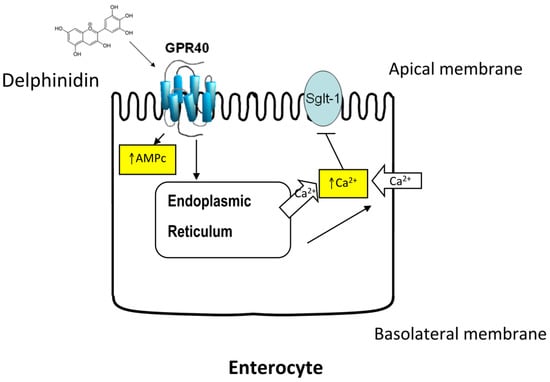
Delphinidin Reduces Glucose Uptake in Mice Jejunal Tissue and Human Intestinal Cells Lines through FFA1/GPR40
Abstract
:1. Introduction
2. Results
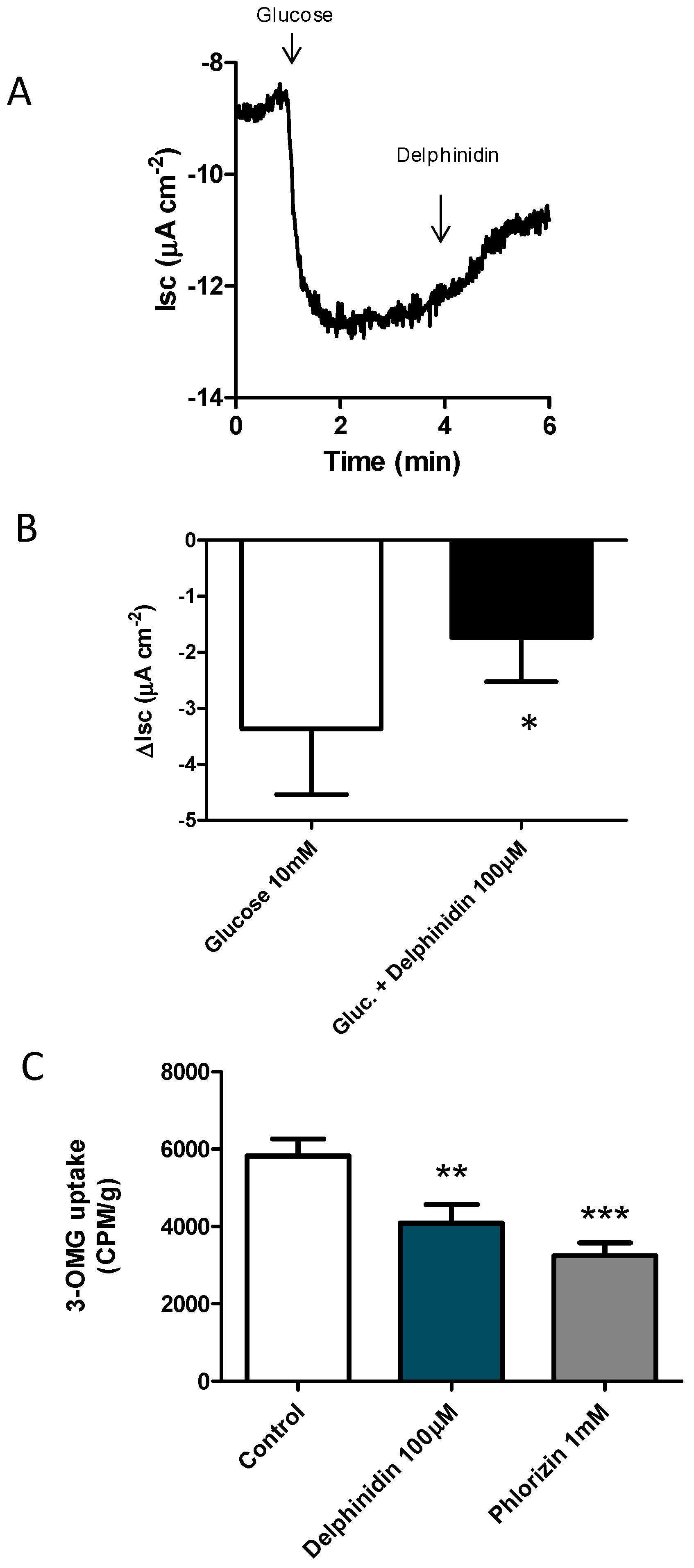
2.1. Delphinidin Inhibits Glucose Absorption in Mouse Jejunal
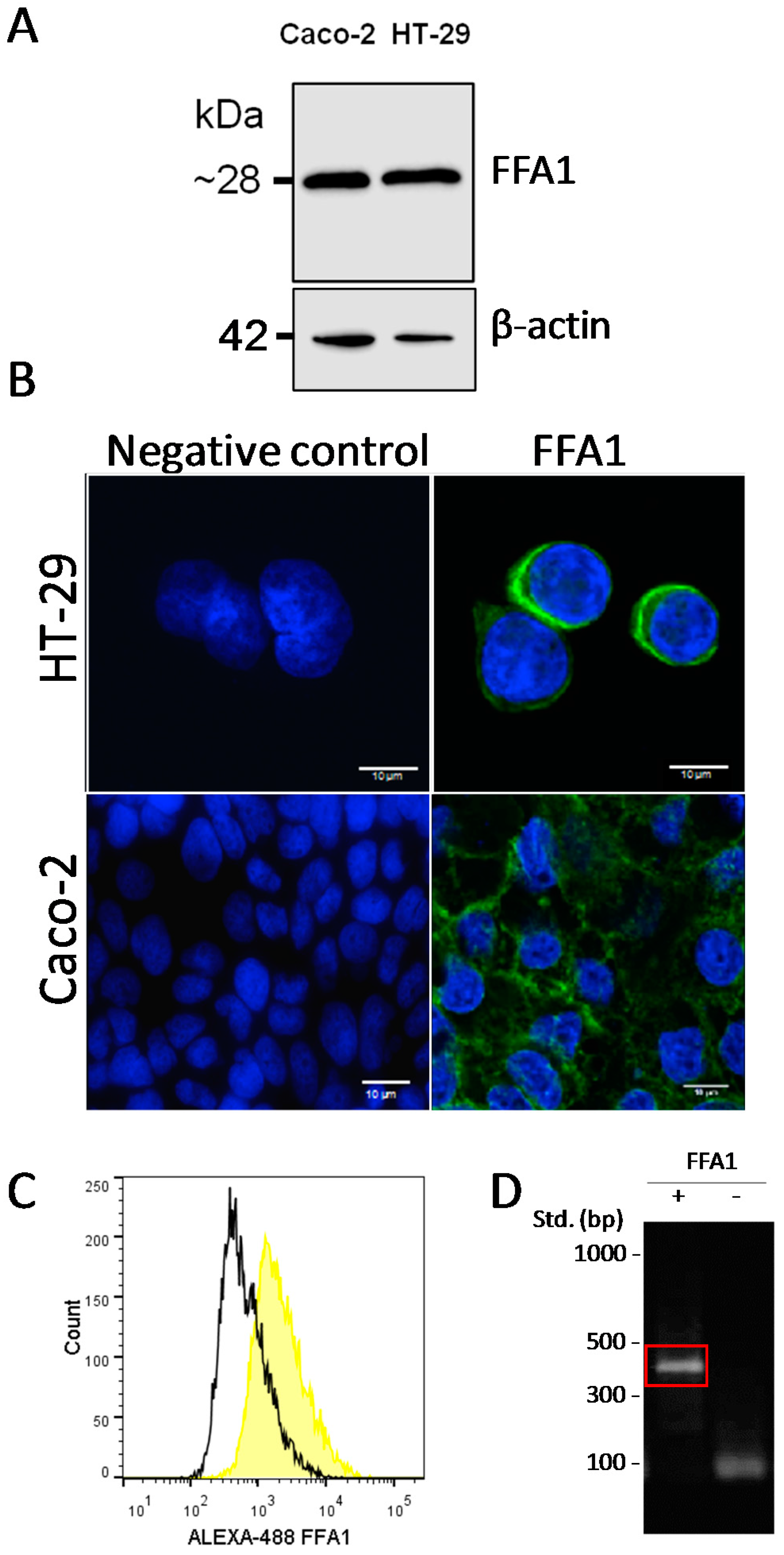
2.2. Presence of FFA1 in Caco-2 and HT-29
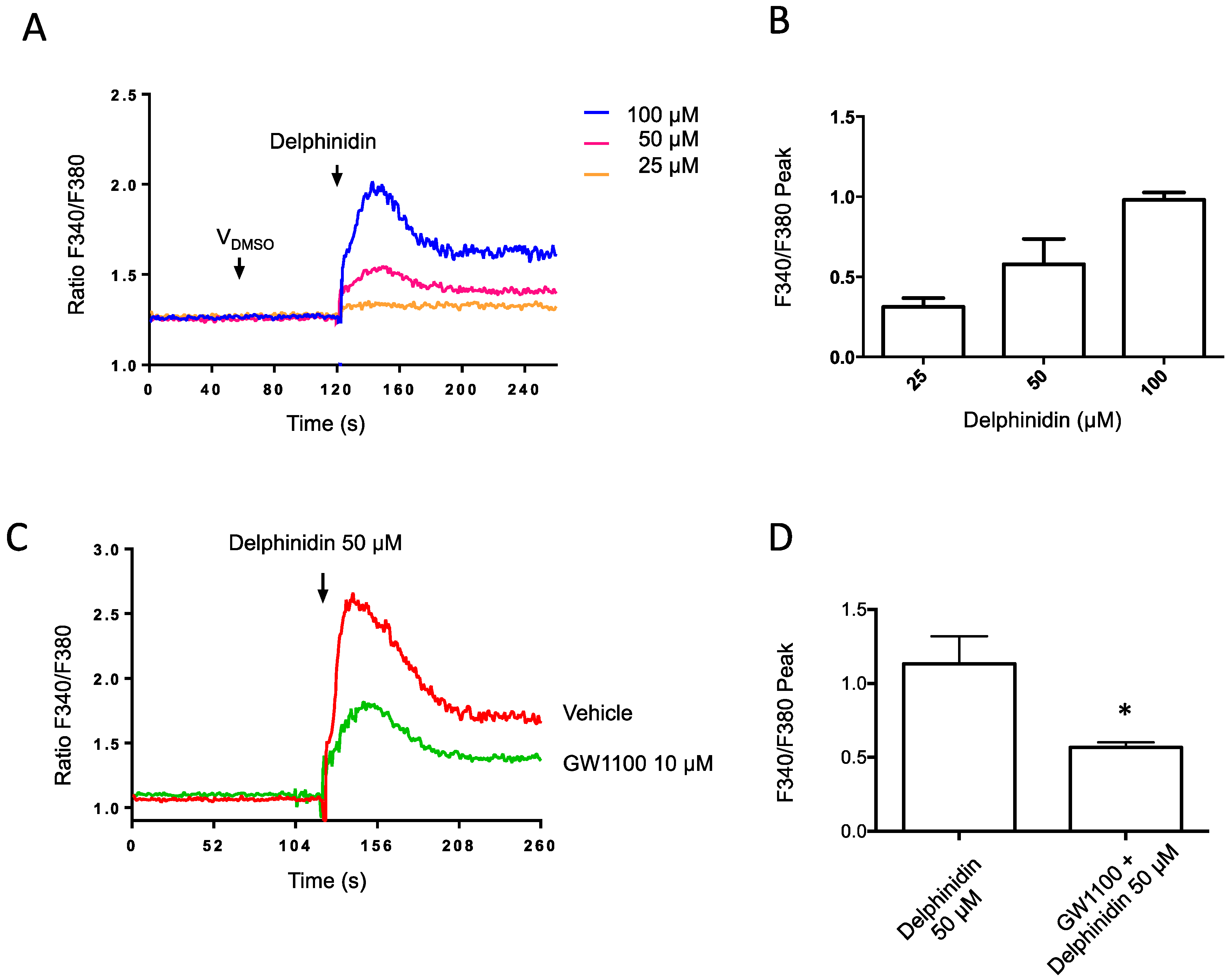
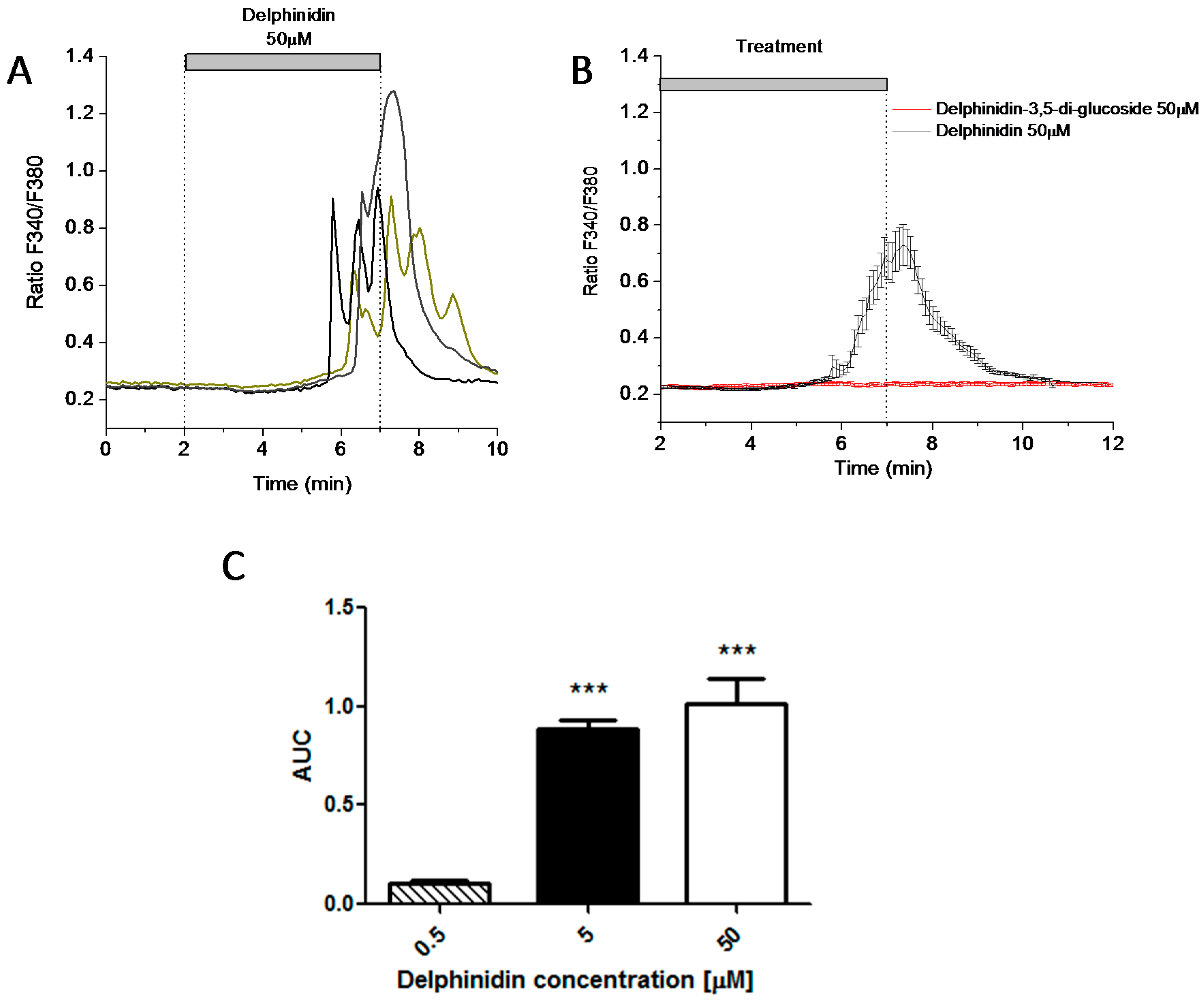
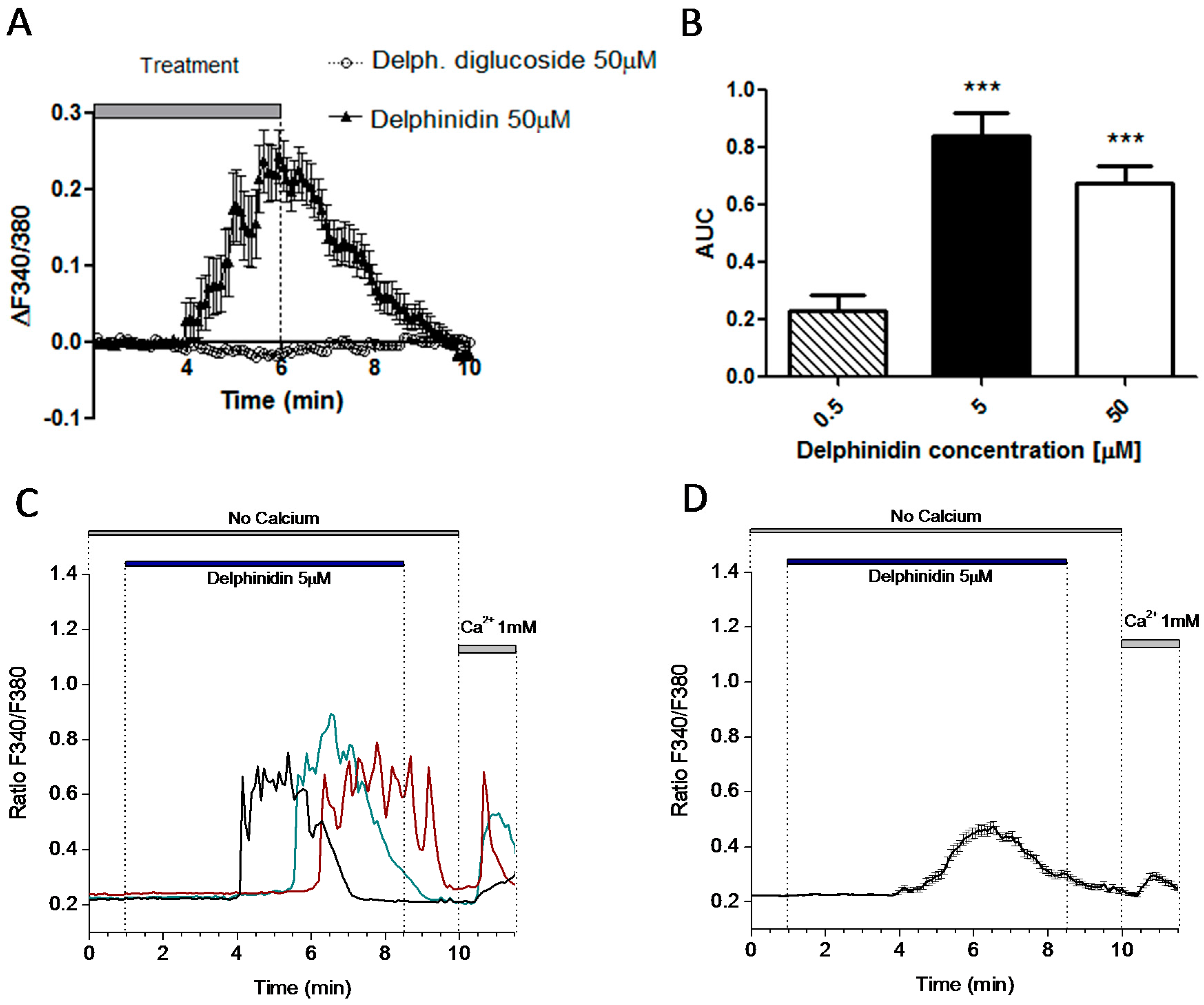
2.3. Delphinidin Induces Intracellular Calcium Release in HT-29 Cells via FFA1
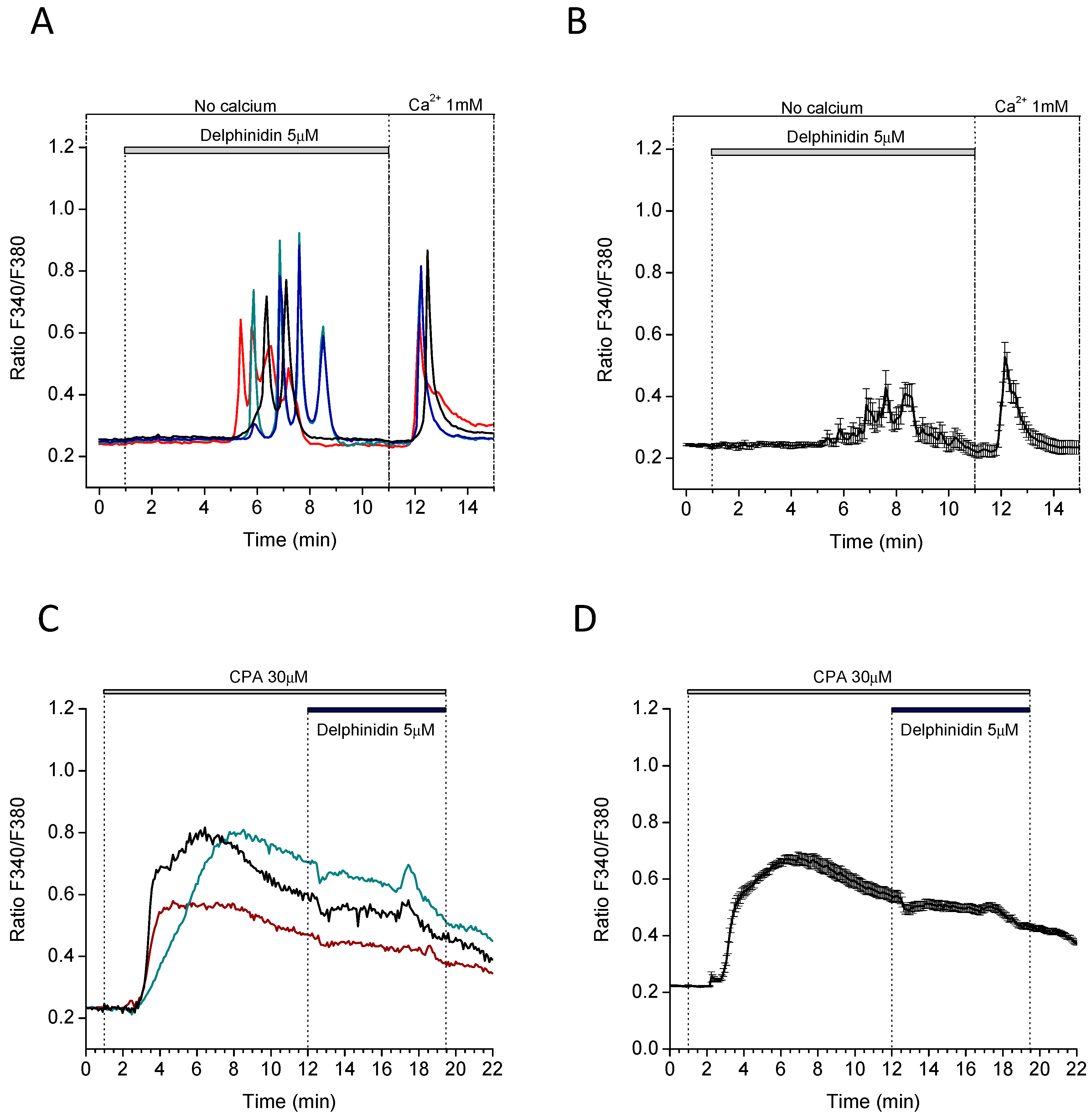
2.4. Delphinidin Induces Intracellular Calcium Oscilations in HT-29 Cells in a Ca2+ Store-Dependent Manner
2.5. Delphinidin Induces Intracellular Calcium Oscilations via FFA1 in Colon Normal Cells
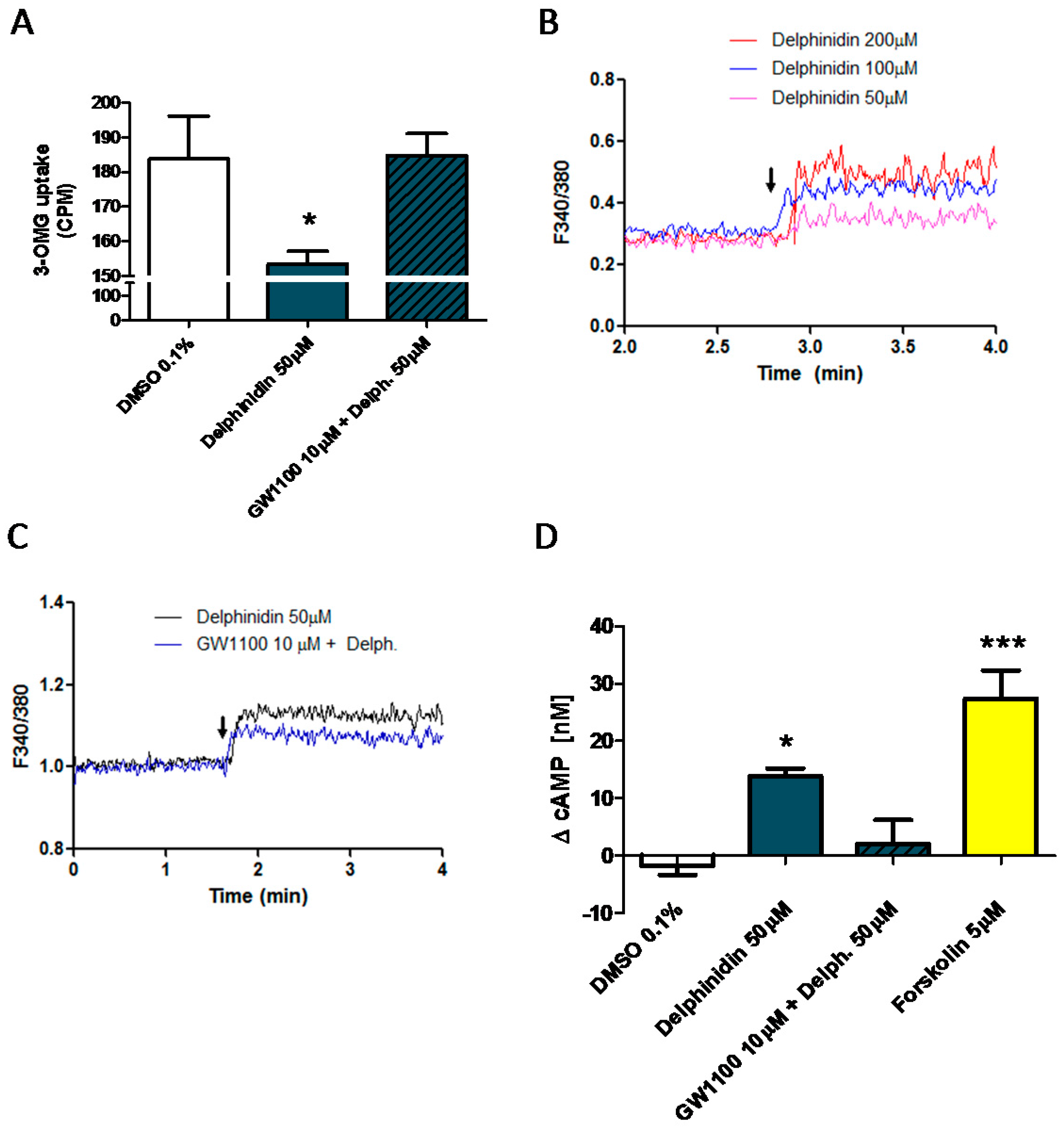
2.6. Delphinidin Induces Intracellular Calcium Release, cAMP, and Glucose Uptake via FFA1
3. Discussion
4. Materials and Methods
4.1. Ussing Chamber Experiments
4.2. Flow Cytometry Analysis of FFA1 Expression
4.3. Glucose Uptake Experiments—Tissue
4.4. Glucose Uptake Experiments—Cultured Cells
4.5. Western Blot
4.6. Immunofluorescence
4.7. Cytosolic Ca2+ Monitoring in Cell Suspensions
4.8. qRT-PCR of FFA1 in HT-29 Cells
4.9. Cytosolic Ca2+ Imaging in Adhered Cells
4.10. cAMP Measurements
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SGLT1 | Sodium-glucose cotransporter1 |
| DM2 | Diabetes mellitus type 2 |
| FFA1 | Free fatty acid receptor 1 |
| 3-OMG | 3-O-methyl-glucose [3H] |
References
