A Comparison of the Effects of Benzalkonium Chloride on Ocular Surfaces between C57BL/6 and BALB/c Mice
Abstract
:1. Introduction
2. Results
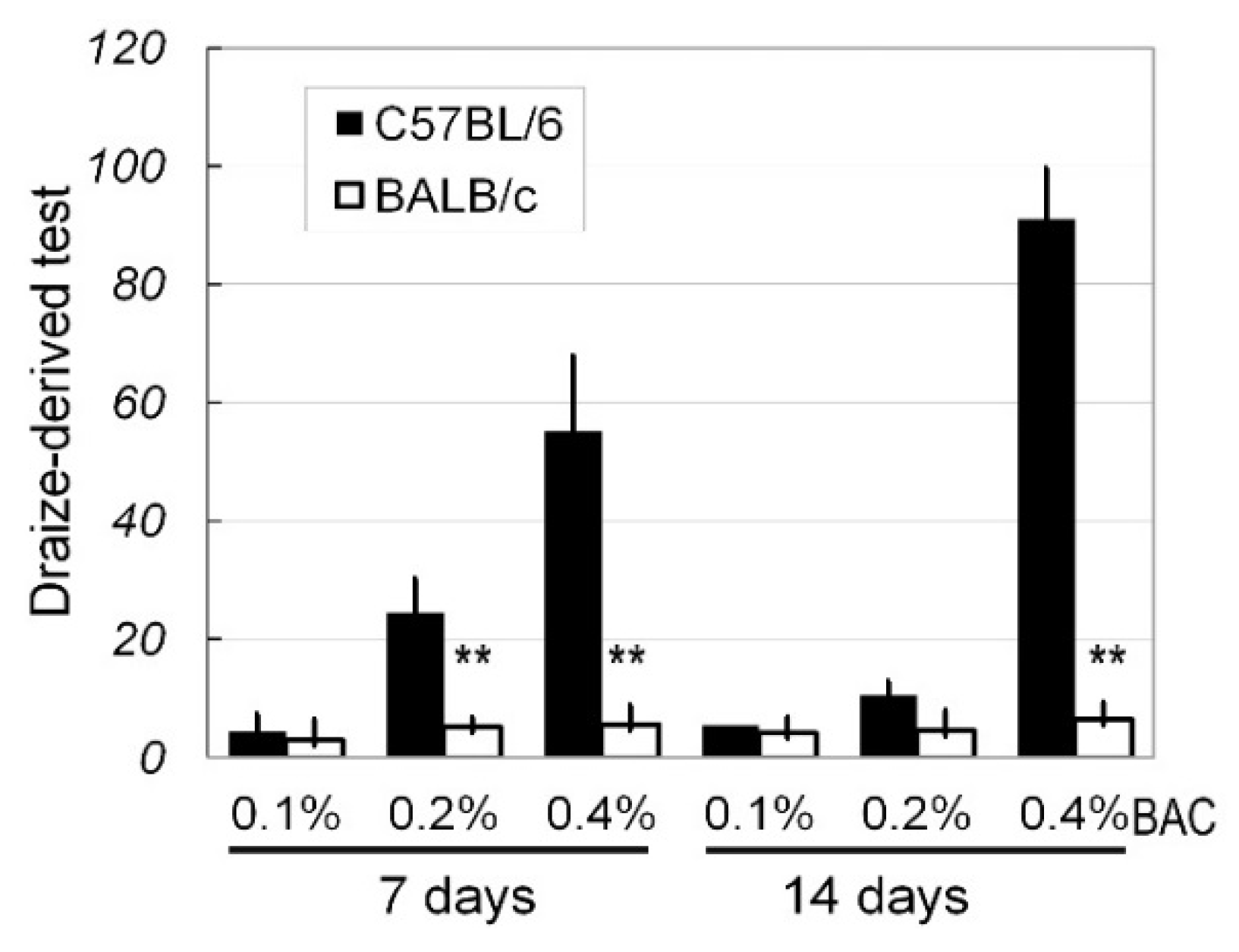
2.1. In Vivo Ocular Surface Manifestation in Two Strains of Mice
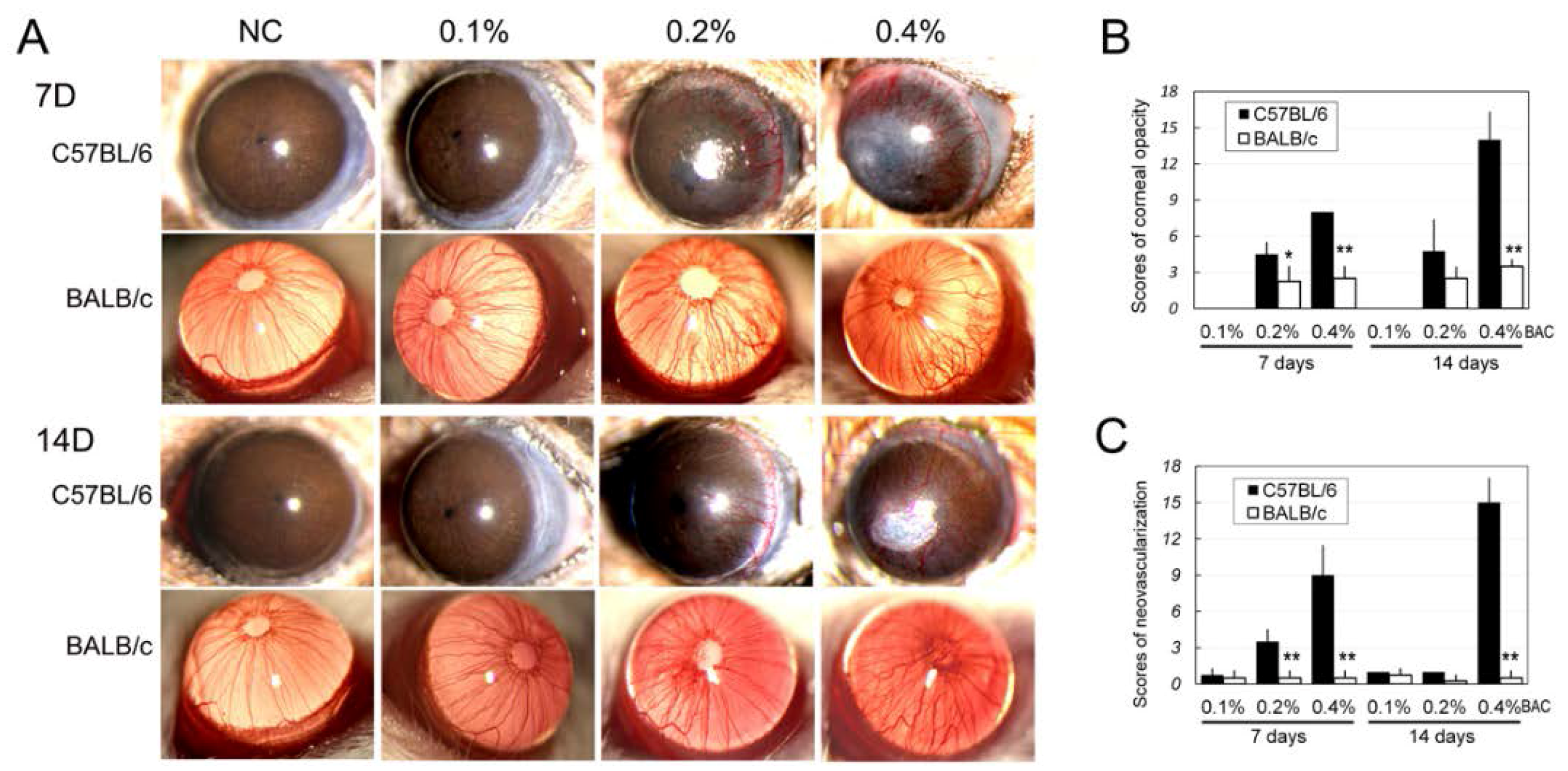
2.2. Corneal Edema, Opacity and Neovascularization
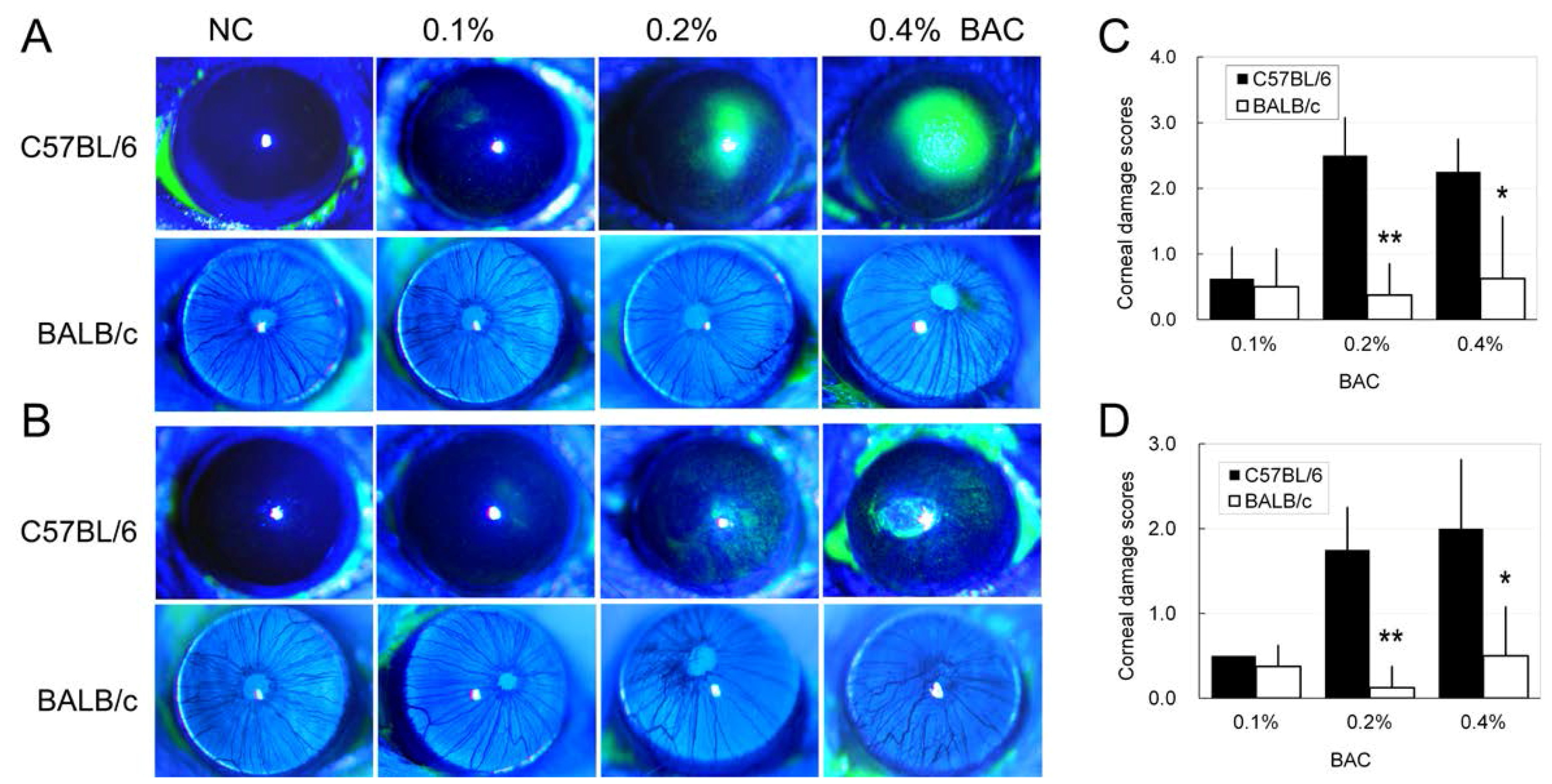
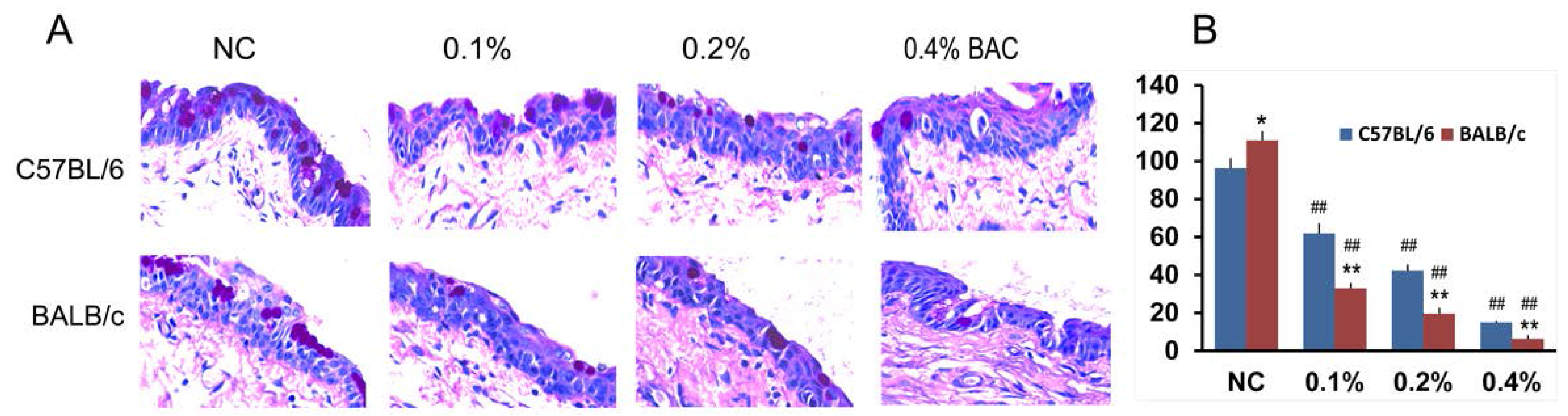
2.3. Disruption of the Corneal Epithelium Induced by Benzalkonium Chloride (BAC) Eye Drops
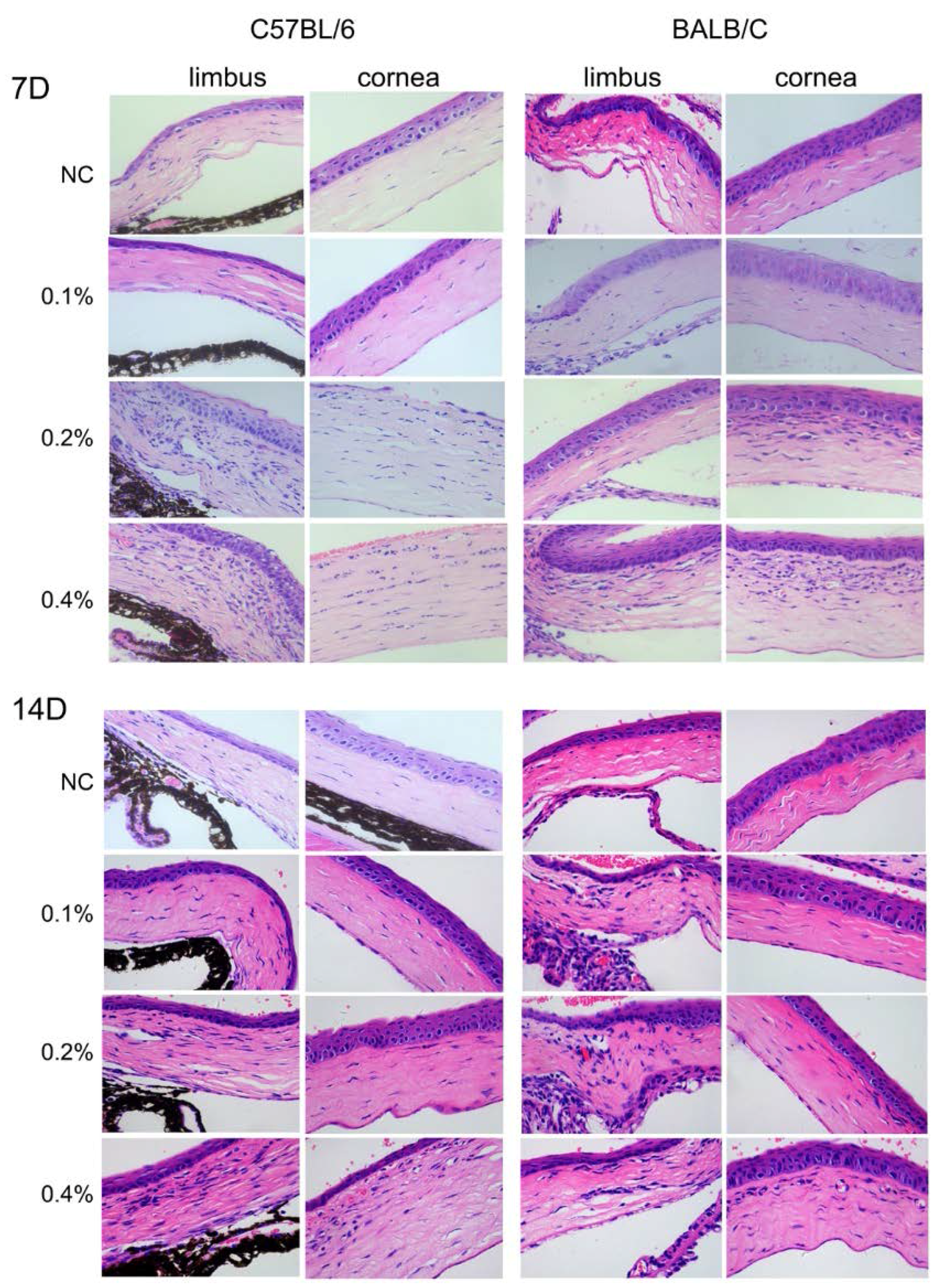
2.4. Histological Changes in the Cornea after BAC Treatment
2.5. Goblet Cell Changes in Conjunctiva Treated with BAC
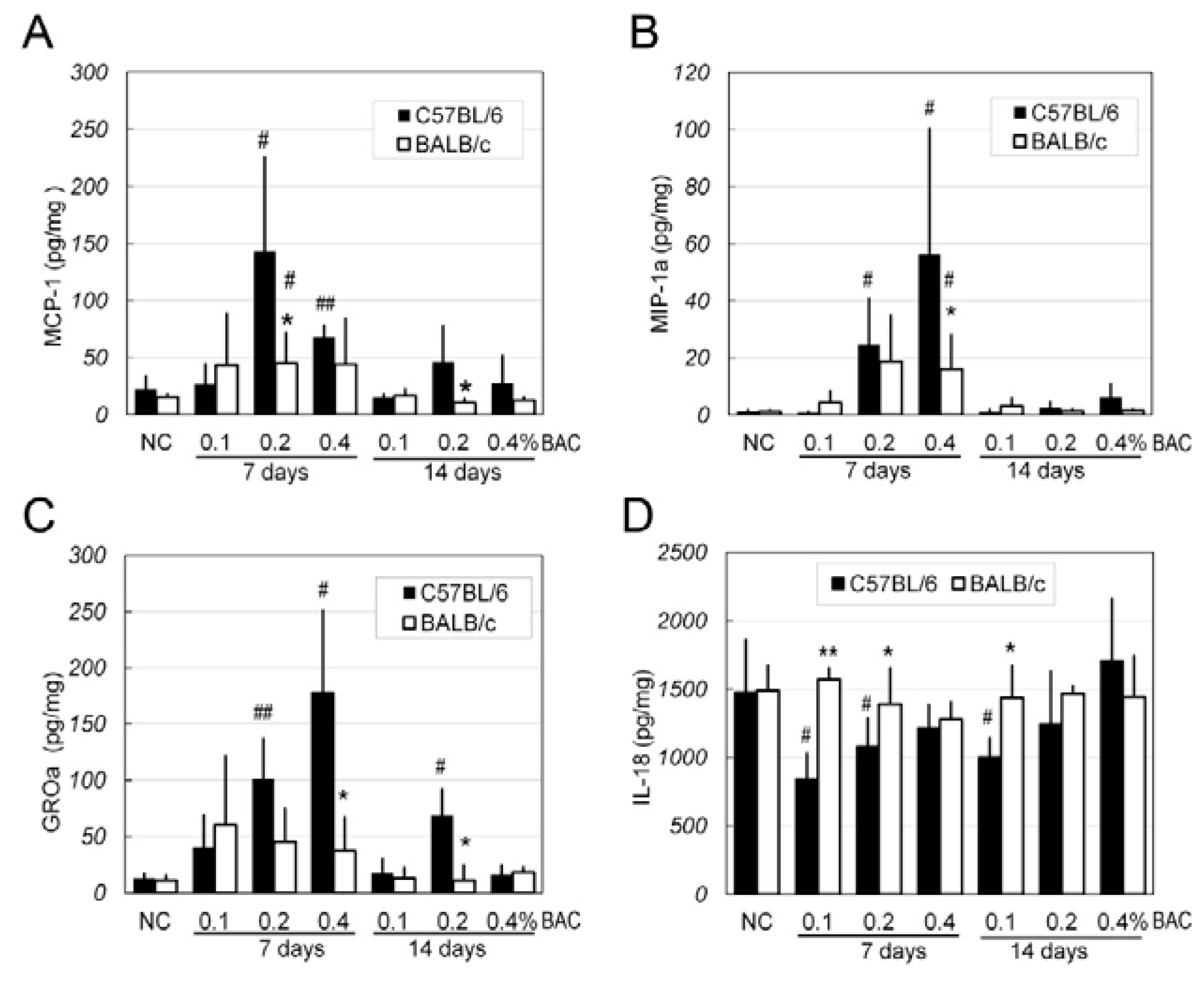
2.6. Changes of Inflammatory Factors in the Corneas after BAC Irritation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. BAC Treatment
4.3. Ocular Surface Alterations and the Draize Test
4.4. Measurement of Corneal Neovascularization and Opacity
4.5. Evaluation of Fluorescein Staining
4.6. Histologic Analysis and Assessment of Conjunctival Goblet Cells
4.7. Detection of Corneal Inflammatory Factors
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baudouin, C.; Labbe, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef] [PubMed]
- Pisella, P.J.; Debbasch, C.; Hamard, P.; Creuzot-Garcher, C.; Rat, P.; Brignole, F.; Baudouin, C. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: An ex vivo and in vitro study. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1360–1368. