The Regulations of Deubiquitinase USP15 and Its Pathophysiological Mechanisms in Diseases
Abstract
:1. Introduction
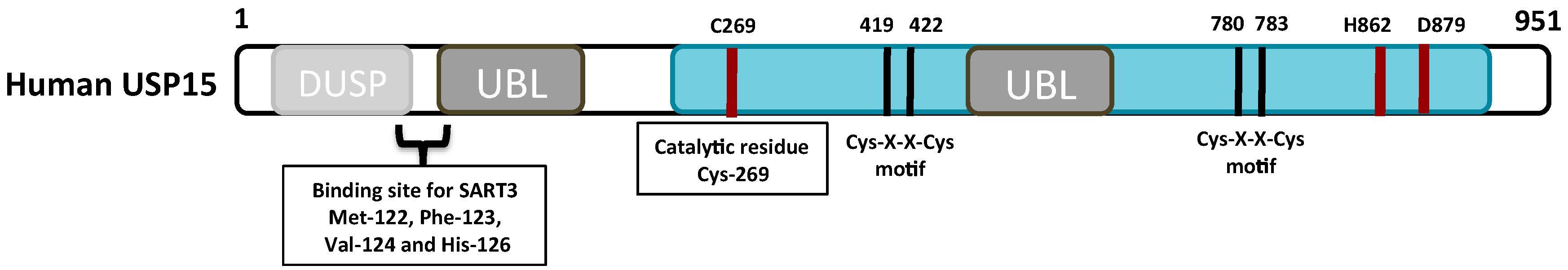
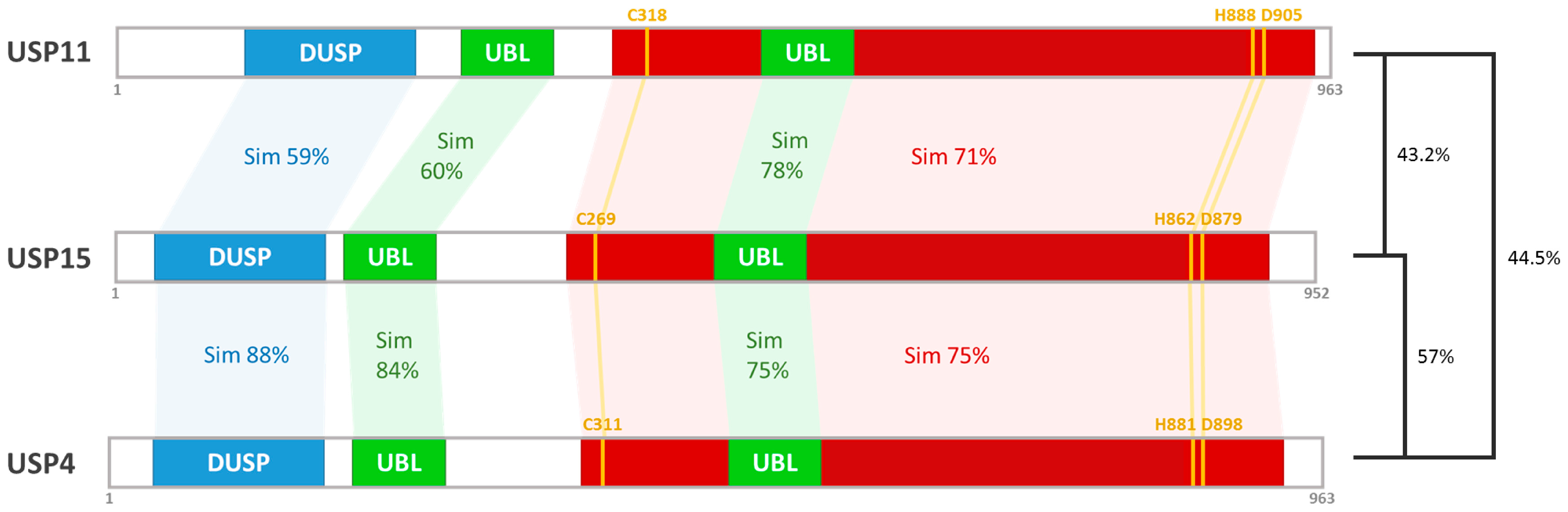
2. The Structure and Function of the USP15 Domains
2.1. The Catalytic Domain
2.2. The DUSP Domain
2.3. The UBL Domain
3. Chromosomal Location and Isoforms of USP15
4. Expression and Subcellular Localization of USP15 Protein
5. USP15 and Oncogenic Signaling
5.1. COP9-Signalosome/Cullin-RING E3 Ubiquitin Ligase
5.2. COP9-Signalosome/The IκBα-Mediated Regulation of NF-κB
5.3. COP9-Signalosome/The APC-Mediated Regulation of β-Catenin Stability
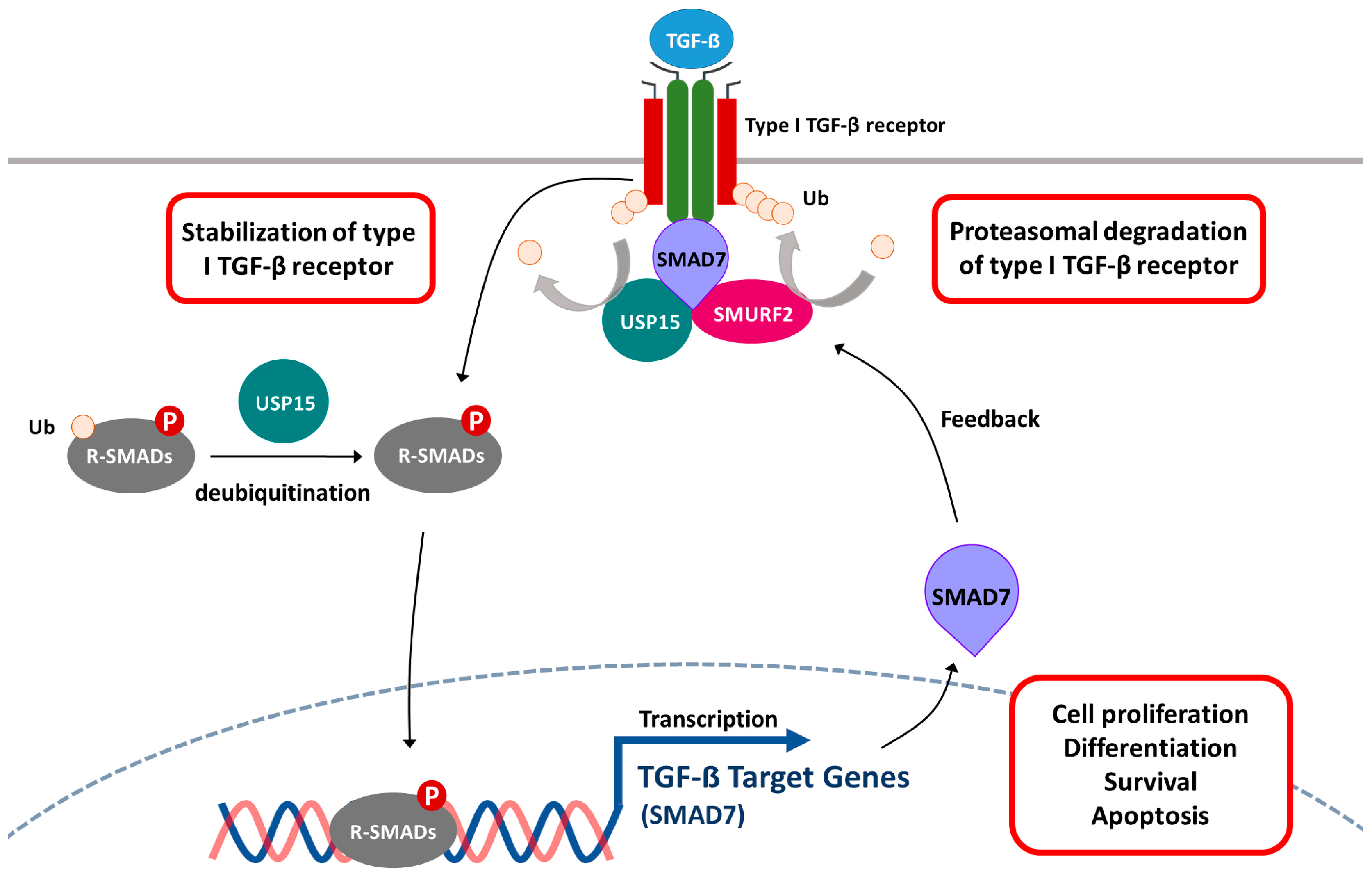
5.4. Transforming Growth Factor β (TGF-β) Signaling Pathway
5.5. The Regulation of Mouse Double Minute 2 Homolog (MDM2) and p53
5.6. Regulation of p53 through Stabilizing HPV/E6 Oncoprotein
6. Acquired Chemoresistance of Cancer Cells
7. Diseases Associated with Dysregulation of USP15
7.1. USP15 and Parkinson’s Disease
7.2. USP15 as Regulator of Antiviral Innate Immune Responses
8. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- D’Azzo, A.; Bongiovanni, A.; Nastasi, T. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic 2005, 6, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Haglund, K.; Dikic, I. Ubiquitylation and cell signaling. EMBO J. 2005, 24, 3353–3359. [Google Scholar] [CrossRef] [PubMed]
- Swatek, K.N.; Komander, D. Ubiquitin modifications. Cell Res. 2016, 26, 399–422. [Google Scholar] [CrossRef] [PubMed]
- Neutzner, M.; Neutzner, A. Enzymes of ubiquitination and deubiquitination. Essays Biochem. 2012, 52, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef] [PubMed]
- Deshaies, R.J.; Joazeiro, C.A. Ring domain E3 ubiquitin ligases. Annu. Rev. Biochem. 2009, 78, 399–434. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Pellman, D. Deubiquitinating enzymes: A new class of biological regulators. Crit. Rev. Biochem. Mol. Biol. 1998, 33, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.D. Ubiquitination and deubiquitination: Targeting of proteins for degradation by the proteasome. Semin. Cell. Dev. Biol. 2000, 11, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Pickart, C.M.; Rose, I.A. Ubiquitin carboxyl-terminal hydrolase acts on ubiquitin carboxyl-terminal amides. J. Biol. Chem. 1985, 260, 7903–7910. [Google Scholar] [PubMed]
- Amerik, A.Y.; Hochstrasser, M. Mechanism and function of deubiquitinating enzymes. Biochim. Biophys. Acta 2004, 1695, 189–207. [Google Scholar] [CrossRef] [PubMed]
- De Jong, R.N.; Ab, E.; Diercks, T.; Truffault, V.; Daniels, M.; Kaptein, R.; Folkers, G.E. Solution structure of the human ubiquitin-specific protease 15 DUSP domain. J. Biol. Chem. 2006, 281, 5026–5031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Berger, F.G.; Yang, J.; Lu, X. USP4 inhibits p53 through deubiquitinating and stabilizing ARF-BP1. EMBO J. 2011, 30, 2177–2189. [Google Scholar] [CrossRef] [PubMed]