- Marin-Penalver, J.J.; Martin-Timon, I.; Sevillano-Collantes, C.; Del Canizo-Gomez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Mynett, K.J.; Page, T. Importance of the intestine as a site of metformin-stimulated glucose utilization. Br. J. Pharmacol. 1994, 112, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Sakar, Y.; Meddah, B.; Faouzi, M.A.; Cherrah, Y.; Bado, A.; Ducroc, R. Metformin-induced regulation of the intestinal d-glucose transporters. J. Physiol. Pharmacol. 2010, 61, 301–307. [Google Scholar] [PubMed]
- Bailey, C.J.; Wilcock, C.; Scarpello, J.H. Metformin and the intestine. Diabetologia 2008, 51, 1552–1553. [Google Scholar] [CrossRef] [PubMed]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, B.; Tan, J.; Jiang, J.; Li, D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016, 70, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Torronen, R.; Kolehmainen, M.; Sarkkinen, E.; Poutanen, K.; Mykkanen, H.; Niskanen, L. Berries reduce postprandial insulin responses to wheat and rye breads in healthy women. J. Nutr. 2013, 143, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; van Dam, R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. 2005, 53, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.H.; Lila, M.A.; Raskin, I. In Vitro and in Vivo Anti-Diabetic Effects of Anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem. 2012, 131, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- McGhie, T.K.; Walton, M.C. The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007, 51, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Dreiseitel, A.; Oosterhuis, B.; Vukman, K.V.; Schreier, P.; Oehme, A.; Locher, S.; Hajak, G.; Sand, P.G. Berry anthocyanins and anthocyanidins exhibit distinct affinities for the efflux transporters BCRP and MDR1. Br. J. Pharmacol. 