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jung, J.C.; Jung, S.Y.; Yu, S.; Lee, K.W.; Park, Y.J. Comparison of the Efficacy of Fluorometholone With and Without Benzalkonium Chloride in Ocular Surface Disease. Cornea 2016, 35, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Song, Y.; Luan, S.; Wan, P.; Li, N.; Tang, J.; Han, Y.; Xiong, C.; Wang, Z. Research on the stability of a rabbit dry eye model induced by topical application of the preservative benzalkonium chloride. PLoS ONE 2012, 7, e33688. [Google Scholar]
- Pauly, A.; Brignole-Baudouin, F.; Labbe, A.; Liang, H.; Warnet, J.M.; Baudouin, C. New tools for the evaluation of toxic ocular surface changes in the rat. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5473–5483. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.C.; Tinelli, C.; Pasinetti, G.M.; Milano, G.; Bianchi, P.E. Dry eye syndrome-related quality of life in glaucoma patients. Eur. J. Ophthalmol. 2009, 19, 572–579. [Google Scholar] [PubMed]
- De Jong, C.; Stolwijk, T.; Kuppens, E.; de Keizer, R.; van Best, J. Topical timolol with and without benzalkonium chloride: Epithelial permeability and autofluorescence of the cornea in glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 1994, 232, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Liang, H.; Hamard, P.; Riancho, L.; Creuzot-Garcher, C.; Warnet, J.M.; Brignole-Baudouin, F. The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-helper 