- Cotto-Rios, X.M.; Bekes, M.; Chapman, J.; Ueberheide, B.; Huang, T.T. Deubiquitinases as a signaling target of oxidative stress. Cell Rep. 2012, 2, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Komander, D.; Clague, M.J.; Urbe, S. Breaking the chains: Structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 2009, 10, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Scheel, H.; Hofmann, K.; Komander, D. Dissection of USP catalytic domains reveals five common insertion points. Mol. Biosyst. 2009, 5, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Tencer, A.H.; Liang, Q.; Zhuang, Z. Divergence in ubiquitin interaction and catalysis among the ubiquitin-specific protease family deubiquitinating enzymes. Biochemistry 2016, 55, 4708–4719. [Google Scholar] [CrossRef] [PubMed]
- Renatus, M.; Parrado, S.G.; D’Arcy, A.; Eidhoff, U.; Gerhartz, B.; Hassiepen, U.; Pierrat, B.; Riedl, R.; Vinzenz, D.; Worpenberg, S.; et al. Structural basis of ubiquitin recognition by the deubiquitinating protease USP2. Structure 2006, 14, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Hetfeld, B.K.; Helfrich, A.; Kapelari, B.; Scheel, H.; Hofmann, K.; Guterman, A.; Glickman, M.; Schade, R.; Kloetzel, P.M.; Dubiel, W. The zinc finger of the CSN-associated deubiquitinating enzyme USP15 is essential to rescue the E3 ligase RBX1. Curr. Biol. 2005, 15, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Avvakumov, G.; Tong, J.; Fan, Y.; Zhao, Y.; Alberts, P.; Persaud, A.; Walker, J.R.; Neculai, A.M.; Neculai, D.; et al. A strategy for modulation of enzymes in the ubiquitin system. Science 2013, 339, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Faesen, A.C.; Luna-Vargas, M.P.; Sixma, T.K. The role of UBL domains in ubiquitin-specific proteases. Biochem. Soc. Trans. 2012, 40, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Menard, R.; Sulea, T. High incidence of ubiquitin-like domains in human ubiquitin-specific proteases. Proteins 2007, 69, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Langelotz, C.; Hetfeld-Pechoc, B.K.; Schwenk, W.; Dubiel, W. The COP9 signalosome mediates β-catenin degradation by deneddylation and blocks adenomatous polyposis coli destruction via USP15. J. Mol. Biol. 2009, 391, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Vlasschaert, C.; Xia, X.; Coulombe, J.; Gray, D.A. Evolution of the highly networked deubiquitinating enzymes USP4, USP15, and USP11. BMC Evol. Biol. 2015, 15, 230. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.S.; Grishin, N.V. The finger domain of the human deubiquitinating enzyme hausp is a zinc ribbon. Cell Cycle 2004, 3, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.T.; Wang, X.W.; Woollatt, E.; White, J.A.; Sutherland, G.R. Identification, functional characterization, and chromosomal localization of USP15, a novel human ubiquitin-specific protease related to the UNP oncoprotein, and a systematic nomenclature for human ubiquitin-specific proteases. Genomics 1999, 59, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Clerici, M.; Luna-Vargas, M.P.; Faesen, A.C.; Sixma, T.K. The DUSP-Ubl domain of USP4 enhances its catalytic efficiency by promoting ubiquitin exchange. Nat. Commun. 2014, 5, 5399. [Google Scholar]
