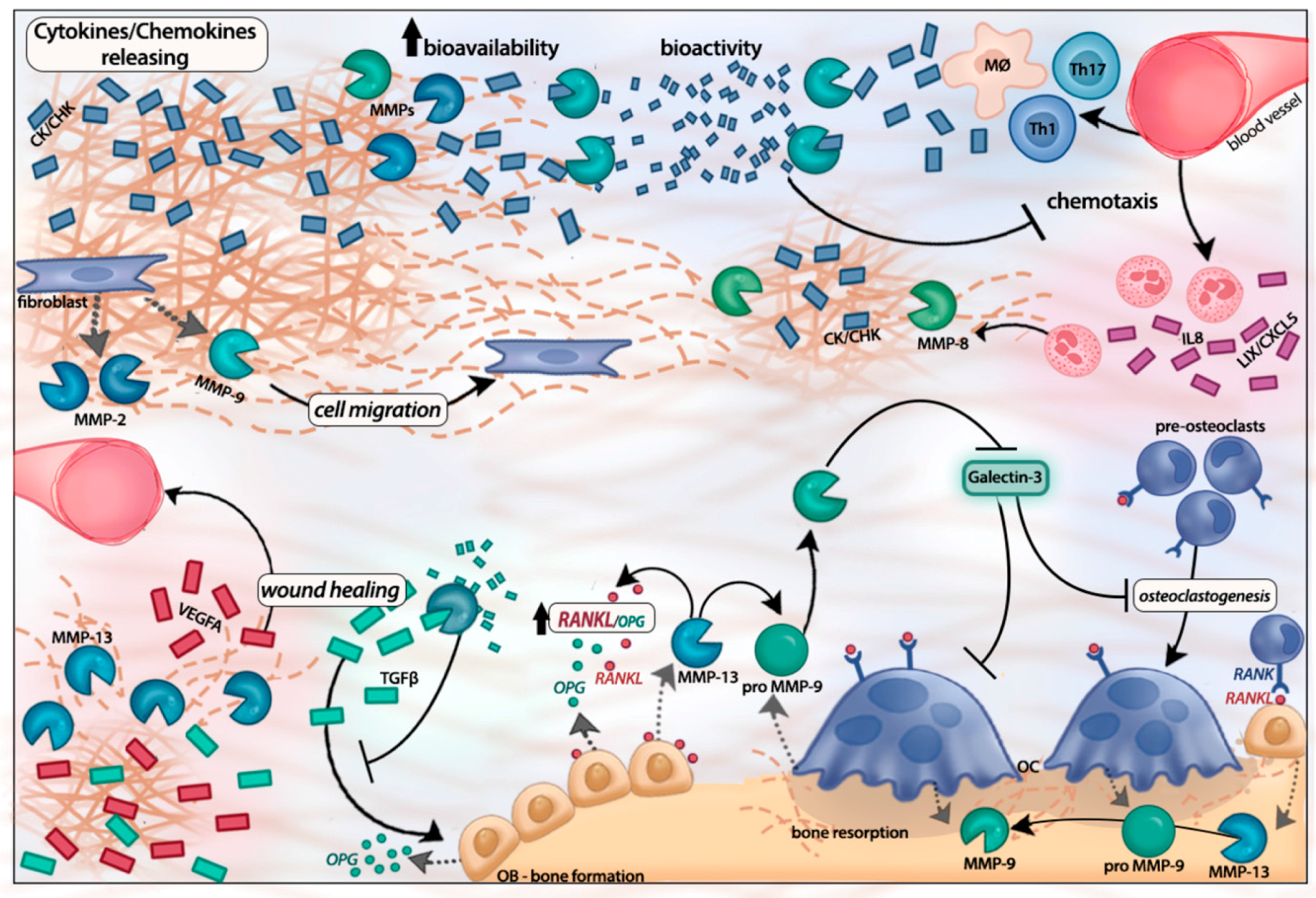
Matrix Metalloproteinases as Regulators of Periodontal Inflammation
Abstract
:1. Introduction
2. Matrix Metalloproteinase Activation Cascades
3. MMP-Mediated Regulation of Inflammatory Response
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhavsar, A.P.; Guttman, J.A.; Finlay, B.B. Manipulation of host-cell pathways by bacterial pathogens. Nature 2007, 449, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Graves, D. Cytokines that promote periodontal tissue destruction. J. Periodontol. 2008, 79, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Houri-Haddad, Y.; Wilensky, A.; Shapira, L. T-cell phenotype as a risk factor for periodontal disease. Periodontol. 2000 2007, 45, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Nitta, H.; Katagiri, S.; Nagasawa, T.; Izumi, Y.; Ishikawa, I.; Izumiyama, H.; Uchimura, I.; Kanazawa, M.; Chiba, H.; Matsuo, A.; et al. The number of microvascular complications is associated with an increased risk for severity of periodontitis in type 2 diabetic patients: Results of a multicenter hospital-based cross-sectional study. J. Diabetes Investig. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pussinen, P.J.; Paju, S.; Mantyla, P.; Sorsa, T. Serum microbial- and host-derived markers of periodontal diseases: A review. Curr. Med. Chem. 2007, 14, 2402–2412. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.V.R.; Mäntyla, P.; Tervahartiala, T.; Sorsa, T.; Gamonal, J. Chronic Periodontitis: The Role of Immuno-Inflammatory Response in the Pathogenesis of Chronic Periodontitis and Development of Chair-Side Point of Care Diagnostics in Periodontitis and Related Systemic Inflammation. In Pathogenesis and Treatment of Periodontitis; Buduneli, N., Ed.; InTech: Rijeka, Croatia, 2012; pp. 33–54. [Google Scholar]
- Buduneli, N.; Kinane, D.F. Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J. Clin. Periodontol. 2011, 38, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Mantyla, P.; Tervahartiala, T.; Pussinen, P.J.; Gamonal, J.; Hernandez, M. MMP activation in diagnostics of periodontitis and systemic inflammation. J. Clin. Periodontol. 2011, 38, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.S.; Overall, C.M. Matrix metalloproteinase processing of signaling molecules to regulate inflammation. Periodontol. 2000 2013, 63, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Sbardella, D.; Fasciglione, G.F.; Gioia, M.; Ciaccio, C.; Tundo, G.R.; Marini, S.; Coletta, M. Human matrix metalloproteinases: An ubiquitarian class of enzymes involved in several pathological processes. Mol. Asp. Med. 2012, 33, 119–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol. 2000 2016, 70, 142–163. [Google Scholar] [CrossRef] [PubMed]
- Folgueras, A.R.; Pendas, A.M.; Sanchez, L.M.; Lopez-Otin, C. Matrix metalloproteinases in cancer: From new functions to improved inhibition strategies. Int. J. Dev. Biol. 2004, 48, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.S.; Overall, C.M. Updated biological roles for matrix metalloproteinases and new “intracellular” substrates revealed by degradomics. Biochemistry 2009, 48, 10830–10845. [Google Scholar] [CrossRef] [PubMed]
