Pannexin- and Connexin-Mediated Intercellular Communication in Platelet Function
Abstract
:1. Introduction
2. Platelet Function: From Plaque Rupture to Thrombus Formation
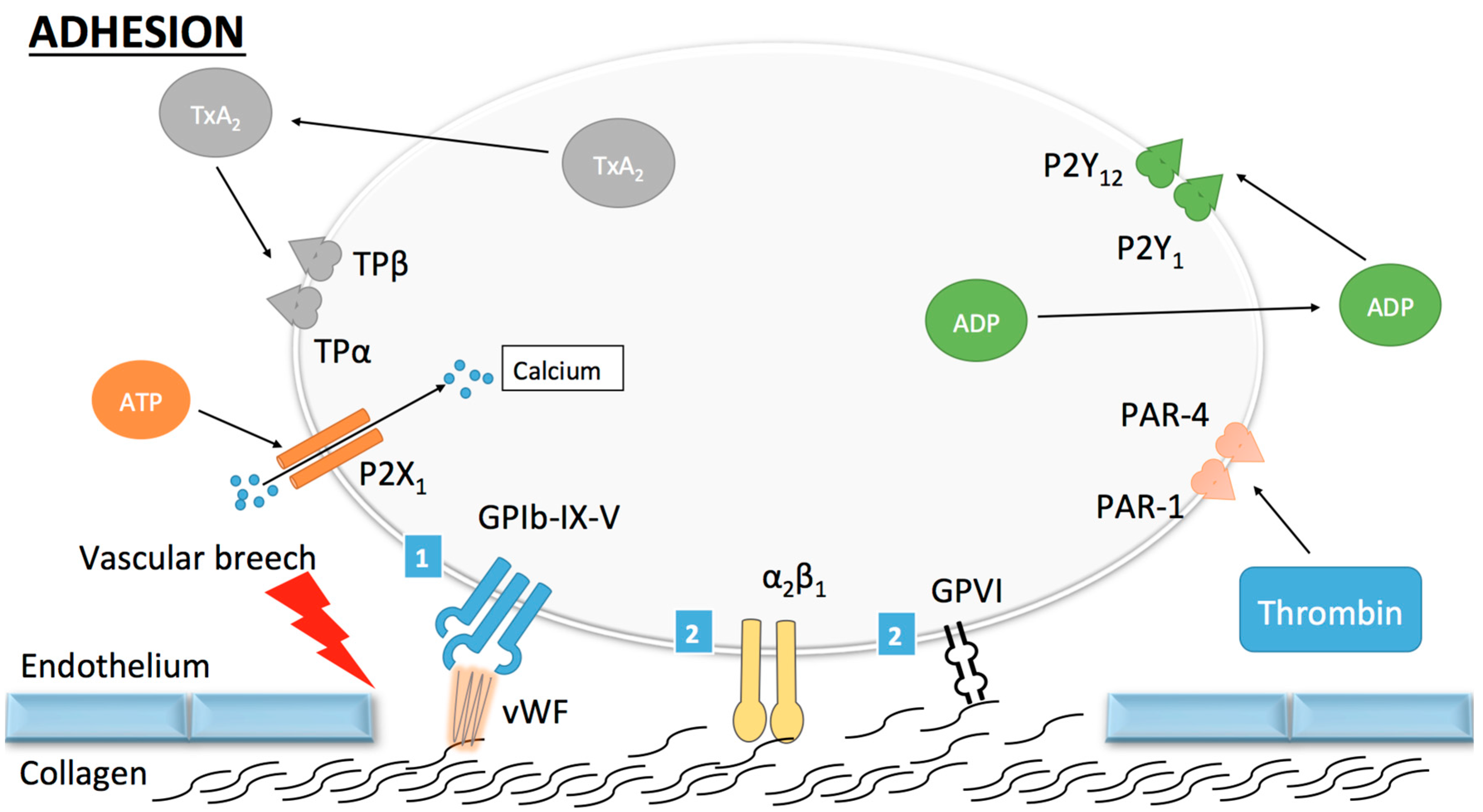
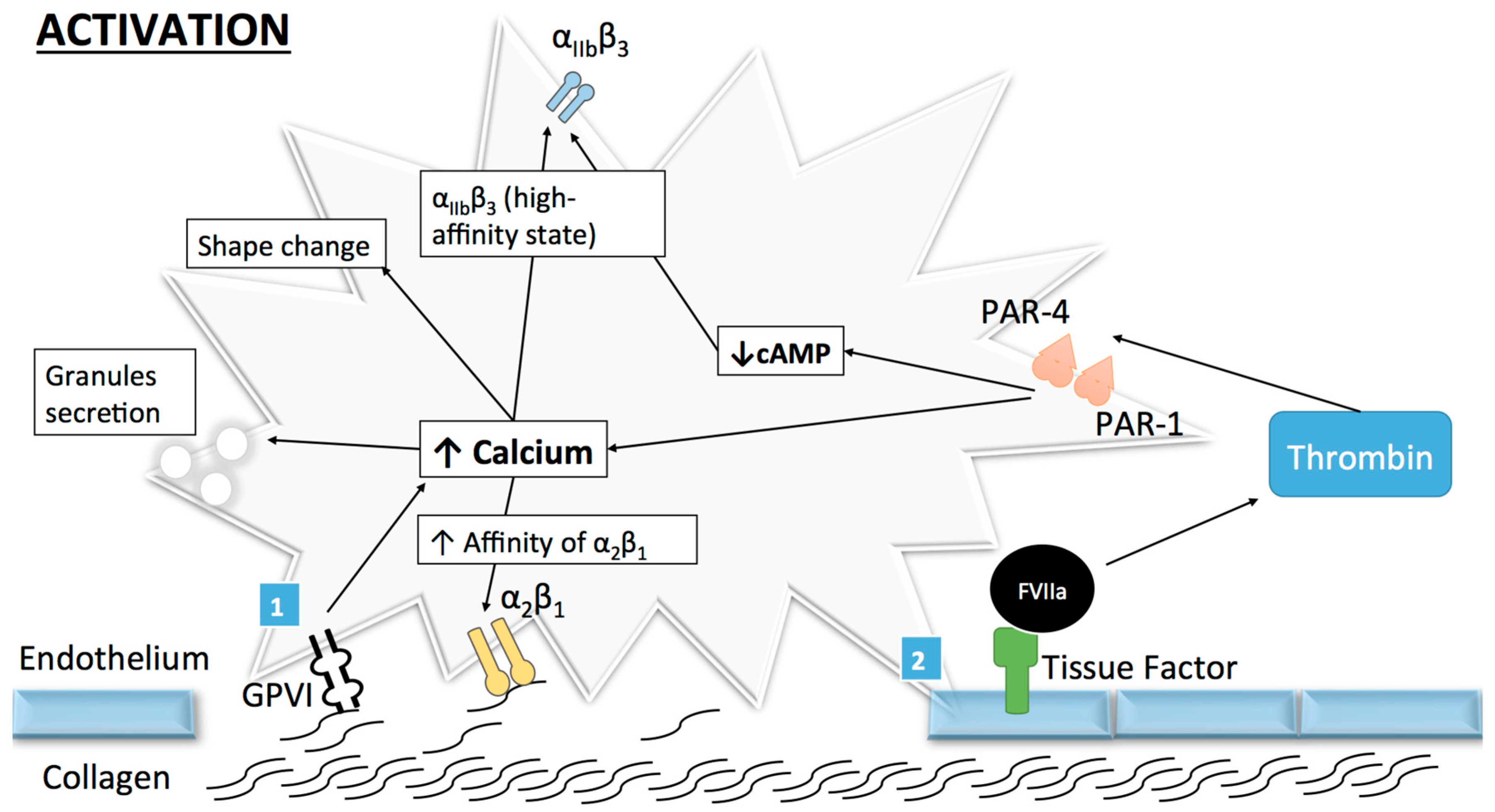
2.1. Platelet Adhesion and Activation
2.1.1. Collagen
Glycoprotein VI
Integrin α2β1
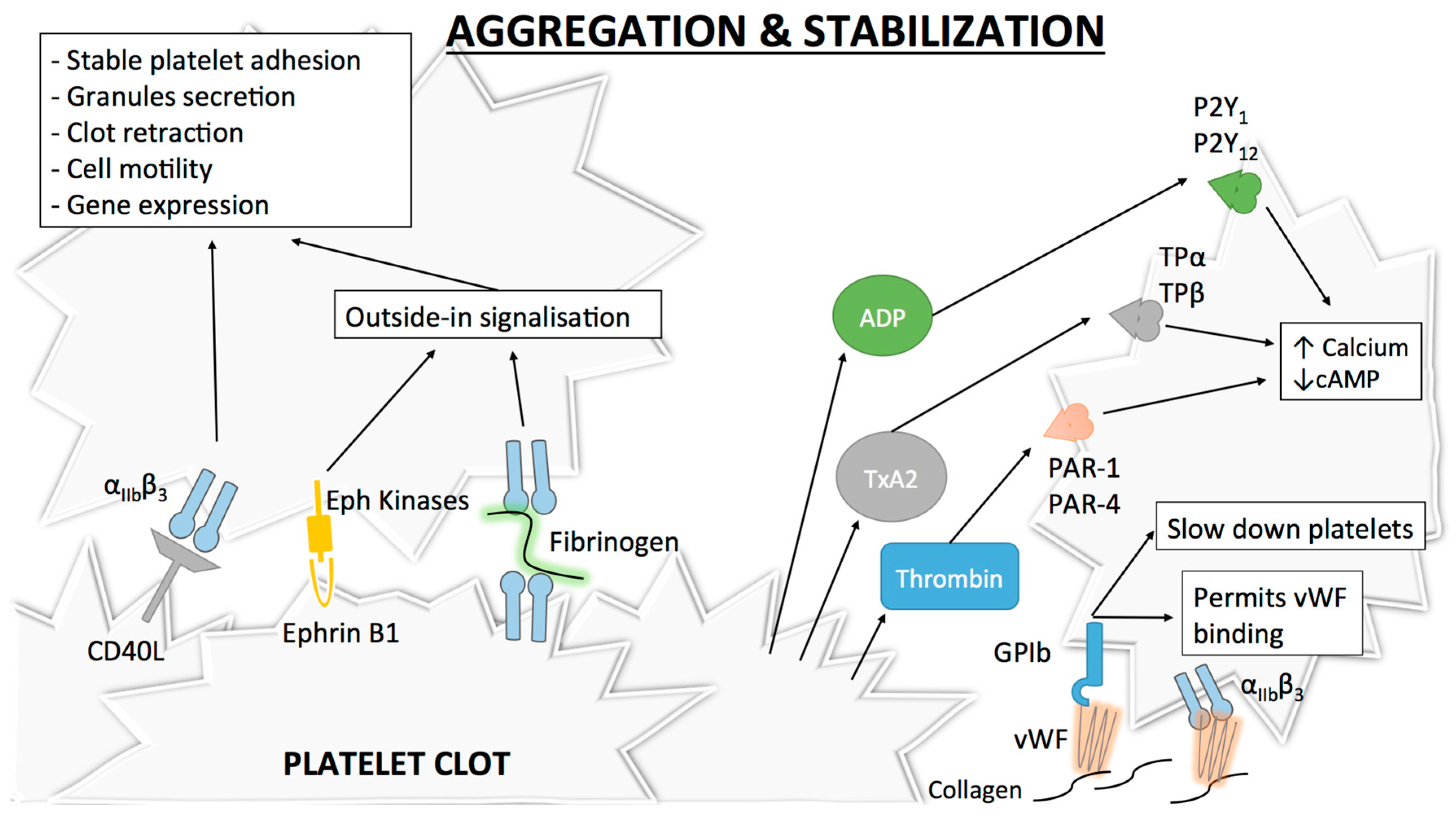
2.2. Platelet Aggregation, Thrombus Growth and Stabilization
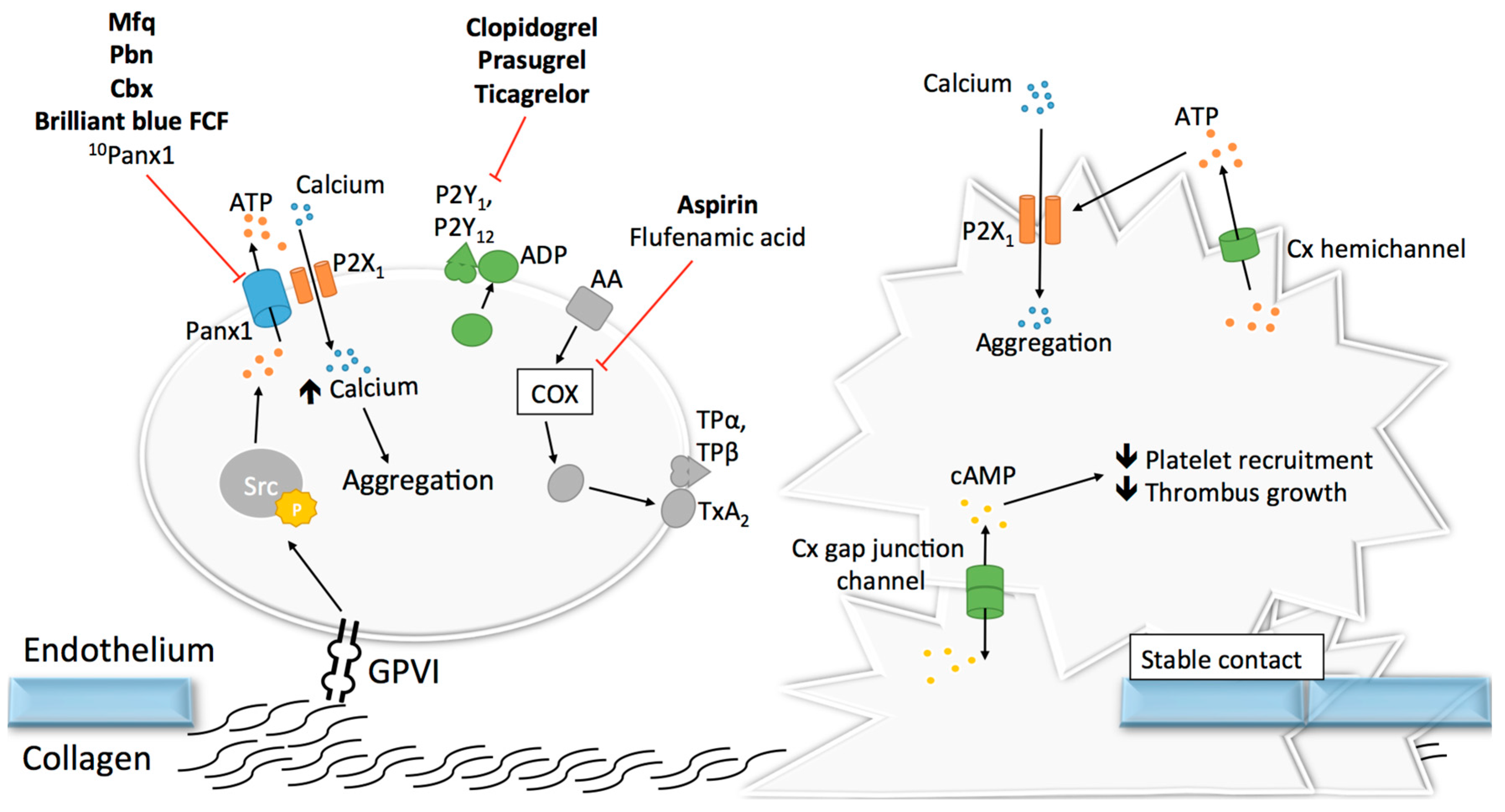
2.3. Towards New Antiplatelet Agents
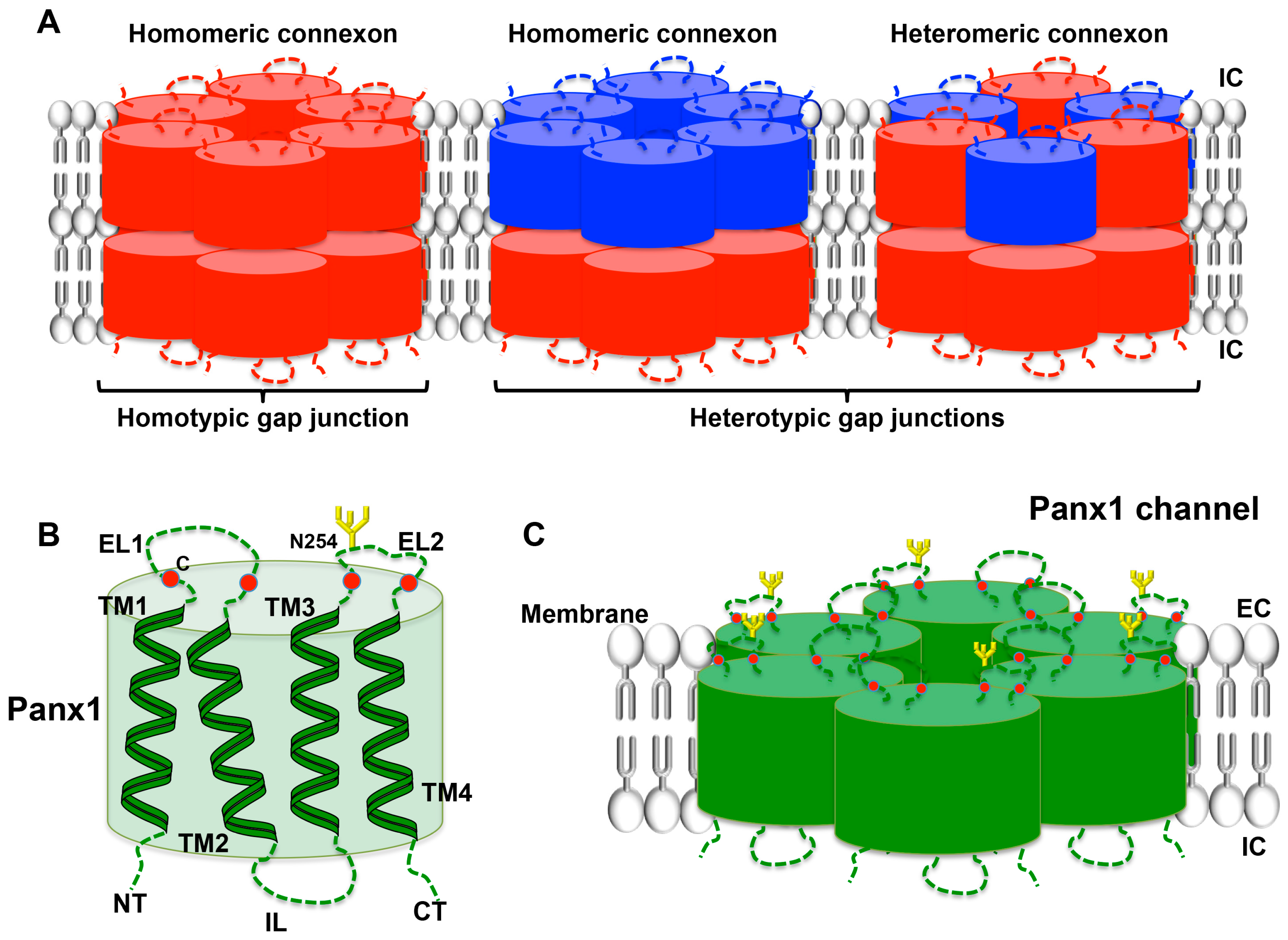
3. Connexins and Pannexins
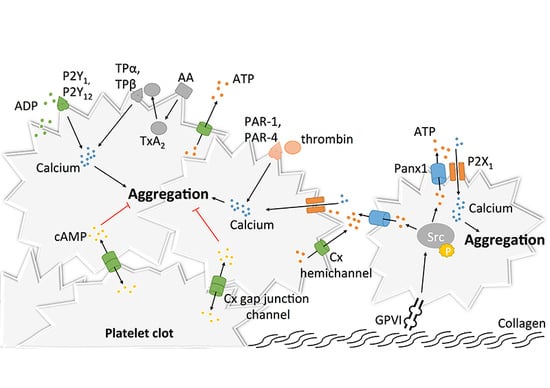
3.1. Pannexin1 and Platelet Aggregation
3.2. Connexins and Platelet Aggregation
4. “Drug-Ability” of Panx-Cx Targets
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMPA | α-Amino-3-hydroxyl-5-methyl-4-isoxazole-propionate |
| NMDA | N-methyl-d-aspartate |
| GP | Glycoprotein |
| vWF | Von Willebrand |
| PAR | Protease-activated receptor |
| cAMP | Cyclic adenosine monophosphate |
| FcR | Fc receptor |
| ITAM | Immunoreceptor tyrosine-based activation motif |
| Syk | Spleen tyrosine kinase |
| PLCγ2 | Phospholipase Cγ2 |
| PI3 | Phosphoinositide-3 |
| IP3 | Inositol 1,4,5-trisphosphate |
| DAG | Diacylglycerol |
| PKC | Protein kinase C |
| TxA2 | Thromboxane A2 |
| PIP3 | Phosphatidylinositol 3,4,5-trisphopsphate |
| CRP | Collagen-related peptide |
| MIDAS | Metal ion-dependent adhesion site |
| TF | Tissue factor |
| RIAM | Rap1-GTP-interacting adaptor molecule |
| ADP | Adenosine diphosphate |
| CD40L | CD40 ligand |
| SFK | Src family kinase |
| PI3-K | Phosphoinositide 3-Kinase |
| ATP | Adenosine trisphosphate |
| SLAM | Signaling lymphocyte activation molecule |
| Panx | Pannexin |
| Cx | Connexin |
| TM | Transmembrane |
| NT | Amino-terminal |
| CT | Carboxy-terminal |
| EL | Extracellular loop |
| IL | Intracellular loop |
| ER | Endoplasmic reticulum |
| Pbn | Probenecid |
| Cbx | Carbenoxolone |
| Mfq | Mefloquine |
| SNP | Single nucleotide polymorphism |
| ADRIE | Antiplatelet Drug Resistances and Ischemic Events |
References
- Kumar, V.; Abbas, A.K.; Aster, J.C.; Cotran, R.S.; Robbins, S.L. Robbins and Cotran Pathologic Basis of Disease (Robbins Pathology), 9th ed.; Elsevier: Amsterdam, The Netherlands, 2014; p. 1391. [Google Scholar]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Back, M.; Weber, C.; Lutgens, E. Regulation of atherosclerotic plaque inflammation. J. Intern. Med. 2015, 278, 462–482. [Google Scholar] [CrossRef] [PubMed]
- Lahoute, C.; Herbin, O.; Mallat, Z.; Tedgui, A. Adaptive immunity in atherosclerosis: Mechanisms and future therapeutic targets. Nat. Rev. Cardiol. 2011, 8, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Yla-Herttuala, S.; Bentzon, J.F.; Daemen, M.; Falk, E.; Garcia-Garcia, H.M.; Herrmann, J.; Hoefer, I.; Jauhiainen, S.; Jukema, J.W.; Krams, R.; et al. Stabilization of atherosclerotic plaques: An update. Eur. Heart J. 2013, 34, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- Newby, A.C. Metalloproteinases and vulnerable atherosclerotic plaques. Trends Cardiovasc. Med. 2007, 17, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Edfeldt, K.; Swedenborg, J.; Hansson, G.K.; Yan, Z.Q. Expression of toll-like receptors in human atherosclerotic lesions: A possible pathway for plaque activation. Circulation 2002, 105, 1158–1161. [Google Scholar] [PubMed]
- Berna-Erro, A.; Jardin, I.; Smani, T.; Rosado, J.A. Regulation of platelet function by Orai, STIM and TRP. Adv. Exp. Med. Biol. 2016, 898, 157–181. [Google Scholar] [PubMed]
- Mahaut-Smith, M.P. The unique contribution of ion channels to platelet and megakaryocyte function. J. Thromb. Haemost. 2012, 10, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Nuyttens, B.P.; Thijs, T.; Deckmyn, H.; Broos, K. Platelet adhesion to collagen. Thromb. Res. 2011, 127 (Suppl. S2), S26–S29. [Google Scholar] [CrossRef]
- Ruggeri, Z.M. Von willebrand factor: Looking back and looking forward. Thromb. Haemost. 2007, 98, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ulrichts, H.; Udvardy, M.; Lenting, P.J.; Pareyn, I.; Vandeputte, N.; Vanhoorelbeke, K.; Deckmyn, H. Shielding of the a1 domain by the D’D3 domains of von Willebrand factor modulates its interaction with platelet glycoprotein Ib-IX-V. J. Biol. Chem. 2006, 281, 4699–4707. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, Z.M.; Mendolicchio, G.L. Adhesion mechanisms in platelet function. Circ. Res. 2007, 100, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Brass, L.F. Thrombin and platelet activation. Chest 2003, 124, 18S–25S. [Google Scholar] [CrossRef] [PubMed]
