Taste Receptors Mediate Sinonasal Immunity and Respiratory Disease
Abstract
:1. Introduction
2. Genetic Variability of TAS2R38
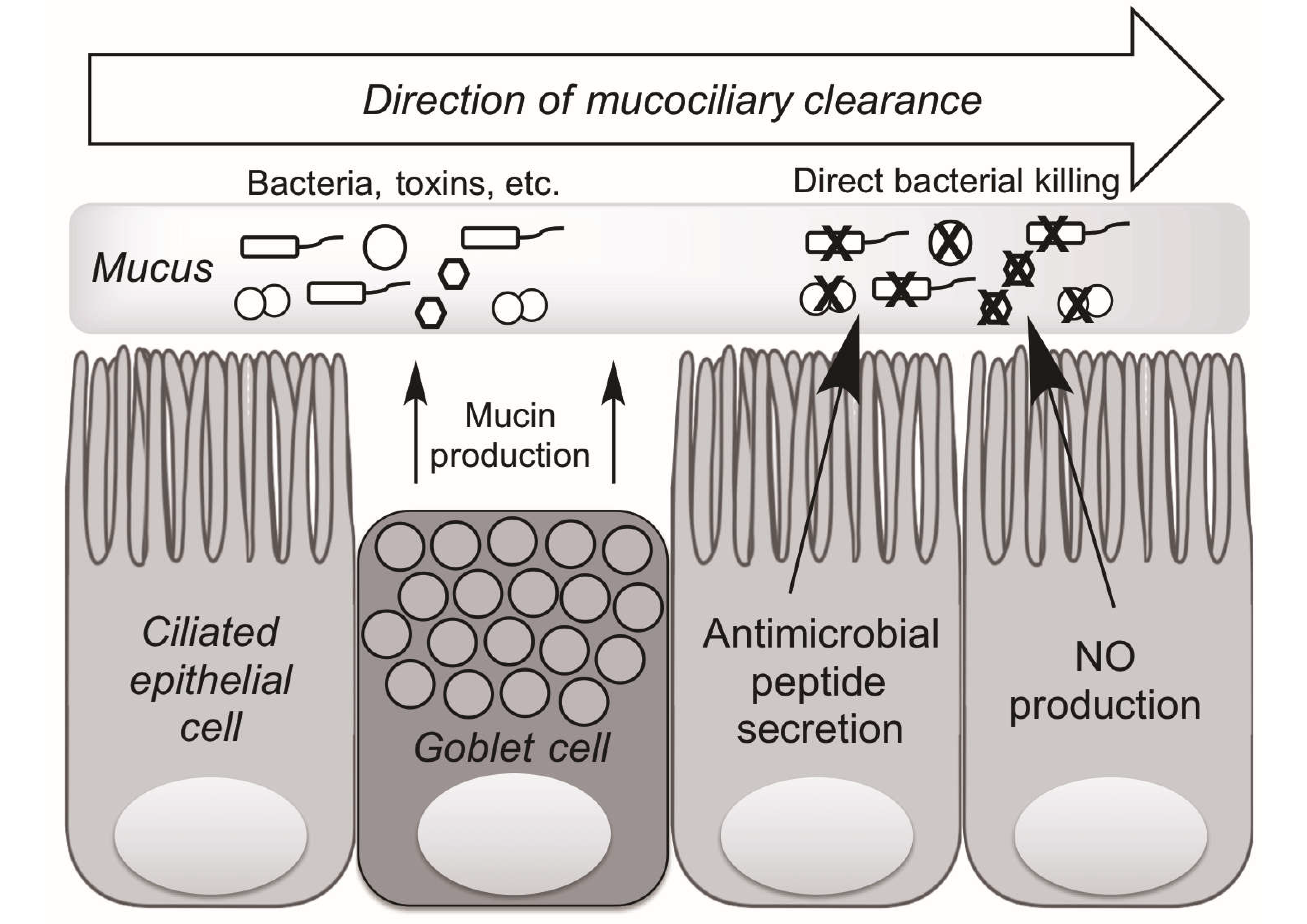
3. Mechanisms of Upper Airway Immunity
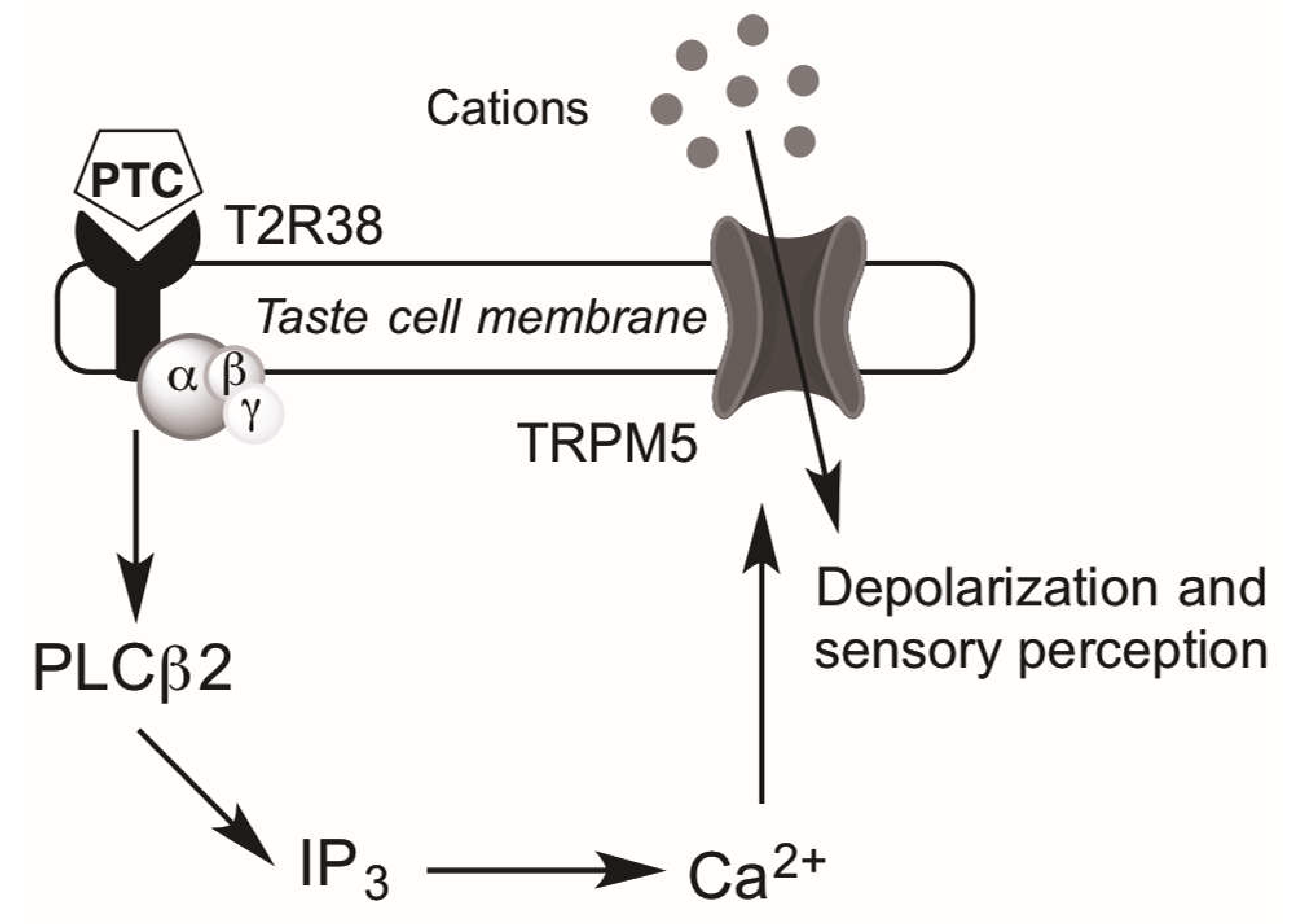
4. Taste Receptors and Upper Airway Immunity
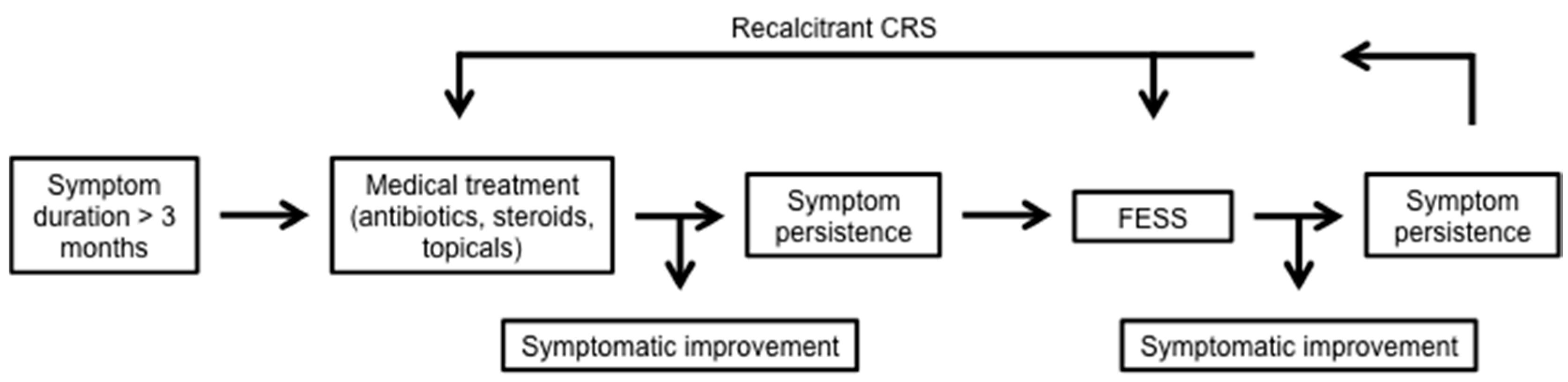
5. Conditions of Defective Airway Immunity
6. Taste Influence on Biofilm
7. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| CRS | Chronic rhinosinusitis |
| GPCRs | G protein-coupled receptors |
| AHLs | Acyl-homoserine lactones |
| PTC | Phenylthiocarbamide |
| QOL | Quality of life |
| PLCβ2 | Phosopholipase C isoform β2 |
| IP3 | Inositol 1,4,5-trisphosphate |
| Ca2+ | Calcium ions |
| TRPM5 | Transient receptor potential cation channel subfamily M member 5 |
| PROP | Propylthiouracil |