- Cauwe, B.; Martens, E.; Proost, P.; Opdenakker, G. Multidimensional degradomics identifies systemic autoantigens and intracellular matrix proteins as novel gelatinase B/MMP-9 substrates. Integr. Biol. 2009, 1, 404–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, K.W.; Wei, C.; Lung, W.Y.; Wei, X.Y.; Cheng, B.H.; Cai, Z.M.; Huang, W.R. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-κB and MMP-9. J. Nutr. Biochem. 2016, 41, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Stawowczyk, M.; Wellenstein, M.D.; Lee, S.B.; Yomtoubian, S.; Durrans, A.; Choi, H.; Huang, W.R. Matrix Metalloproteinase 14 promotes lung cancer by cleavage of Heparin-Binding EGF-like Growth Factor. Neoplasia 2016, 19, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Shintani, T.; Kusuhara, Y.; Daizumoto, K.; Dondoo, T.O.; Yamamoto, H.; Mori, H.; Fukawa, T.; Nakatsuji, H.; Fukumori, T.; Takahashi, M.; et al. The Involvement of Hepatocyte Growth Factor-MET-Matrix Metalloproteinase 1 Signaling in Bladder Cancer Invasiveness and Proliferation. Effect of the MET Inhibitor, Cabozantinib (XL184), on Bladder Cancer Cells. Urology 2016. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, P.; Wielockx, B.; Puimege, L.; Noel, A.; Lopez-Otin, C.; Libert, C. Resistance of collagenase-2 (matrix metalloproteinase-8)-deficient mice to TNF-induced lethal hepatitis. J. Immunol. 2005, 175, 7642–7649. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Modulation of MMP-2 and MMP-9 secretion by cytokines, inducers and inhibitors in human glioblastoma T-98G cells. Oncol. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Naruse, T.; Ishida, Y.; Shigeishi, H.; Nakagawa, T.; Fukui, A.; Nishi, H.; Sasaki, K.; Ogawa, I.; Takechi, M. TNF-α-induced IL-6 and MMP-9 expression in immortalized ameloblastoma cell line established by hTERT. Oral Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.M.; Moilanen, J.A.; Sorsa, T.; Kivela-Rajamaki, M.; Tervahartiala, T.; Vesaluoma, M.H.; Tervo, T.M. Activation of matrix metalloproteinase-8 by membrane type 1-MMP and their expression in human tears after photorefractive keratectomy. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2550–2556. [Google Scholar] [CrossRef]
- Han, Y.P.; Yan, C.; Zhou, L.; Qin, L.; Tsukamoto, H. A matrix metalloproteinase-9 activation cascade by hepatic stellate cells in trans-differentiation in the three-dimensional extracellular matrix. J. Biol. Chem. 2007, 282, 12928–12939. [Google Scholar] [CrossRef] [PubMed]
- Dreier, R.; Grassel, S.; Fuchs, S.; Schaumburger, J.; Bruckner, P. Pro-MMP-9 is a specific macrophage product and is activated by osteoarthritic chondrocytes via MMP-3 or a MT1-MMP/MMP-13 cascade. Exp. Cell Res. 2004, 297, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Knauper, V.; Will, H.; Lopez-Otin, C.; Smith, B.; Atkinson, S.J.; Stanton, H.; Hembry, R.M.; Murphy, G. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J. Biol. Chem. 1996, 271, 17124–17131. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Dutzan, N.; Garcia-Sesnich, J.; Abusleme, L.; Dezerega, A.; Silva, N.; González, F.E.; Vernal, R.; Sorsa, T.; Gamonal, J. Host-pathogen interactions in progressive chronic periodontitis. J. Dent. Res. 2011, 90, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Van Wart, H.E.; Birkedal-Hansen, H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA 1990, 87, 5578–5582. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Tjaderhane, L.; Konttinen, Y.T.; Lauhio, A.; Salo, T.; Lee, H.M.; Golub, L.M.; Brown, D.L.; Mäntylä, P. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann. Med. 2006, 38, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Mantyla, P.; Stenman, M.; Kinane, D.; Salo, T.; Suomalainen, K.; Tikanoja, S.; Sorsa, T. Monitoring periodontal disease status in smokers and nonsmokers using a gingival crevicular fluid matrix metalloproteinase-8-specific chair-side test. J. Periodontal Res. 2006, 41, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, A.M.; Sorsa, T.; Pitkaniemi, J.; Tervahartiala, T.; Kari, K.; Broms, U.; Koskenvuo, M.; Meurman, J.H. Smoking affects diagnostic salivary periodontal disease biomarker levels in adolescents. J. Periodontol. 2010, 81, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Valenzuela, M.A.; Lopez-Otin, C.; Alvarez, J.; Lopez, J.M.; Vernal, R.; Gamonal, J. Matrix metalloproteinase-13 is highly expressed in destructive periodontal disease activity. J. Periodontol. 2006, 77, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Gamonal, J.; Tervahartiala, T.; Mantyla, P.; Rivera, O.; Dezerega, A.; Dutzan, N.; Sorsa, T. Associations between matrix metalloproteinase-8 and -14 and myeloperoxidase in gingival crevicular fluid from subjects with progressive chronic periodontitis: A longitudinal study. J. Periodontol. 2010, 81, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Rios, M.; Sorsa, T.; Obregon, F.; Tervahartiala, T.; Valenzuela, M.A.; Pozo, P.; Dutzan, N.; Lesaffre, E.; Molas, M.; Gamonal, J. Proteolytic roles of matrix metalloproteinase (MMP)-13 during progression of chronic periodontitis: Initial evidence for MMP-13/MMP-9 activation cascade. J. Clin. Periodontol. 2009, 36, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Ingman, T.; Suomalainen, K.; Haapasalo, M.; Konttinen, Y.T.; Lindy, O.; Saari, H.; Uitto, V.J. Identification of proteases from periodontopathogenic bacteria as activators of latent human neutrophil and fibroblast-type interstitial collagenases. Infect Immun. 1992, 60, 4491–4495. [Google Scholar] [PubMed]
- Overall, C.M. Molecular determinants of metalloproteinase substrate specificity: Matrix metalloproteinase substrate binding domains, modules, and exosites. Mol. Biotechnol. 2002, 22, 51–86. [Google Scholar] [CrossRef]
- Leppilahti, J.M.; Hernandez-Rios, P.A.; Gamonal, J.A.; Tervahartiala, T.; Brignardello-Petersen, R.; Mantyla, P.; Sorsa, T.; Hernández, M. Matrix metalloproteinases and myeloperoxidase in gingival crevicular fluid provide site-specific diagnostic value for chronic periodontitis. J. Clin. Periodontol. 2014, 41, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Gamonal, J.; Salo, T.; Tervahartiala, T.; Hukkanen, M.; Tjaderhane, L.; Sorsa, T. Reduced expression of lipopolysaccharide-induced CXC chemokine in Porphyromonas gingivalis-induced experimental periodontitis in matrix metalloproteinase-8 null mice. J. Periodontal Res. 2011, 46, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Saari, H.; Suomalainen, K.; Lindy, O.; Konttinen, Y.T.; Sorsa, T. Activation of latent human neutrophil collagenase by reactive oxygen species and serine proteases. Biochem. Biophys. Res. Commun. 1990, 171, 979–987. [Google Scholar] [CrossRef]