- Harper, S.; Besong, T.M.; Emsley, J.; Scott, D.J.; Dreveny, I. Structure of the USP15 N-terminal domains: A β-hairpin mediates close association between the DUSP and UBL domains. Biochemistry 2011, 50, 7995–8004. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.R.; Liu, H.; Pastok, M.W.; Grossmann, G.J.; Rigden, D.J.; Clague, M.J.; Urbe, S.; Barsukov, I.L. Structural variability of the ubiquitin specific protease DUSP-UBL double domains. FEBS Lett. 2011, 585, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Harding, R.; Hou, F.; Dong, A.; Walker, J.R.; Bteich, J.; Tong, Y. Structural basis of the recruitment of ubiquitin-specific protease USP15 by spliceosome recycling factor SART3. J. Biol. Chem. 2016, 291, 17283–17292. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Das, T.; Song, E.J.; Kim, E.E. Structural basis for recruiting and shuttling of the spliceosomal deubiquitinase USP4 by SART3. Nucleic Acids Res. 2016, 44, 5424–5437. [Google Scholar] [CrossRef] [PubMed]
- Angelats, C.; Wang, X.W.; Jermiin, L.S.; Copeland, N.G.; Jenkins, N.A.; Baker, R.T. Isolation and characterization of the mouse ubiquitin-specific protease USP15. Mamm. Genome 2003, 14, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Park, K.C.; Choi, E.J.; Min, S.W.; Chung, S.S.; Kim, H.; Suzuki, T.; Tanaka, K.; Chung, C.H. Tissue-specificity, functional characterization and subcellular localization of a rat ubiquitin-specific processing protease, UBP109, whose mrna expression is developmentally regulated. Biochem. J. 2000, 349, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Soboleva, T.A.; Jans, D.A.; Johnson-Saliba, M.; Baker, R.T. Nuclear-cytoplasmic shuttling of the oncogenic mouse UNP/USP4 deubiquitylating enzyme. J. Biol. Chem. 2005, 280, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Thelen, J.P.; Furgason, M.; Haj-Yahya, M.; Brik, A.; Cheng, D.; Peng, J.; Yao, T. The U4/U6 recycling factor SART3 has histone chaperone activity and associates with USP15 to regulate H2B deubiquitination. J. Biol. Chem. 2014, 289, 8916–8930. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, P.J.; Rodon, L.; Gonzalez-Junca, A.; Dirac, A.; Gili, M.; Martinez-Saez, E.; Aura, C.; Barba, I.; Peg, V.; Prat, A.; et al. USP15 stabilizes TGF-β receptor I and promotes oncogenesis through the activation of TGF-β signaling in glioblastoma. Nat. Med. 2012, 18, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, P.V.; Jaynes, P.; Rodon, L.; Lama, D.; Law, K.P.; Lim, Y.P.; Verma, C.; Seoane, J.; Eichhorn, P.J. USP15 regulates SMURF2 kinetics through C-lobe mediated deubiquitination. Sci. Rep. 2015, 5, 14733. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, K.; Bozko, P.M.; Dubiel, W.; Naumann, M. CSN controls NF-κB by deubiquitinylation of IκBα. EMBO J. 2007, 26, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Jin, J.; Hu, H.; Li, H.S.; Romano, S.; Xiao, Y.; Nakaya, M.; Zhou, X.; Cheng, X.; Yang, P.; et al. USP15 stabilizes MDM2 to mediate cancer-cell survival and inhibit antitumor T cell responses. Nat. Immunol. 2014, 15, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Inui, M.; Manfrin, A.; Mamidi, A.; Martello, G.; Morsut, L.; Soligo, S.; Enzo, E.; Moro, S.; Polo, S.; Dupont, S.; et al. USP15 is a deubiquitylating enzyme for receptor-activated smads. Nat. Cell Biol. 2011, 13, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Serino, G.; Deng, X.W. The COP9 signalosome: More than a protease. Trends Biochem. Sci. 2008, 33, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wee, S.; Rhee, E.; Naumann, M.; Dubiel, W.; Wolf, D.A. Fission yeast COP9/signalosome suppresses cullin activity through recruitment of the deubiquitylating enzyme Ubp12p. Mol. Cell 2003, 11, 927–938. [Google Scholar] [CrossRef]
- Wei, D.; Sun, Y. Small ring finger proteins RBX1 and RBX2 of SCF E3 ubiquitin ligases: The role in cancer and as cancer targets. Genes Cancer 2010, 1, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Peth, A.; Berndt, C.; Henke, W.; Dubiel, W. Downregulation of COP9 signalosome subunits differentially affects the CSN complex and target protein stability. BMC Biochem. 2007, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Michel, J.J.; Xiong, Y. Association with cullin partners protects ROC proteins from proteasome-dependent degradation. Oncogene 1999, 18, 6758–6766. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Zhao, R.; Phan, L.; Yeung, S.C. Roles of COP9 signalosome in cancer. Cell Cycle 2011, 10, 3057–3066. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xie, L.; Gu, Y.; Li, J.; Xie, J. Roles of multifunctional COP9 signalosome complex in cell fate and implications for drug discovery. J. Cell. Physiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.S.; Zundel, W. The emerging role of the COP9 signalosome in cancer. Mol. Cancer Res. 2005, 3, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt breakers in colon cancer. Cancer Cell 2004, 5, 5–6. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Morrison, E.E. The APC-EB1 interaction. Adv. Exp. Med. Biol. 2009, 656, 41–50. [Google Scholar] [PubMed]