- Gremmel, T.; Frelinger, A.L., 3rd; Michelson, A.D. Platelet physiology. Semin. Thromb. Hemost. 2016, 42, 191–204. [Google Scholar] [PubMed]
- Lawson, J.H.; Kalafatis, M.; Stram, S.; Mann, K.G. A model for the tissue factor pathway to thrombin. I. An empirical study. J. Biol. Chem. 1994, 269, 23357–23366. [Google Scholar] [PubMed]
- Kahn, M.L.; Nakanishi-Matsui, M.; Shapiro, M.J.; Ishihara, H.; Coughlin, S.R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Investig. 1999, 103, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.K.; Hung, D.T.; Wheaton, V.I.; Coughlin, S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 1991, 64, 1057–1068. [Google Scholar] [CrossRef]
- Miura, Y.; Takahashi, T.; Jung, S.M.; Moroi, M. Analysis of the interaction of platelet collagen receptor glycoprotein VI (GPVI) with collagen. A dimeric form of GPVI, but not the monomeric form, shows affinity to fibrous collagen. J. Biol. Chem. 2002, 277, 46197–46204. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Tao, L.; Lin, S.; Calingasan, N.Y.; Li, J.; Tandon, N.N.; Yoshitake, M.; Kambayashi, J. Expression of glycoprotein VI in vascular endothelial cells. Platelets 2003, 14, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Okuma, M.; Ushikubi, F.; Sensaki, S.; Kanaji, K.; Uchino, H. A novel platelet aggregating factor found in a patient with defective collagen-induced platelet aggregation and autoimmune thrombocytopenia. Blood 1987, 69, 1712–1720. [Google Scholar] [PubMed]
- Berlanga, O.; Tulasne, D.; Bori, T.; Snell, D.C.; Miura, Y.; Jung, S.; Moroi, M.; Frampton, J.; Watson, S.P. The fc receptor γ-chain is necessary and sufficient to initiate signaling through glycoprotein VI in transfected cells by the snake c-type lectin, convulxin. Eur. J. Biochem. 2002, 269, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Ezumi, Y.; Arai, M.; Takayama, H. A novel association of fc receptor γ-chain with glycoprotein VI and their co-expression as a collagen receptor in human platelets. J. Biol. Chem. 1997, 272, 23528–23531. [Google Scholar] [CrossRef] [PubMed]
- Quek, L.S.; Pasquet, J.M.; Hers, I.; Cornall, R.; Knight, G.; Barnes, M.; Hibbs, M.L.; Dunn, A.R.; Lowell, C.A.; Watson, S.P. Fyn and lyn phosphorylate the fc receptor γ chain downstream of glycoprotein VI in murine platelets, and lyn regulates a novel feedback pathway. Blood 2000, 96, 4246–4253. [Google Scholar] [PubMed]
- Moroi, M.; Jung, S.M. Platelet glycoprotein VI: Its structure and function. Thromb. Res. 2004, 114, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Reny, J.L.; Berdague, P.; Poncet, A.; Barazer, I.; Nolli, S.; Fabbro-Peray, P.; Schved, J.F.; Bounameaux, H.; Mach, F.; de Moerloose, P.; et al. Antiplatelet drug response status does not predict recurrent ischemic events in stable cardiovascular patients: Results of the antiplatelet drug resistances and ischemic events study. Circulation 2012, 125, 3201–3210. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.P.; Reep, B.; McConnell, R.T.; Lapetina, E.G. Collagen stimulates [3H]inositol trisphosphate formation in indomethacin-treated human platelets. Biochem. J. 1985, 226, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Rittenhouse, S.E.; Allen, C.L. Synergistic activation by collagen and 15-hydroxy-9α,11α-peroxidoprosta-5,13-dienoic acid (PGH2) of phosphatidylinositol metabolism and arachidonic acid release in human platelets. J. Clin. Investig. 1982, 70, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Brass, L.F.; Joseph, S.K. A role for inositol triphosphate in intracellular Ca2+ mobilization and granule secretion in platelets. J. Biol. Chem. 1985, 260, 15172–15179. [Google Scholar] [PubMed]
- Nishizuka, Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 1984, 308, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, D.; Theoret, J.F.; Villeneuve, L.; Abou-Saleh, H.; Mourad, W.; Allen, B.G.; Merhi, Y. Essential role of protein kinase Cδ in platelet signaling, α IIb β3 activation, and thromboxane A2 release. J. Biol. Chem. 2006, 281, 30024–30035. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, J.M.; Bobe, R.; Gross, B.; Gratacap, M.P.; Tomlinson, M.G.; Payrastre, B.; Watson, S.P. A collagen-related peptide regulates phospholipase cγ2 via phosphatidylinositol 3-kinase in human platelets. Biochem. J. 1999, 342 Pt 1, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Santoro, S.A. Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell 1986, 46, 913–920. [Google Scholar] [CrossRef]