- Miyasaki, K.T.; Iofel, R.; Lehrer, R.I. Sensitivity of periodontal pathogens to the bactericidal activity of synthetic protegrins, antibiotic peptides derived from porcine leukocytes. J. Dent. Res. 1997, 76, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Krauss, J. Neutrophils in periodontal inflammation. Front. Oral Biol. 2012, 15, 56–83. [Google Scholar] [PubMed]
- Alfakry, H.; Malle, E.; Koyani, C.N.; Pussinen, P.J.; Sorsa, T. Neutrophil proteolytic activation cascades: A possible mechanistic link between chronic periodontitis and coronary heart disease. Innate Immun. 2016, 22, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Peppin, G.J.; Weiss, S.J. Activation of the endogenous metalloproteinase, gelatinase, by triggered human neutrophils. Proc. Natl. Acad. Sci. USA 1986, 83, 4322–4326. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J.; Peppin, G.; Ortiz, X.; Ragsdale, C.; Test, S.T. Oxidative autoactivation of latent collagenase by human neutrophils. Science 1985, 227, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.F.; Ho, K.Y.; Ho, Y.P.; Wu, Y.M.; Yang, Y.H.; Tsai, C.C. The investigation of glutathione peroxidase, lactoferrin, myeloperoxidase and interleukin-1β in gingival crevicular fluid: Implications for oxidative stress in human periodontal diseases. J. Periodontal Res. 2004, 39, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Marcaccini, A.M.; Meschiari, C.A.; Zuardi, L.R.; de Sousa, T.S.; Taba, M., Jr.; Teofilo, J.M.; Jacob-Ferreira, A.L.; Tanus-Santos, J.E.; Novaes, A.B., Jr.; Gerlach, R.F. Gingival crevicular fluid levels of MMP-8, MMP-9, TIMP-2, and MPO decrease after periodontal therapy. J. Clin. Periodontol. 2010, 37, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Baeza, M.; Garrido, M.; Hernandez-Rios, P.; Dezerega, A.; Garcia-Sesnich, J.; Strauss, F.; Aitken, J.P.; Lesaffre, E.; Vanbelle, S.; Gamonal, J.; et al. Diagnostic accuracy for apical and chronic periodontitis biomarkers in gingival crevicular fluid: An exploratory study. J. Clin. Periodontol. 2016, 43, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Shibanuma, M.; Nose, K. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res. 2004, 64, 7464–7472. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.O.; Park, S.J.; Yoon, S.Y.; Yun, C.H.; Chung, A.S. Sustained production of H2O2 activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-κB pathway. J. Biol. Chem. 2002, 277, 30271–30282. [Google Scholar] [CrossRef] [PubMed]
- Siwik, D.A.; Pagano, P.J.; Colucci, W.S. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am. J. Physiol. Cell Physiol. 2001, 280, C53–C60. [Google Scholar] [PubMed]
- Yu, M.; Sato, H.; Seiki, M.; Spiegel, S.; Thompson, E.W. Calcium influx inhibits MT1-MMP processing and blocks MMP-2 activation. FEBS Lett. 1997, 412, 568–572. [Google Scholar] [CrossRef]