2009, 158, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Tani, T.; Terahara, N.; Tsuda, T. The Anthocyanin Delphinidin 3-Rutinoside Stimulates Glucagon-Like Peptide-1 Secretion in Murine GLUTag Cell Line via the Ca2+/Calmodulin-Dependent Kinase II Pathway. PLoS ONE 2015, 10, e0126157. [Google Scholar]
- Hoggard, N.; Cruickshank, M.; Moar, K.M.; Bestwick, C.; Holst, J.J.; Russell, W.; Horgan, G. A single supplement of a standardised bilberry (Vaccinium myrtillus L.) extract (36% wet weight anthocyanins) modifies glycaemic response in individuals with type 2 diabetes controlled by diet and lifestyle. J. Nutr. Sci. 2013, 2, e22. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Andriambeloson, E.; Takeda, K.; Andriantsitohaina, R. Red wine polyphenols increase calcium in bovine aortic endothelial cells: A basis to elucidate signalling pathways leading to nitric oxide production. Br. J. Pharmacol. 2002, 135, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Jara, E.; Hidalgo, M.A.; Hancke, J.L.; Hidalgo, A.I.; Brauchi, S.; Nunez, L.; Villalobos, C.; Burgos, R.A. Delphinidin activates NFAT and induces IL-2 production through SOCE in T cells. Cell Biochem. Biophys. 2014, 68, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Farshchi, A.; Nikfar, S.; Seyedifar, M. Effect of chromium on glucose and lipid profiles in patients with type 2 diabetes; a meta-analysis review of randomized trials. J. Pharm. Pharm. Sci. 2013, 16, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tuo, B.; Dong, H. Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Hyson, D.A.; Thomson, A.B.; Kappagoda, C.T. Calcium channel blockers modify jejunal uptake of d-galactose in rabbits. Dig. Dis. Sci. 1996, 41, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Maekawa, F.; Yada, T. Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet beta-cells: Mediation by PLC and L-type Ca2+ channel and link to insulin release. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E670–E677. [Google Scholar] [CrossRef] [PubMed]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; Zucco, F.; Felsani, A. Good Caco-2 cell culture practices. Toxicol. In Vitro 2012, 26, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Manzano, S.; Williamson, G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Mol. Nutr. Food Res. 2010, 54, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Kottra, G.; Daniel, H. Flavonoid glycosides are not transported by the human Na+/glucose transporter when expressed in Xenopus laevis oocytes, but effectively inhibit electrogenic glucose uptake. J. Pharmacol. Exp. Ther. 2007, 322, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, C.P.; Tadayyon, M.; Andrews, J.L.; Benson, W.G.; Chambers, J.K.; Eilert, M.M.; Ellis, C.; Elshourbagy, N.A.; Goetz, A.S.; Minnick, D.T.; et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 2003, 278, 11303–11311. [Google Scholar] [CrossRef] [PubMed]
- Edfalk, S.; Steneberg, P.; Edlund, H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 2008, 57, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Kashihara, D.; Ichimura, A.; Kimura, I.; Tsujimoto, G.; Hirasawa, A. Role of free fatty acid receptors in the regulation of energy metabolism. Biochim. Biophys. Acta 2014, 1841, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, H.; Tsujihata, Y.; Takeuchi, K.; Hazama, M.; Johnson, P.R.; Rorsman, P. The