- Kunicki, T.J.; Nugent, D.J.; Staats, S.J.; Orchekowski, R.P.; Wayner, E.A.; Carter, W.G. The human fibroblast class II extracellular matrix receptor mediates platelet adhesion to collagen and is identical to the platelet glycoprotein Ia-IIa complex. J. Biol. Chem. 1988, 263, 4516–4519. [Google Scholar] [PubMed]
- Sixma, J.J.; van Zanten, G.H.; Huizinga, E.G.; van der Plas, R.M.; Verkley, M.; Wu, Y.P.; Gros, P.; de Groot, P.G. Platelet adhesion to collagen: An update. Thromb. Haemost. 1997, 78, 434–438. [Google Scholar] [PubMed]
- Elices, M.J.; Hemler, M.E. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc. Natl. Acad. Sci. USA 1989, 86, 9906–9910. [Google Scholar] [CrossRef] [PubMed]
- Emsley, J.; Knight, C.G.; Farndale, R.W.; Barnes, M.J.; Liddington, R.C. Structural basis of collagen recognition by integrin α2β1. Cell 2000, 101, 47–56. [Google Scholar] [CrossRef]
- Calderwood, D.A.; Fujioka, Y.; de Pereda, J.M.; Garcia-Alvarez, B.; Nakamoto, T.; Margolis, B.; McGlade, C.J.; Liddington, R.C.; Ginsberg, M.H. Integrin β cytoplasmic domain interactions with phosphotyrosine-binding domains: A structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. USA 2003, 100, 2272–2277. [Google Scholar] [CrossRef] [PubMed]
- Banno, A.; Ginsberg, M.H. Integrin activation. Biochem. Soc. Trans. 2008, 36, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Wegener, K.L.; Partridge, A.W.; Han, J.; Pickford, A.R.; Liddington, R.C.; Ginsberg, M.H.; Campbell, I.D. Structural basis of integrin activation by talin. Cell 2007, 128, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, S.; Shattil, S.J.; Eto, K.; Tai, V.; Liddington, R.C.; de Pereda, J.M.; Ginsberg, M.H.; Calderwood, D.A. Talin binding to integrin β tails: A final common step in integrin activation. Science 2003, 302, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Moroi, M. Activation of the platelet collagen receptor integrin α2β1: Its mechanism and participation in the physiological functions of platelets. Trends Cardiovasc. Med. 2000, 10, 285–292. [Google Scholar] [CrossRef]
- Inoue, O.; Suzuki-Inoue, K.; Dean, W.L.; Frampton, J.; Watson, S.P. Integrin α2β1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCγ2. J. Cell Biol. 2003, 160, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Herr, A.B.; Farndale, R.W. Structural insights into the interactions between platelet receptors and fibrillar collagen. J. Biol. Chem. 2009, 284, 19781–19785. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Rieu, P.; Arnaout, M.A.; Liddington, R. Crystal structure of the A domain from the α subunit of integrin CR3 (CD11b/CD18). Cell 1995, 80, 631–638. [Google Scholar] [CrossRef]
- Kamiguti, A.S.; Hay, C.R.; Zuzel, M. Inhibition of collagen-induced platelet aggregation as the result of cleavage of α2β1-integrin by the snake venom metalloproteinase jararhagin. Biochem. J. 1996, 320 Pt 2, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S.; Markland, F.S.; Zhou, Q.; Laing, G.D.; Theakston, R.D.; Zuzel, M. Proteolytic cleavage of the β1 subunit of platelet α2β1 integrin by the metalloproteinase jararhagin compromises collagen-stimulated phosphorylation of pp72syk. J. Biol. Chem. 1997, 272, 32599–32605. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.P. Collagen receptor signaling in platelets and megakaryocytes. Thromb. Haemost. 1999, 82, 365–376. [Google Scholar] [PubMed]
- Knight, C.G.; Morton, L.F.; Onley, D.J.; Peachey, A.R.; Messent, A.J.; Smethurst, P.A.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. Identification in collagen type I of an integrin α2β1-binding site containing an essential ger sequence. J. Biol. Chem. 1998, 273, 33287–33294. [Google Scholar] [CrossRef] [PubMed]
- Rudd, C.E.; Janssen, O.; Cai, Y.C.; da Silva, A.J.; Raab, M.; Prasad, K.V. Two-step TCRζ/CD3-CD4 and CD28 signaling in T cells: SH2/SH3 domains, protein-tyrosine and lipid kinases. Immunol. Today 1994, 15, 225–234. [Google Scholar] [CrossRef]
- Farndale, R.W.; Sixma, J.J.; Barnes, M.J.; de Groot, P.G. The role of collagen in thrombosis and hemostasis. J. Thromb. Haemost. 2004, 2, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Coller, B.S.; Shattil, S.J. The GPIIb/IIIa (integrin αIIbβ3) odyssey: A technology-driven saga of a receptor with twists, turns, and even a bend. Blood 2008, 112, 3011–3025. [Google Scholar] [CrossRef] [PubMed]
- Shattil, S.J.; Kim, C.; Ginsberg, M.H. The final steps of integrin activation: The end game. Nat. Rev. Mol. Cell Biol. 2010, 11, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Savage, B.; Shattil, S.J.; Ruggeri, Z.M. Modulation of platelet function through adhesion receptors. A dual role for glycoprotein IIb-IIIa (integrin αIIbβ3) mediated by fibrinogen and glycoprotein Ib-von Willebrand factor. J. Biol. Chem. 1992, 267, 11300–11306. [Google Scholar] [PubMed]