1 and T-helper 2 pathways. Ophthalmology 2008, 115, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, A.; Godefroy, D.; Celerier, I.; Frugier, J.; Riancho, L.; Baudouin, F.; Rostene, W.; Baudouin, C. CX3CL1 expression in the conjunctiva is involved in immune cell trafficking during toxic ocular surface inflammation. Mucosal Immunol. 2012, 5, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liu, X.; Zhou, T.; Wang, Y.; Bai, L.; He, H.; Liu, Z. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol. Vis. 2011, 17, 257–264. [Google Scholar] [PubMed]
- Pauly, A.; Labbe, A.; Baudouin, C.; Liang, H.; Warnet, J.M.; Brignole-Baudouin, F. In vivo confocal microscopic grading system for standardized corneal evaluation: Application to toxic-induced damage in rat. Curr. Eye Res. 2008, 33, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Bates, N.; Edwards, N. Benzalkonium chloride exposure in cats: A retrospective analysis of 245 cases reported to the Veterinary Poisons Information Service (VPIS). Vet. Rec. 2015, 176, 229. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Baudouin, C.; Pauly, A.; Brignole-Baudouin, F. Conjunctival and corneal reactions in rabbits following short- and repeated exposure to preservative-free tafluprost, commercially available latanoprost and 0.02% benzalkonium chloride. Br. J. Ophthalmol. 2008, 92, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Chen, D.; Liu, J.; Liu, B.; Li, N.; Zhou, Y.; Liang, X.; Ma, P.; Ye, C.; Ge, J.; et al. A rabbit dry eye model induced by topical medication of a preservative benzalkonium chloride. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Barki, W.H.; Tahir, M. Effects of topical benzalkonium chloride on corneal epithelium. Biomedica 2007, 23, 65–70. [Google Scholar]
- Durand-Cavagna, G.; Delort, P.; Duprat, P.; Bailly, Y.; Plazonnet, B.; Gordon, L.R. Corneal toxicity studies in rabbits and dogs with hydroxyethyl cellulose and benzalkonium chloride. Fundam. Appl. Toxicol. 1989, 13, 500–508. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Q.; Zhou, T.; Lin, Z.; Zong, R.; Liu, Z.; Sun, F.; Shao, Y.; Liu, X.; Ma, J.X.; et al. Modulation of the canonical Wnt pathway by Benzalkonium Chloride in corneal epithelium. Exp. Eye Res. 2011, 93, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, H.; Wang, C.; Wu, Y.; Xie, J.; Jin, X.; Yang, J.; Ye, J. Genoprotective effect of hyaluronic acid against benzalkonium chloride-induced DNA damage in human corneal epithelial cells. Mol. Vis. 2011, 17, 3364–3370. [Google Scholar] [PubMed]
- Sarkar, J.; Chaudhary, S.; Namavari, A.; Ozturk, O.; Chang, J.H.; Yco, L.; Sonawane, S.; Khanolkar, V.; Hallak, J.; Jain, S. Corneal neurotoxicity due to topical benzalkonium chloride. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- DryEye; WorkShop. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Tseng, C.L.; Seghatchian, J.; Burnouf, T. Animal models to assess the therapeutic efficacy of human serum and serum-converted platelet lysates for dry eye syndrome: Seeing is believing. Transfus. Apher. Sci. 2015, 53, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; He, H.; Lin, Z.; Luo, P.; He, H.; Zhou, T.; Zhou, Y.; Liu, Z. Therapeutic effects of epidermal growth factor on benzalkonium chloride-induced dry eye in a mouse model. Investig. Ophthalmol. Vis. Sci. 2012, 53, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, W.Z.; Zhu, Z.Z.; Hu, Q.Q.; Chen, Y.F.; He, H.; Chen, Y.X.; Liu, Z.G. Therapeutic effects of topical doxycycline in a benzalkonium chloride-induced mouse dry eye model. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2963–2974. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Hyun, L.C.; Jung, S.H.; Yang, S.J. The leaves of Diospyros kaki exert beneficial effects on a benzalkonium chloride-induced murine dry eye model. Mol. Vis. 2016, 22, 284–293. [Google Scholar] [PubMed]
- Barabino, S.; Antonelli, S.; Cimbolini, N.; Mauro, V.; Bouzin, M. The effect of preservatives and antiglaucoma treatments on the ocular surface of mice with dry eye. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6499–6504. [Google Scholar] [CrossRef] [PubMed]
- Yingfang, F.; Zhuang, B.; Wang, C.; Xu, X.; Xu, W.; Lv, Z. Pimecrolimus micelle exhibits excellent therapeutic effect for Keratoconjunctivitis Sicca. Colloids Surf. B Biointerfaces 2016, 140, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, E.J.; Kim, Y.H.; Kim, Y.I.; Lee, S.H.; Jung, J.C.; Lee, K.W.; Park, Y.J. In Vivo Effects of Preservative-free and Preserved Prostaglandin Analogs: Mouse Ocular Surface Study. Korean J. Ophthalmol. 