effects of TAK-875, a selective G protein-coupled receptor 40/free fatty acid 1 agonist, on insulin and glucagon secretion in isolated rat and human islets. J. Pharmacol. Exp. Ther. 2012, 340, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.W.; Kuhre, R.E.; Janus, C.; Svendsen, B.; Holst, J.J. Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 secretion from isolated perfused rat small intestine. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, P.; Fritz, N. Biochemistry of calcium oscillations. Biochem. Biophys. Res. Commun. 2010, 396, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Dadsetan, S.; Zakharova, L.; Molinski, T.F.; Fomina, A.F. Store-operated Ca2+ influx causes Ca2+ release from the intracellular Ca2+ channels that is required for T cell activation. J. Biol. Chem. 2008, 283, 12512–12519. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Cavia Mdel, M.; Alonso-Torre, S.R. Oleic acid inhibits store-operated calcium entry in human colorectal adenocarcinoma cells. Eur. J. Nutr. 2012, 51, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Sobradillo, D.; Hernandez-Morales, M.; Ubierna, D.; Moyer, M.P.; Nunez, L.; Villalobos, C. A reciprocal shift in transient receptor potential channel 1 (TRPC1) and stromal interaction molecule 2 (STIM2) contributes to Ca2+ remodeling and cancer hallmarks in colorectal carcinoma cells. J. Biol. Chem. 2014, 289, 28765–28782. [Google Scholar] [CrossRef] [PubMed]