- Peth, A.; Boettcher, J.P.; Dubiel, W. Ubiquitin-dependent proteolysis of the microtubule end-binding protein 1, EB1, is controlled by the COP9 signalosome: Possible consequences for microtubule filament stability. J. Mol. Biol. 2007, 368, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, L.; Wan, Y.Y.; Zhu, B. Mechanism of action of IL-7 and its potential applications and limitations in cancer immunotherapy. Int. J. Mol. Sci. 2015, 16, 10267–10280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Xie, F.; Zhang, Z.; van Dam, H.; Zhang, L.; Zhou, F. The regulation of TGF-β/SMAD signaling by protein deubiquitination. Protein Cell 2014, 5, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Itoh, S.; ten Dijke, P. Negative regulation of TGF-β receptor/SMAD signal transduction. Curr. Opin. Cell Biol. 2007, 19, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Wicks, S.J.; Grocott, T.; Haros, K.; Maillard, M.; ten Dijke, P.; Chantry, A. Reversible ubiquitination regulates the SMAD/TGF-β signalling pathway. Biochem. Soc. Trans. 2006, 34, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Kamadurai, H.B.; Souphron, J.; Scott, D.C.; Duda, D.M.; Miller, D.J.; Stringer, D.; Piper, R.C.; Schulman, B.A. Insights into ubiquitin transfer cascades from a structure of a UbcH5B approximately ubiquitin-HECTNEDD4L complex. Mol. Cell 2009, 36, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Ogunjimi, A.A.; Wiesner, S.; Briant, D.J.; Varelas, X.; Sicheri, F.; Forman-Kay, J.; Wrana, J.L. The ubiquitin binding region of the SMURF HECT domain facilitates polyubiquitylation and binding of ubiquitylated substrates. J. Biol. Chem. 2010, 285, 6308–6315. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lozano, G. Molecular pathways: Targeting MDM2 and MDM4 in cancer therapy. Clin. Cancer Res. 2013, 19, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wade, M.; Li, Y.C.; Wahl, G.M. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat. Rev. Cancer 2013, 13, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Rayburn, E.; Zhang, R.; He, J.; Wang, H. MDM2 and human malignancies: Expression, clinical pathology, prognostic markers, and implications for chemotherapy. Curr. Cancer Drug Targets 2005, 5, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Zur Hausen, H. Papillomaviruses in the causation of human cancers—A brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Pim, D.; Banks, L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene 1999, 18, 7690–7700. [Google Scholar] [CrossRef] [PubMed]
- Vos, R.M.; Altreuter, J.; White, E.A.; Howley, P.M. The ubiquitin-specific peptidase USP15 regulates human papillomavirus type 16 E6 protein stability. J. Virol. 2009, 83, 8885–8892. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, R.; Mialon, A.; Pitkanen, H.; Westermarck, J.; Hietanen, S. Activation of p53 in cervical cancer cells by human papillomavirus E6 RNA interference is transient, but can be sustained by inhibiting endogenous nuclear export-dependent p53 antagonists. Cancer Res. 2006, 66, 11817–11824. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.B. Taxol (paclitaxel): Mechanisms of action. Ann. Oncol. 1994, 5 (Suppl. 6), S3–S6. [Google Scholar] [PubMed]