2015, 29, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.P.; Chen, D.; Asbell, P.A. Evaluation of biomarkers of inflammation in response to benzalkonium chloride on corneal and conjunctival epithelial cells. J. Ocul. Pharmacol. Ther. 2009, 25, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.A.; Kaufman, P.L.; Kiland, J.A. Benzalkonium chloride and glaucoma. J. Ocul. Pharmacol. Ther. 2014, 30, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Droy-Lefaix, M.T.; Bueno, L.; Caron, P.; Belot, E.; Roche, O. Ocular inflammation and corneal permeability alteration by benzalkonium chloride in rats: A protective effect of a myosin light chain kinase inhibitor. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2705–2710. [Google Scholar] [CrossRef] [PubMed]
- Galletti, J.G.; Gabelloni, M.L.; Morande, P.E.; Sabbione, F.; Vermeulen, M.E.; Trevani, A.S.; Giordano, M.N. Benzalkonium chloride breaks down conjunctival immunological tolerance in a murine model. Mucosal Immunol. 2013, 6, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Pisella, P.J.; Pouliquen, P.; Baudouin, C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br. J. Ophthalmol. 2002, 86, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.M.; Baryla, J.; Liu, H.; Laurie, G.W.; McKown, R.L.; Ashki, N.; Bhayana, D.; Hutnik, C.M. Cytoprotective effect of lacritin on human corneal epithelial cells exposed to benzalkonium chloride in vitro. Curr. Eye Res. 2014, 39, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Clouzeau, C.; Godefroy, D.; Riancho, L.; Rostene, W.; Baudouin, C.; Brignole-Baudouin, F. Hyperosmolarity potentiates toxic effects of benzalkonium chloride on conjunctival epithelial cells in vitro. Mol. Vis. 2012, 18, 851–863. [Google Scholar] [PubMed]
- Okahara, A.; Kawazu, K. Local toxicity of benzalkonium chloride in ophthalmic solutions following repeated applications. J. Toxicol. Sci. 2013, 38, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jun, R.M.; Cho, M.S.; Choi, K.R. Comparison of the ocular surface changes following the use of two different prostaglandin F2α analogues containing benzalkonium chloride or polyquad in rabbit eyes. Cutan. Ocul. Toxicol. 2015, 34, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; He, H.; Zhou, T.; Liu, X.; Wang, Y.; He, H.; Wu, H.; Liu, Z. A mouse model of limbal stem cell deficiency induced by topical medication with the preservative benzalkonium chloride. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6314–6325. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Brignole-Baudouin, F.; Rabinovich-Guilatt, L.; Mao, Z.; Riancho, L.; Faure, M.O.; Warnet, J.M.; Lambert, G.; Baudouin, C. Reduction of quaternary ammonium-induced ocular surface toxicity by emulsions: An in vivo study in rabbits. Mol. Vis. 2008, 14, 204–216. [Google Scholar] [PubMed]