- Alzaid, F.; Cheung, H.M.; Preedy, V.R.; Sharp, P.A. Regulation of glucose transporter expression in human intestinal Caco-2 cells following exposure to an anthocyanin-rich berry extract. PLoS ONE 2013, 8, e78932. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Ogi, K.; Hosoya, M.; Tanaka, Y.; Uejima, H.; et al. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Lefkimmiatis, K.; Moyer, M.P.; Curci, S.; Hofer, A.M. The ω-3 fatty acid eicosapentaenoic acid elicits cAMP generation in colonic epithelial cells via a “store-operated” mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G715–G722. [Google Scholar] [CrossRef] [PubMed]
- Hauge, M.; Vestmar, M.A.; Husted, A.S.; Ekberg, J.P.; Wright, M.J.; Di Salvo, J.; Weinglass, A.B.; Engelstoft, M.S.; Madsen, A.N.; Luckmann, M.; et al. GPR40 (FFAR1)—Combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol. Metab. 2015, 4, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Hauge, M.; Ekberg, J.P.; Engelstoft, M.S.; Timshel, P.; Madsen, A.N.; Schwartz, T.W. Gq and Gs signaling acting in synergy to control GLP-1 secretion. Mol. Cell Endocrinol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mace, O.J.; Schindler, M.; Patel, S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J. Physiol. 2012, 590, 2917–2936. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, K.P.; Park, S.J.; Kang, S.; Huang, J.; Lee, J.M.; Sato, K.; Chung, H.Y.; Okajima, F.; Im, D.S. ω-3 fatty acids induce Ca2+ mobilization responses in human colon epithelial cell lines endogenously expressing FFA4. Acta Pharmacol. Sin. 2015, 36, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, J.L.; Leschot, A.; Olivera-Nappa, A.; Salgado, A.M.; Rioseco, H.; Lyon, C.; Vigil, P. Delphinidin-Rich Maqui Berry Extract (Delphinol(R)) Lowers Fasting and Postprandial Glycemia and Insulinemia in Prediabetic Individuals during Oral Glucose Tolerance Tests. Biomed. Res. Int. 2016, 2016, 9070537. [Google Scholar] [CrossRef] [PubMed]