- Wagner, C.L.; Mascelli, M.A.; Neblock, D.S.; Weisman, H.F.; Coller, B.S.; Jordan, R.E. Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood 1996, 88, 907–914. [Google Scholar] [PubMed]
- Shattil, S.J.; Kashiwagi, H.; Pampori, N. Integrin signaling: The platelet paradigm. Blood 1998, 91, 2645–2657. [Google Scholar] [PubMed]
- Weisel, J.W.; Nagaswami, C.; Vilaire, G.; Bennett, J.S. Examination of the platelet membrane glycoprotein IIb-IIIa complex and its interaction with fibrinogen and other ligands by electron microscopy. J. Biol. Chem. 1992, 267, 16637–16643. [Google Scholar] [PubMed]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Calderwood, D.A.; Ginsberg, M.H. Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 2000, 113 Pt 20, 3563–3571. [Google Scholar] [PubMed]
- Ma, Y.Q.; Qin, J.; Wu, C.; Plow, E.F. Kindlin-2 (Mig-2): A co-activator of β3 integrins. J. Cell Biol. 2008, 181, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.L. Linking rap to cell adhesion. Curr. Opin. Cell Biol. 2005, 17, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lim, C.J.; Puzon-McLaughlin, W.; Shattil, S.J.; Ginsberg, M.H. RIAM activates integrins by linking talin to ras GTPase membrane-targeting sequences. J. Biol. Chem. 2009, 284, 5119–5127. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowska-Wodnicka, M.; Smyth, S.S.; Schoenwaelder, S.M.; Fischer, T.H.; White, G.C., 2nd. Rap1b is required for normal platelet function and hemostasis in mice. J. Clin. Invest. 2005, 115, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Leisner, T.M.; Wencel-Drake, J.D.; Wang, W.; Lam, S.C. Bidirectional transmembrane modulation of integrin αIIbβ3 conformations. J. Biol. Chem. 1999, 274, 12945–12949. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, M.H.; Partridge, A.; Shattil, S.J. Integrin regulation. Curr. Opin. Cell Biol. 2005, 17, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Shattil, S.J.; Newman, P.J. Integrins: Dynamic scaffolds for adhesion and signaling in platelets. Blood 2004, 104, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Gong, H.; Shen, B.; Flevaris, P.; Chow, C.; Lam, S.C.; Voyno-Yasenetskaya, T.A.; Kozasa, T.; Du, X. G protein subunit Gα13 binds to integrin αIIbβ3 and mediates integrin “outside-in” signaling. Science 2010, 327, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.M.; Brugge, J.S. Cellular functions regulated by Src family kinases. Annu Rev. Cell Dev. Biol. 1997, 13, 513–609. [Google Scholar] [CrossRef] [PubMed]
- Law, D.A.; DeGuzman, F.R.; Heiser, P.; Ministri-Madrid, K.; Killeen, N.; Phillips, D.R. Integrin cytoplasmic tyrosine motif is required for outside-in αIIbβ3 signalling and platelet function. Nature 1999, 401, 808–811. [Google Scholar] [PubMed]
- Anthis, N.J.; Haling, J.R.; Oxley, C.L.; Memo, M.; Wegener, K.L.; Lim, C.J.; Ginsberg, M.H.; Campbell, I.D. β integrin tyrosine phosphorylation is a conserved mechanism for regulating talin-induced integrin activation. J. Biol. Chem. 2009, 284, 36700–36710. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Flevaris, P.; Stojanovic, A.; Chishti, A.; Phillips, D.R.; Lam, S.C.; Du, X. Tyrosine phosphorylation of the integrin β3 subunit regulates β3 cleavage by calpain. J. Biol. Chem. 2006, 281, 29426–29430. [Google Scholar] [CrossRef] [PubMed]
- Kunapuli, S.P.; Ding, Z.; Dorsam, R.T.; Kim, S.; Murugappan, S.; Quinton, T.M. Adp receptors—Targets for developing antithrombotic agents. Curr. Pharm. Des. 2003, 9, 2303–2316. [Google Scholar] [CrossRef] [PubMed]
- Arthur, W.T.; Petch, L.A.; Burridge, K. Integrin engagement suppresses rhoa activity via a c-Src-dependent mechanism. Curr. Biol. 2000, 10, 719–722. [Google Scholar] [CrossRef]
- Arthur, W.T.; Burridge, K. Rhoa inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell 2001, 12, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Obergfell, A.; Eto, K.; Mocsai, A.; Buensuceso, C.; Moores, S.L.; Brugge, J.S.; Lowell, C.A.; Shattil, S.J. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J. Cell Biol. 2002, 157, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Boylan, B.; Gao, C.; Rathore, V.; Gill, J.C.; Newman, D.K.; Newman, P.J. Identification of FcγRIIa as the ITAM-bearing receptor mediating αIIbβ3 outside-in integrin signaling in human platelets. Blood 2008, 112, 2780–2786. [Google Scholar] [CrossRef] [PubMed]
- Hechler, B.; Gachet, C. Purinergic receptors in thrombosis and inflammation. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Daniel, J.L.; Kunapuli, S.P. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J. Biol. Chem. 1998, 273, 2030–2034. [Google Scholar] [CrossRef] [PubMed]