- Yusuf, R.Z.; Duan, Z.; Lamendola, D.E.; Penson, R.T.; Seiden, M.V. Paclitaxel resistance: Molecular mechanisms and pharmacologic manipulation. Curr. Cancer Drug Targets 2003, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, K.N. Microtubule-targeted anticancer agents and apoptosis. Oncogene 2003, 22, 9075–9086. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs that target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef] [PubMed]
- Rensen, W.M.; Roscioli, E.; Tedeschi, A.; Mangiacasale, R.; Ciciarello, M.; di Gioia, S.A.; Lavia, P. RanBP1 downregulation sensitizes cancer cells to taxol in a caspase-3-dependent manner. Oncogene 2009, 28, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Gallegos, J.R.; Gu, Q.; Huang, Y.; Li, J.; Jin, Y.; Lu, H.; Sun, Y. SAG/ROC-SCFβ-TrCP E3 ubiquitin ligase promotes pro-caspase-3 degradation as a mechanism of apoptosis protection. Neoplasia 2006, 8, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Takanashi, M.; Oikawa, K.; Tanaka, M.; Nishi, H.; Isaka, K.; Kudo, M.; Kuroda, M. USP15 plays an essential role for caspase-3 activation during paclitaxel-induced apoptosis. Biochem. Biophys. Res. Commun. 2009, 388, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Corti, O.; Lesage, S.; Brice, A. What genetics tells us about the causes and mechanisms of Parkinson’s disease. Physiol. Rev. 2011, 91, 1161–1218. [Google Scholar] [CrossRef]
- Cornelissen, T.; Haddad, D.; Wauters, F.; van Humbeeck, C.; Mandemakers, W.; Koentjoro, B.; Sue, C.; Gevaert, K.; de Strooper, B.; Verstreken, P.; et al. The deubiquitinase USP15 antagonizes Parkin-mediated mitochondrial ubiquitination and mitophagy. Hum. Mol. Genet. 2014, 23, 5227–5242. [Google Scholar] [CrossRef] [PubMed]
- Veeriah, S.; Taylor, B.S.; Meng, S.; Fang, F.; Yilmaz, E.; Vivanco, I.; Janakiraman, M.; Schultz, N.; Hanrahan, A.J.; Pao, W.; et al. Somatic mutations of the Parkinson’s disease-associated gene PARK2 in glioblastoma and other human malignancies. Nat. Genet. 2010, 42, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Pichlmair, A.; Schulz, O.; Tan, C.P.; Naslund, T.I.; Liljestrom, P.; Weber, F.; Reis e Sousa, C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 2006, 314, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Gack, M.U.; Shin, Y.C.; Joo, C.H.; Urano, T.; Liang, C.; Sun, L.; Takeuchi, O.; Akira, S.; Chen, Z.; Inoue, S.; et al. TRIM25 ring-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 2007, 446, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Inn, K.S.; Gack, M.U.; Tokunaga, F.; Shi, M.; Wong, L.Y.; Iwai, K.; Jung, J.U. Linear ubiquitin assembly complex negatively regulates RIG-I- and TRIM25-mediated type I interferon induction. Mol. Cell 2011, 41, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Pauli, E.K.; Chan, Y.K.; Davis, M.E.; Gableske, S.; Wang, M.K.; Feister, K.F.; Gack, M.U. The ubiquitin-specific protease USP15 promotes RIG-I-mediated antiviral signaling by deubiquitylating TRIM25. Sci. Signal. 2014, 7, ra3. [Google Scholar] [CrossRef] [PubMed]