- Castro-Acosta, M.L.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Berries and anthocyanins: Promising functional food ingredients with postprandial glycaemia-lowering effects. Proc. Nutr. Soc. 2016, 75, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. J. Nutr. Biochem. 2016, 38, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Torronen, R.; Sarkkinen, E.; Niskanen, T.; Tapola, N.; Kilpi, K.; Niskanen, L. Postprandial glucose, insulin and glucagon-like peptide 1 responses to sucrose ingested with berries in healthy subjects. Br. J. Nutr. 2012, 107, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Fatima, J.; Iqbal, C.W.; Houghton, S.G.; Kasparek, M.S.; Duenes, J.A.; Zheng, Y.; Sarr, M.G. Hexose transporter expression and function in mouse small intestine: Role of diurnal rhythm. J. Gastrointest. Surg. 2009, 13, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, J.; Flores, C.; Hidalgo, M.A.; Perez, M.; Yanez, A.; Quinones, L.; Caceres, D.D.; Burgos, R.A. Delphinol(R) standardized maqui berry extract reduces postprandial blood glucose increase in individuals with impaired glucose regulation by novel mechanism of sodium glucose cotransporter inhibition. Panminerva Med. 2014, 56, 1–7. [Google Scholar] [PubMed]
- Gouyon, F.; Caillaud, L.; Carriere, V.; Klein, C.; Dalet, V.; Citadelle, D.; Kellett, G.L.; Thorens, B.; Leturque, A.; Brot-Laroche, E. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: A study in GLUT2-null mice. J. Physiol. 