- Yang, Q.; Chen, Z.Y.; Liu, X.P.; Wu, K.L. Overexpression of carbonic anhydrase 1 in pterygium. Int. J. Ophthalmol. 2016, 9, 931–932. [Google Scholar] [PubMed]
- Chen, Z.; Shamsi, F.A.; Li, K.; Huang, Q.; Al-Rajhi, A.A.; Chaudhry, I.A.; Wu, K. Comparison of camel tear proteins between summer and winter. Mol. Vis. 2011, 17, 323–331. [Google Scholar] [PubMed]
| BAC% | C57BL/6 (%) # | BALB/c (%) # | ||
|---|---|---|---|---|
| 7 Days | 14 Days | 7 Days | 14 Days | |
| 0.10 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 0.20 * | 9 (90.0) | 10 (100.0) | 4 (40.0) | 6 (60.0) |
| 0.40 | 10 (100.0) | 10 (100.0) | 10 (100.0) | 10 (100.0) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Zhang, Y.; Liu, X.; Wang, N.; Song, Z.; Wu, K. A Comparison of the Effects of Benzalkonium Chloride on Ocular Surfaces between C57BL/6 and BALB/c Mice. Int. J. Mol. Sci. 2017, 18, 509. https://doi.org/10.3390/ijms18030509
Yang Q, Zhang Y, Liu X, Wang N, Song Z, Wu K. A Comparison of the Effects of Benzalkonium Chloride on Ocular Surfaces between C57BL/6 and BALB/c Mice. International Journal of Molecular Sciences. 2017; 18(3):509. https://doi.org/10.3390/ijms18030509
Chicago/Turabian StyleYang, Qian, Yafang Zhang, Xiuping Liu, Nan Wang, Zhenyu Song, and Kaili Wu. 2017. "A Comparison of the Effects of Benzalkonium Chloride on Ocular Surfaces between C57BL/6 and BALB/c Mice" International Journal of Molecular Sciences 18, no. 3: 509. https://doi.org/10.3390/ijms18030509