- Tiranathanagul, S.; Yongchaitrakul, T.; Pattamapun, K.; Pavasant, P. Actinobacillus actinomycetemcomitans lipopolysaccharide activates matrix metalloproteinase-2 and increases receptor activator of nuclear factor-κB ligand expression in human periodontal ligament cells. J. Periodontol. 2004, 75, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Osorio, C.; Cavalla, F.; Paula-Lima, A.; Diaz-Araya, G.; Vernal, R.; Ahumada, P.; Gamonal, J.; Hernández, M. H2O2 activates matrix metalloproteinases through the nuclear factor κB pathway and Ca2+ signals in human periodontal fibroblasts. J. Periodontal Res. 2015, 50, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Korpi, J.T.; Astrom, P.; Lehtonen, N.; Tjaderhane, L.; Kallio-Pulkkinen, S.; Siponen, M.; Sorsa, T.; Pirilä, E.; Salo, T. Healing of extraction sockets in collagenase-2 (matrix metalloproteinase-8)-deficient mice. Eur. J. Oral Sci. 2009, 117, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Jackson, P.; Tester, A.M.; Diaconu, E.; Overall, C.M.; Blalock, J.E.; Pearlman, E. Matrix metalloproteinase-8 facilitates neutrophil migration through the corneal stromal matrix by collagen degradation and production of the chemotactic peptide Pro–Gly–Pro. Am. J. Pathol. 2008, 173, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Tester, A.M.; Cox, J.H.; Connor, A.R.; Starr, A.E.; Dean, R.A.; Puente, X.S.; López-Otín, C.; Overall, C.M. LPS responsiveness and neutrophil chemotaxis in vivo require PMN MMP-8 activity. PLoS ONE 2007, 2, e312. [Google Scholar] [CrossRef] [PubMed]
- Van Den Steen, P.E.; Wuyts, A.; Husson, S.J.; Proost, P.; van Damme, J.; Opdenakker, G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur. J. Biochem. 2003, 270, 3739–3749. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Fernandez, A.; Inada, M.; Balbin, M.; Fueyo, A.; Pitiot, A.S.; Astudillo, A.; Hirose, K.; Hirata, M.; Shapiro, S.D.; Noël, A.; et al. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8). FASEB J. 2007, 21, 2580–2591. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, T.A.; Silva, M.J.B.; Alves, P.M.; Chica, J.E.L.; Barcelos, E.Z.; Giani, M.A.A.; Garlet, G.P.; da Silva, J.S.; Júnior, V.R.; Rodrigues, D.B.R.; et al. Inflammation Biomarkers of Advanced Disease in Nongingival Tissues of Chronic Periodontitis Patients. Mediat. Inflamm. 2015. [Google Scholar] [CrossRef] [PubMed]
- Cavalla, F.; Araujo-Pires, A.C.; Biguetti, C.C.; Garlet, G.P. Cytokine Networks Regulating Inflammation and Immune Defense in the Oral Cavity. Curr. Oral Health Rep. 2014, 1, 104–113. [Google Scholar] [CrossRef]
- Stadler, A.F.; Angst, P.D.; Arce, R.M.; Gomes, S.C.; Oppermann, R.V.; Susin, C. Gingival crevicular fluid levels of cytokines/chemokines in chronic periodontitis: A meta-analysis. J. Clin. Periodontol. 2016, 43, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S.; Page, R.C.; Tonetti, M.S. The host response to the microbial challenge in periodontitis: Assembling the players. Periodontol. 2000 1997, 14, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, A.J.; Gottardi, R.; Yoshizawa, S.; Cavalla, F.; Garlet, G.P.; Sfeir, C.; Little, S.R. Strategies to Direct the Enrichment, Expansion, and Recruitment of Regulatory Cells for the Treatment of Disease. Ann. Biomed. Eng. 2015, 43, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Finn, R. Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2016, 44, D343–D350. [Google Scholar] [CrossRef] [PubMed]