2003, 552 Pt 3, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Debnam, E.S.; Grimble, G.K. Methods for assessing intestinal absorptive function in relation to enteral nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Yamashita, S.; Konishi, K.; Yamazaki, Y.; Taki, Y.; Sakane, T.; Sezaki, H.; Furuyama, Y. New and better protocols for a short-term Caco-2 cell culture system. J. Pharm. Sci. 2002, 91, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Nunez, L.; Valero, R.A.; Senovilla, L.; Sanz-Blasco, S.; Garcia-Sancho, J.; Villalobos, C. Cell proliferation depends on mitochondrial Ca2+ uptake: Inhibition by salicylate. J. Physiol. 2006, 571 Pt 1, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Lew, M. Good statistical practice in pharmacology. Problem 2. Br. J. Pharmacol. 2007, 152, 299–303. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, J.; Teuber, S.; Morera, F.J.; Ojeda, C.; Flores, C.A.; Hidalgo, M.A.; Núñez, L.; Villalobos, C.; Burgos, R.A. Delphinidin Reduces Glucose Uptake in Mice Jejunal Tissue and Human Intestinal Cells Lines through FFA1/GPR40. Int. J. Mol. Sci. 2017, 18, 750. https://doi.org/10.3390/ijms18040750
Hidalgo J, Teuber S, Morera FJ, Ojeda C, Flores CA, Hidalgo MA, Núñez L, Villalobos C, Burgos RA. Delphinidin Reduces Glucose Uptake in Mice Jejunal Tissue and Human Intestinal Cells Lines through FFA1/GPR40. International Journal of Molecular Sciences. 2017; 18(4):750. https://doi.org/10.3390/ijms18040750
Chicago/Turabian StyleHidalgo, Jorge, Stefanie Teuber, Francisco J. Morera, Camila Ojeda, Carlos A. Flores, María A. Hidalgo, Lucía Núñez, Carlos Villalobos, and Rafael A. Burgos. 2017. "Delphinidin Reduces Glucose Uptake in Mice Jejunal Tissue and Human Intestinal Cells Lines through FFA1/GPR40" International Journal of Molecular Sciences 18, no. 4: 750. https://doi.org/10.3390/ijms18040750