- Gachet, C. Regulation of platelet functions by P2 receptors. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Hourani, S.M.; Cusack, N.J. Pharmacological receptors on blood platelets. Pharmacol. Rev. 1991, 43, 243–298. [Google Scholar] [PubMed]
- Ohlmann, P.; Laugwitz, K.L.; Nurnberg, B.; Spicher, K.; Schultz, G.; Cazenave, J.P.; Gachet, C. The human platelet ADP receptor activates GI2 proteins. Biochem. J. 1995, 312 Pt 3, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.Z.; Jin, J.; Kunapuli, S.P. Molecular mechanism of thromboxane A2-induced platelet aggregation. Essential role for P2TAC and α2A receptors. J. Biol. Chem. 1999, 274, 29108–29114. [Google Scholar] [CrossRef] [PubMed]
- Andre, P.; Prasad, K.S.; Denis, C.V.; He, M.; Papalia, J.M.; Hynes, R.O.; Phillips, D.R.; Wagner, D.D. CD40L stabilizes arterial thrombi by a β3 integrin—Dependent mechanism. Nat. Med. 2002, 8, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Prevost, N.; Woulfe, D.S.; Jiang, H.; Stalker, T.J.; Marchese, P.; Ruggeri, Z.M.; Brass, L.F. Eph kinases and ephrins support thrombus growth and stability by regulating integrin outside-in signaling in platelets. Proc. Natl. Acad. Sci. USA 2005, 102, 9820–9825. [Google Scholar] [CrossRef] [PubMed]
- Swieringa, F.; Kuijpers, M.J.; Heemskerk, J.W.; van der Meijden, P.E. Targeting platelet receptor function in thrombus formation: The risk of bleeding. Blood Rev. 2014, 28, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Mangin, P.; Loyau, S.; Hechler, B.; Billiald, P.; Gachet, C.; Jandrot-Perrus, M. The future of glycoprotein VI as an antithrombotic target. J. Thromb. Haemost. 2012, 10, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Molica, F.; Meens, M.J.; Morel, S.; Kwak, B.R. Mutations in cardiovascular connexin genes. Biol Cell. 2014, 106, 269–293. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.J.; Simek, J.; Laird, D.W. Mechanisms linking connexin mutations to human diseases. Cell Tissue Res. 2015, 360, 701–721. [Google Scholar] [CrossRef] [PubMed]
- Abascal, F.; Zardoya, R. Evolutionary analyses of gap junction protein families. Biochim. Biophys. Acta 2013, 1828, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W. Life cycle of connexins in health and disease. Biochem. J. 2006, 394, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Thevenin, A.F.; Kowal, T.J.; Fong, J.T.; Kells, R.M.; Fisher, C.G.; Falk, M.M. Proteins and mechanisms regulating gap-junction assembly, internalization, and degradation. Physiology 2013, 28, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Koval, M.; Molina, S.A.; Burt, J.M. Mix and match: Investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. FEBS Lett. 2014, 588, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Zhang, X.; Veenstra, R.D. Connexin hemichannel and pannexin channel electrophysiology: How do they differ? FEBS Lett. 2014, 588, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Saez, J.C.; Leybaert, L. Hunting for connexin hemichannels. FEBS Lett. 2014, 588, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.S.; Axelsen, L.N.; Sorgen, P.L.; Verma, V.; Delmar, M.; Holstein-Rathlou, N.H. Gap junctions. Compr. Physiol. 2012, 2, 1981–2035. [Google Scholar] [PubMed]
- Solan, J.L.; Lampe, P.D. Connexin43 phosphorylation: Structural changes and biological effects. Biochem. J. 2009, 419, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Begandt, D.; Good, M.E.; Keller, A.S.; DeLalio, L.J.; Rowley, C.; Isakson, B.E.; Figueroa, X.F. Pannexin channel and connexin hemichannel expression in vascular function and inflammation. BMC Cell. Biol. 2017, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Laird, D.W. The gap junction proteome and its relationship to disease. Trends Cell. Biol. 2010, 20, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Agullo-Pascual, E.; Cerrone, M.; Delmar, M. Arrhythmogenic cardiomyopathy and Brugada syndrome: Diseases of the connexome. FEBS Lett. 2014, 588, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Pfenniger, A.; Wohlwend, A.; Kwak, B.R. Mutations in connexin genes and disease. Eur. J. Clin. Investig. 2011, 41, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Panchin, Y.; Kelmanson, I.; Matz, M.; Lukyanov, K.; Usman, N.; Lukyanov, S. A ubiquitous family of putative gap junction molecules. Curr. Biol. 2000, 10, R473–R474. [Google Scholar] [CrossRef]
- Shestopalov, V.I.; Panchin, Y. Pannexins and gap junction protein diversity. Cell Mol. Life Sci. 2008, 65, 376–394. [Google Scholar] [CrossRef] [PubMed]