- Cavalla, F.; Osorio, C.; Paredes, R.; Valenzuela, M.A.; Garcia-Sesnich, J.; Sorsa, T.; Tervahartiala, T.; Hernández, M. Matrix metalloproteinases regulate extracellular levels of SDF-1/CXCL12, IL-6 and VEGF in hydrogen peroxide-stimulated human periodontal ligament fibroblasts. Cytokine 2015, 73, 114–121. [Google Scholar] [CrossRef] [PubMed]
- McQuibban, G.A.; Butler, G.S.; Gong, J.H.; Bendall, L.; Power, C.; Clark-Lewis, I.; Overall, C.M. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J. Biol. Chem. 2001, 276, 43503–43508. [Google Scholar] [CrossRef] [PubMed]
- Bauvois, B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: Outside-in signaling and relationship to tumor progression. Biochim. Biophys. Acta 2012, 1825, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kuula, H.; Salo, T.; Pirila, E.; Tuomainen, A.M.; Jauhiainen, M.; Uitto, V.J.; Tjäderhane, L.; Pussinen, P.J.; Sorsa, T. Local and systemic responses in matrix metalloproteinase 8-deficient mice during Porphyromonas gingivalis-induced periodontitis. Infect. Immun. 2009, 77, 850–859. [Google Scholar] [CrossRef] [PubMed]
- McQuibban, G.A.; Gong, J.H.; Wong, J.P.; Wallace, J.L.; Clark-Lewis, I.; Overall, C.M. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood 2002, 100, 1160–1167. [Google Scholar] [PubMed]
- Dezerega, A.; Pozo, P.; Hernandez, M.; Oyarzun, A.; Rivera, O.; Dutzan, N.; Gutiérrez-Fernández, A.; Overall, C.M.; Garrido, M.; Alcota, M.; Ortiz, E.; et al. Chemokine monocyte chemoattractant protein-3 in progressive periodontal lesions in patients with chronic periodontitis. J. Periodontol. 2010, 81, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Adhikary, R.; Nandi, A.; Bishayi, B. Neutralization of MMP-2 protects Staphylococcus aureus infection induced septic arthritis in mice and regulates the levels of cytokines. Microb. Pathog. 2016, 99, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Astrom, P.; Pirila, E.; Lithovius, R.; Heikkola, H.; Korpi, J.T.; Hernandez, M.; Sorsa, T.; Salo, T. Matrix metalloproteinase-8 regulates transforming growth factor-β1 levels in mouse tongue wounds and fibroblasts in vitro. Exp. Cell Res. 2014, 328, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, U.W.; Jonsson, J.A.; Dabrosin, C. Tamoxifen decreases extracellular TGF-β1 secreted from breast cancer cells—A post-translational regulation involving matrix metalloproteinase activity. Exp. Cell Res. 2009, 315, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Balbin, M.; Fueyo, A.; Tester, A.M.; Pendas, A.M.; Pitiot, A.S.; Astudillo, A.; Overall, C.M.; Shapiro, S.D.; López-Otín, C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat. Genet. 2003, 35, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Hill, P.A.; Docherty, A.J.; Bottomley, K.M.; O’Connell, J.P.; Morphy, J.R.; Reynolds, J.J.; Meikle, M.C. Inhibition of bone resorption in vitro by selective inhibitors of gelatinase and collagenase. Biochem. J. 1995, 308, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, E.; Scapolan, M.; Pecolo, M.; Wassermann, B.; Abu-Rumeileh, I.; Balestreri, L.; Borsatti, E.; Tripodo, C.; Colombatti, A.; Spessotto, P. MMP-13 stimulates osteoclast differentiation and activation in tumour breast bone metastases. Breast Cancer Res. 2011, 13, R105. [Google Scholar] [CrossRef] [PubMed]