| mRNA Transcripts | Transcript ID | Length (bp) | Exon | Protein Isoform | Protein ID | Protein Size (aa) | Mass (kDa) |
|---|---|---|---|---|---|---|---|
| USP15-001 | ENST00000353364 | 14,950 | 21 | ubiquitin carboxyl-terminal hydrolase 15 isoform 2 | NP_006304 | 952 | 109.297 |
| USP15-002 | ENST00000280377 | 4,748 | 22 | ubiquitin carboxyl-terminal hydrolase 15 isoform 1 | NP_001239007 | 981 | 112.419 |
| USP15-003 | ENST00000312635 | 2,226 | 7 | ubiquitin carboxyl-terminal hydrolase 15 isoform 3 | NP_001239008 | 235 | 27.094 |
| Organs | Protein Expression Overview (Score) | Organs | Protein Expression Overview (Score) | ||
|---|---|---|---|---|---|
| Adipose tissue/Soft tissue | Adipose tissue | + | Immune system | Appendix | +++ |
| Soft tissue | ++ | Bone marrow | ++ | ||
| Brain | Cerebral cortex | + | Lymph node | ++ | |
| Hippocampus | + | Tonsil | ++ | ||
| Caudate | + | Spleen | ++ | ||
| Cerebellum | + | Kidney | Kidney | ++ | |
| Endocrine tissues | Thyroid gland | ++ | Urinary bladder | + | |
| Parathyroid gland | +++ | Liver/Gallbladder | Liver | + | |
| Adrenal gland | ++ | Gallbladder | +++ | ||
| Female tissues | Breast | + | |||
| Vagina | - | Lung | Nasopharynx | ++ | |
| Cervix, uterine | + | Bronchus | + | ||
| Endometrium | + | Lung | ++ | ||
| Fallopian tube | ++ | Male tissues | Testis | ++ | |
| Ovary | + | Epididymis | ++ | ||
| Placenta | ++ | Prostate | ++ | ||
| Gastrointestinal tract | Oral mucosa | + | Seminal vesicle | ++ | |
| Salivary gland | + | Muscle tissues | Heart muscle | + | |
| Esophagus | + | Skeletal muscle | ++ | ||
| Stomach | ++ | Smooth muscle | ++ | ||
| Duodenum | +++ | Pancreatic tissues | Pancreas | ++ | |
| Small intestine | +++ | Skin | skin | ++ | |
| Colon | ++ | ||||
| Rectum | +++ |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, C.-K.; Chang, Y.-T.; Korinek, M.; Chen, Y.-T.; Yang, Y.-T.; Leu, S.; Lin, I.-L.; Tang, C.-J.; Chiu, C.-C. The Regulations of Deubiquitinase USP15 and Its Pathophysiological Mechanisms in Diseases. Int. J. Mol. Sci. 2017, 18, 483. https://doi.org/10.3390/ijms18030483
Chou C-K, Chang Y-T, Korinek M, Chen Y-T, Yang Y-T, Leu S, Lin I-L, Tang C-J, Chiu C-C. The Regulations of Deubiquitinase USP15 and Its Pathophysiological Mechanisms in Diseases. International Journal of Molecular Sciences. 2017; 18(3):483. https://doi.org/10.3390/ijms18030483
Chicago/Turabian StyleChou, Chon-Kit, Yu-Ting Chang, Michal Korinek, Yei-Tsung Chen, Ya-Ting Yang, Steve Leu, I-Ling Lin, Chin-Ju Tang, and Chien-Chih Chiu. 2017. "The Regulations of Deubiquitinase USP15 and Its Pathophysiological Mechanisms in Diseases" International Journal of Molecular Sciences 18, no. 3: 483. https://doi.org/10.3390/ijms18030483