- Bond, S.R.; Naus, C.C. The pannexins: Past and present. Front. Physiol. 2014, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G.; Muller, K.J. Innexin and pannexin channels and their signaling. FEBS Lett. 2014, 588, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Penuela, S.; Harland, L.; Simek, J.; Laird, D.W. Pannexin channels and their links to human disease. Biochem. J. 2014, 461, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Boassa, D.; Ambrosi, C.; Qiu, F.; Dahl, G.; Gaietta, G.; Sosinsky, G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J. Biol. Chem. 2007, 282, 31733–31743. [Google Scholar] [CrossRef] [PubMed]
- Esseltine, J.L.; Laird, D.W. Next-generation connexin and pannexin cell biology. Trends Cell. Biol. 2016, 26, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, R.; Hormuzdi, S.G.; Barbe, M.T.; Herb, A.; Monyer, H. Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13644–13649. [Google Scholar] [CrossRef] [PubMed]
- Sosinsky, G.E.; Boassa, D.; Dermietzel, R.; Duffy, H.S.; Laird, D.W.; MacVicar, B.; Naus, C.C.; Penuela, S.; Scemes, E.; Spray, D.C.; et al. Pannexin channels are not gap junction hemichannels. Channels 2011, 5, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Penuela, S.; Simek, J.; Thompson, R.J. Regulation of pannexin channels by post-translational modifications. FEBS Lett. 2014, 588, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Locovei, S.; Wang, J.; Dahl, G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006, 580, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Scemes, E. Nature of plasmalemmal functional “hemichannels”. Biochim. Biophys. Acta 2012, 1818, 1880–1883. [Google Scholar] [CrossRef] [PubMed]
- Kunapuli, S.P.; Dorsam, R.T.; Kim, S.; Quinton, T.M. Platelet purinergic receptors. Curr. Opin. Pharmacol. 2003, 3, 175–180. [Google Scholar] [CrossRef]
- Adamson, S.E.; Leitinger, N. The role of pannexin1 in the induction and resolution of inflammation. FEBS Lett. 2014, 588, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Meens, M.J.; Kwak, B.R.; Duffy, H.S. Role of connexins and pannexins in cardiovascular physiology. Cell Mol. Life Sci. 2015, 72, 2779–2792. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.J. Pannexin channels and ischaemia. J. Physiol. 2015, 593, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.K.; Berndt, M.C. Platelet physiology and thrombosis. Thromb. Res. 2004, 114, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, J.M. Platelet adhesion signalling and the regulation of thrombus formation. J. Cell Sci. 2004, 117, 3415–3425. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.A.; Wright, J.R.; Vial, C.; Evans, R.J.; Mahaut-Smith, M.P. Amplification of human platelet activation by surface pannexin-1 channels. J. Thromb. Haemost. 2014, 12, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Molica, F.; Morel, S.; Meens, M.J.; Denis, J.F.; Bradfield, P.F.; Penuela, S.; Zufferey, A.; Monyer, H.; Imhof, B.A.; Chanson, M.; et al. Functional role of a polymorphism in the pannexin1 gene in collagen-induced platelet aggregation. Thromb. Haemost. 2015, 114, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jackson, D.G.; Dahl, G. The food dye FD&C Blue No. 1 is a selective inhibitor of the ATP release channel Panx1. J. Gen. Physiol. 2013, 141, 649–656. [Google Scholar] [PubMed]
- Molica, F.; Nolli, S.; Fontana, P.; Kwak, B.R. Turbidimetry on human washed platelets: The effect of the pannexin1-inhibitor Brilliant Blue FCF on collagen-induced aggregation. J. Vis. Exp. 2017, in press. [Google Scholar]
- Stierlin, F.B.; Molica, F.; Reny, J.L.; Kwak, B.R.; Fontana, P. Pannexin1 single nucleotide polymorphism and platelet reactivity in a cohort of cardiovascular patients. Cell Commun. Adhes. 2017, 23, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Naus, C.C.; Giaume, C. Bridging the gap to therapeutic strategies based on connexin/pannexin biology. J. Transl. Med. 2016, 14, 330. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Kaissi, S.; Homaidan, F.R.; Naim, H.Y.; El-Sabban, M.E. Cross-talk between intestinal epithelial cells and immune cells in inflammatory bowel disease. Sci. Rep. 2016, 6, 29783. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Christen, T.; Roth, I.; Chadjichristos, C.E.; Derouette, J.P.; Foglia, B.F.; Chanson, M.; Goodenough, D.A.; Kwak, B.R. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat. Med. 2006, 12, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Kameritsch, P.; Pogoda, K.; Pohl, U. Channel-independent influence of connexin 43 on cell migration. Biochim. Biophys. Acta 2012, 1818, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.R. Gap junctions between cells of bone marrow: An ultrastructural study using tannic acid. Anat. Rec. 1980, 196, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.R. Ultrastructural studies of intercellular contacts (junctions) in bone marrow: A review. Scan. Electron. Microsc. 1986, 621–629. [Google Scholar]
