. [Google Scholar] [CrossRef] [PubMed]
- Shoenfeld, Y.; Gallant, L.A.; Shaklai, M.; Livni, E.; Djaldetti, M.; Pinkhas, J. Gaucher’s disease: A disease with chronic stimulation of the immune system. Arch. Pathol. Lab. Med. 1982, 106, 388–391. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2CT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, A.; Woedtke, T.V.; Stenzel, J.; Lindner, T.; Polei, S.; Vollmar, B.; Bekeschus, S. One Year Follow-Up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet. Int. J. Mol. Sci. 2017, 18, 868. https://doi.org/10.3390/ijms18040868
Schmidt A, Woedtke TV, Stenzel J, Lindner T, Polei S, Vollmar B, Bekeschus S. One Year Follow-Up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet. International Journal of Molecular Sciences. 2017; 18(4):868. https://doi.org/10.3390/ijms18040868
Chicago/Turabian StyleSchmidt, Anke, Thomas Von Woedtke, Jan Stenzel, Tobias Lindner, Stefan Polei, Brigitte Vollmar, and Sander Bekeschus. 2017. "One Year Follow-Up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet" International Journal of Molecular Sciences 18, no. 4: 868. https://doi.org/10.3390/ijms18040868