- Nannuru, K.C.; Futakuchi, M.; Varney, M.L.; Vincent, T.M.; Marcusson, E.G.; Singh, R.K. Matrix metalloproteinase (MMP)-13 regulates mammary tumor-induced osteolysis by activating MMP9 and transforming growth factor-β signaling at the tumor-bone interface. Cancer Res. 2010, 70, 3494–3504. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.E.; Watanabe, T.; Chen, C.; Chekenya, M.; Stallcup, W.B.; Kapila, Y.L. NG2, a novel proapoptotic receptor, opposes integrin α4 to mediate anoikis through PKCα-dependent suppression of FAK phosphorylation. Cell Death Differ. 2008, 15, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.E.; Miao, D.; Bermudez, M.; Stallcup, W.B.; Kapila, Y.L. Shedding of NG2 by MMP-13 attenuates anoikis. DNA Cell Biol. 2014, 33, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.; Kirkpatrick, G.; Evans, M.; Gorski, G.; Foster, S.; Borghaei, R.C. IL-4 inhibition of IL-1 induced Matrix metalloproteinase-3 (MMP-3) expression in human fibroblasts involves decreased AP-1 activation via negative crosstalk involving of Jun N-terminal kinase (JNK). Exp. Cell Res. 2013, 319, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Marchant, D.J.; Bellac, C.L.; Moraes, T.J.; Wadsworth, S.J.; Dufour, A.; Butler, G.S.; Bilawchuk, L.M.; Hendry, R.G.; Robertson, A.G.; Cheung, C.T.; et al. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat. Med. 2014, 20, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Kubota, S.; Kawata, K.; Mukudai, Y.; Uehara, J.; Ohgawara, T.; Ibaragi, S.; Sasaki, A.; Kuboki, T.; Takigawa, M. Novel transcription-factor-like function of human matrix metalloproteinase 3 regulating the CTGF/CCN2 gene. Mol. Cell. Biol. 2008, 28, 2391–2413. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Yan, Y.; Jin, Y.H.; Meng, X.Y.; Mo, Y.Y.; Zeng, X.T. Matrix metalloproteinase gene polymorphisms and periodontitis susceptibility: A meta-analysis involving 6162 individuals. Sci. Rep. 2016, 6, 24812. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cai, Q.; Ma, L.; Wang, M.; Ma, J.; Zhang, W.; Pan, Y.; Wang, L. Association between MMP-1 g.-1607dupG polymorphism and periodontitis susceptibility: A meta-analysis. PLoS ONE 2013, 8, e59513. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, C.; Patricia, H.-R.; Timo, S.; Claudia, B.; Marcela, H. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. Int. J. Mol. Sci. 2017, 18, 440. https://doi.org/10.3390/ijms18020440
Franco C, Patricia H-R, Timo S, Claudia B, Marcela H. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. International Journal of Molecular Sciences. 2017; 18(2):440. https://doi.org/10.3390/ijms18020440
Chicago/Turabian StyleFranco, Cavalla, Hernández-Ríos Patricia, Sorsa Timo, Biguetti Claudia, and Hernández Marcela. 2017. "Matrix Metalloproteinases as Regulators of Periodontal Inflammation" International Journal of Molecular Sciences 18, no. 2: 440. https://doi.org/10.3390/ijms18020440