- Allen, T.D.; Dexter, T.M. Ultrastructural aspects of erythropoietic differentiation in long-term bone marrow culture. Differentiation 1982, 21, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Vaiyapuri, S.; Jones, C.I.; Sasikumar, P.; Moraes, L.A.; Munger, S.J.; Wright, J.R.; Ali, M.S.; Sage, T.; Kaiser, W.J.; Tucker, K.L.; et al. Gap junctions and connexin hemichannels underpin hemostasis and thrombosis. Circulation 2012, 125, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Angelillo-Scherrer, A.; Fontana, P.; Burnier, L.; Roth, I.; Sugamele, R.; Brisset, A.; Morel, S.; Nolli, S.; Sutter, E.; Chassot, A.; et al. Connexin 37 limits thrombus propensity by downregulating platelet reactivity. Circulation 2011, 124, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Vaiyapuri, S.; Moraes, L.A.; Sage, T.; Ali, M.S.; Lewis, K.R.; Mahaut-Smith, M.P.; Oviedo-Orta, E.; Simon, A.M.; Gibbins, J.M. Connexin40 regulates platelet function. Nat. Commun. 2013, 4, 2564. [Google Scholar] [CrossRef] [PubMed]
- Derouette, J.P.; Desplantez, T.; Wong, C.W.; Roth, I.; Kwak, B.R.; Weingart, R. Functional differences between human Cx37 polymorphic hemichannels. J. Mol. Cell Cardiol. 2009, 46, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.A. Results of the caprie trial: Efficacy and safety of clopidogrel. Clopidogrel versus aspirin in patients at risk of ischaemic events. Vasc. Med. 1998, 3, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Pina, A.E.; Tapia-Alvarez, G.R.; Reyes-Raminrez, A.; Navarrete, A. Carbenoxolone gastroprotective mechanism: Participation of nitric oxide/cGMP/KATP pathway in ethanol-induced gastric injury in the rat. Fundam. Clin. Pharmacol. 2011, 25, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Furlow, P.W.; Zhang, S.; Soong, T.D.; Halberg, N.; Goodarzi, H.; Mangrum, C.; Wu, Y.G.; Elemento, O.; Tavazoie, S.F. Mechanosensitive pannexin-1 channels mediate microvascular metastatic cell survival. Nat. Cell Biol. 2015, 17, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.J.; Rhodes, J.; Calcraft, B.J. Complications of carbenoxolone therapy. Br. Med. J. 1974, 3, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Mitrou, N.; Braam, B.; Cupples, W.A. A gap junction inhibitor, carbenoxolone, induces spatiotemporal dispersion of renal cortical perfusion and impairs autoregulation. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H582–H591. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, R.; Barbe, M.T.; Jakob, N.J.; Monyer, H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J. Neurochem. 2005, 92, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Balaramnavar, V.M.; Hohlfeld, T.; Saxena, A.K. Drug/drug interaction of common nsaids with antiplatelet effect of aspirin in human platelets. Eur. J. Pharmacol. 2013, 721, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, G.; Nitecki, S.; Harty, G.J.; Camilleri, M.; Szurszewski, J.H. The effect of flufenamic acid on gastrointestinal myoelectrical activity and transit time in dogs. Gut 1998, 42, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Price, R.N.; Nosten, F.; Luxemburger, C.; van Vugt, M.; Phaipun, L.; Chongsuphajaisiddhi, T.; White, N.J. Artesunate/mefloquine treatment of multi-drug resistant falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 574–577. [Google Scholar] [CrossRef]
- Dahl, G.; Qiu, F.; Wang, J. The bizarre pharmacology of the ATP release channel pannexin1. Neuropharmacology 2013, 75, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Connellan, J.M.; Deacon, S.; Thurlow, P.J. Changes in platelet function and reactivity induced by quinine in relation to quinine (drug) induced immune thrombocytopenia. Thromb. Res. 1991, 61, 501–514. [Google Scholar] [CrossRef]
- Ritchie, E.C.; Block, J.; Nevin, R.L. Psychiatric side effects of mefloquine: Applications to forensic psychiatry. J. Am. Acad. Psychiatry Law 2013, 41, 224–235. [Google Scholar] [PubMed]
- Cunningham, R.F.; Israili, Z.H.; Dayton, P.G. Clinical pharmacokinetics of probenecid. Clin. Pharmacokinet 1981, 6, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Packham, M.A.; Rand, M.L.; Perry, D.W.; Ruben, D.H.; Kinlough-Rathbone, R.L. Probenecid inhibits platelet responses to aggregating agents in vitro and has a synergistic inhibitory effect with penicillin G. Thromb. Haemost. 1996, 76, 239–244. [Google Scholar] [PubMed]
- Sjolinder, M.; Tornhamre, S.; Claesson, H.E.; Hydman, J.; Lindgren, J. Characterization of a leukotriene C4 export mechanism in human platelets: Possible involvement of multidrug resistance-associated protein 1. J. Lipid Res. 1999, 40, 439–446. [Google Scholar] [PubMed]
- Ozaki, Y.; Matsumoto, Y.; Yatomi, Y.; Higashihara, M.; Kariya, T.; Shoji, K. Effects of five anion channel blockers on thrombin- and ionomycin-activated platelet functions. Biochem. Pharmacol. 1989, 38, 2147–2152. [Google Scholar] [PubMed]
- Raisch, D.W.; Straight, T.M.; Holodniy, M. Thrombocytopenia from combination treatment with oseltamivir and probenecid: Case report, medwatch data summary, and review of the literature. Pharmacotherapy 2009, 29, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Poornima, V.; Madhupriya, M.; Kootar, S.; Sujatha, G.; Kumar, A.; Bera, A.K. P2X7 receptor-pannexin 1 hemichannel association: Effect of extracellular calcium on membrane permeabilization. J. Mol. Neurosci. 2012, 46, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Boumechache, M.; Robinson, L.E.; Marschall, V.; Gorecki, D.C.; Masin, M.; Murrell-Lagnado, R.D. Splice variants of the P2X7 receptor reveal differential agonist dependence and functional coupling with pannexin-1. J. Cell Sci. 2012, 125, 3776–3789. [Google Scholar] [CrossRef] [PubMed]
- Gettings, S.D.; Blaszcak, D.L.; Roddy, M.T.; Curry, A.S.; McEwen, G.N., Jr. Evaluation of the cumulative (repeated application) eye irritation and corneal staining potential of FD&C Yellow No. 5, FD&C Blue No. 1 and FD&C Blue No. 1 aluminium lake. Food Chem. Toxicol. 1992, 30, 1051–1055. [Google Scholar] [PubMed]
- Borzelleca, J.F.; Depukat, K.; Hallagan, J.B. Lifetime toxicity/carcinogenicity studies of FD&C Blue No. 1 (Brilliant Blue FCF) in rats and mice. Food Chem. Toxicol. 1990, 28, 221–234. [Google Scholar] [PubMed]
- Pelegrin, P.; Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, M.; Locovei, S.; Keane, R.W.; Dahl, G. Modulation of membrane channel currents by gap junction protein mimetic peptides: Size matters. Am. J. Physiol. Cell Physiol. 2007, 293, C1112–C1119. [Google Scholar] [CrossRef] [PubMed]
- Weilinger, N.L.; Lohman, A.W.; Rakai, B.D.; Ma, E.M.; Bialecki, J.; Maslieieva, V.; Rilea, T.; Bandet, M.V.; Ikuta, N.T.; Scott, L.; et al. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat. Neurosci. 2016, 19, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Billaud, M.; Chiu, Y.H.; Lohman, A.W.; Parpaite, T.; Butcher, J.T.; Mutchler, S.M.; DeLalio, L.J.; Artamonov, M.V.; Sandilos, J.K.; Best, A.K.; et al. A molecular signature in the pannexin1 intracellular loop confers channel activation by the α1 adrenoreceptor in smooth muscle cells. Sci. Signal. 2015, 8, ra17. [Google Scholar] [CrossRef] [PubMed]
- Lohman, A.W.; Leskov, I.L.; Butcher, J.T.; Johnstone, S.R.; Stokes, T.A.; Begandt, D.; DeLalio, L.J.; Best, A.K.; Penuela, S.; Leitinger, N.; et al. Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nat. Commun. 2015, 6, 7965. [Google Scholar] [CrossRef] [PubMed]
| Compound’s Name | Clinical Applications | Effects on Platelet Aggregation | Possible Side Effects |
|---|---|---|---|
| Carbenoxolone | Gastric ulcer [139] | Reduces collagen-induced aggregation [122] | Sodium retention, hypokalaemia [141] |
| Flufenamic acid | NA 1 | Reduces aggregation via COX inhibition [144] | Aspirin antagonism [144], GI 2 disturbances [145] |
| Mefloquine | Malaria [146] | Reduces collagen-induced aggregation [123] | Psychiatric diseases [149] |
| Probenecid | Gout; Adjuvant for antibiotics [150] | Inhibits aggregation [122,123,152,153] | Thrombocytopenia [154] |
| Brilliant blue FCF | NA | Reduces collagen-induced aggregation [125] | NA |
| 10Panx1 peptide | NA | Reduces collagen-induced aggregation [123] | NA |
| TAT-Panx1308 | NA | NA | NA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molica, F.; Stierlin, F.B.; Fontana, P.; Kwak, B.R. Pannexin- and Connexin-Mediated Intercellular Communication in Platelet Function. Int. J. Mol. Sci. 2017, 18, 850. https://doi.org/10.3390/ijms18040850
Molica F, Stierlin FB, Fontana P, Kwak BR. Pannexin- and Connexin-Mediated Intercellular Communication in Platelet Function. International Journal of Molecular Sciences. 2017; 18(4):850. https://doi.org/10.3390/ijms18040850
Chicago/Turabian StyleMolica, Filippo, Florian B. Stierlin, Pierre Fontana, and Brenda R. Kwak. 2017. "Pannexin- and Connexin-Mediated Intercellular Communication in Platelet Function" International Journal of Molecular Sciences 18, no. 4: 850. https://doi.org/10.3390/ijms18040850