| SNPs | Single nucleotide polymorphisms |
| PAV | Proline-alanine-valine TAS2R38 haplotype (“tasters”) |
| AVI | Alanine-valine-isoleucine TAS2R38 haplotype (“non-tasters”) |
| ASL | Airway surface liquid |
| MCC | Mucociliary clearance |
| NO | Nitric oxide |
| AMPs | Antimicrobial peptides |
| CBF | Ciliary beat frequency |
| DB | Denatonium benzoate |
| SCCs | Solitary chemosensory cells |
| ALI | Air-liquid interface culture |
| C4HSL | N-butyryl-l-homoserine lactone |
| C12HSL | N-3-oxo-dodecanoyl-l-homoserine lactone |
| glc | Glucose |
| CF | Cystic fibrosis |
| SNOT-22 | Sinonasal outcome test |
| FESS | Functional endoscopic sinus surgery |
References
- Kinnamon, S.C. Taste receptor signalling—From tongues to lungs. Acta Physiol. 2012, 204, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, J.; Mueller, K.L.; Hoon, M.A.; Adler, E.; Feng, L.; Guo, W.; Zuker, C.S.; Ryba, N.J. T2Rs function as bitter taste receptors. Cell 2000, 100, 703–711. [Google Scholar] [CrossRef]
- Adler, E.; Hoon, M.A.; Mueller, K.L.; Chandrashekar, J.; Ryba, N.J.; Zuker, C.S. A novel family of mammalian taste receptors. Cell 2000, 100, 693–702. [Google Scholar] [CrossRef]
- Hoon, M.A.; Adler, E.; Lindemeier, J.; Battey, J.F.; Ryba, N.J.P.; Zuker, C.S. Putative mammalian taste receptors: A class of taste-specific gpcrs with distinct topographic selectivity. Cell 1999, 96, 541–551. [Google Scholar] [CrossRef]
- Clark, A.A.; Liggett, S.B.; Munger, S.D. Extraoral bitter taste receptors as mediators of off-target drug effects. FASEB J. 2012, 26, 4827–4831. [Google Scholar] [CrossRef] [PubMed]
- Laffitte, A.; Neiers, F.; Briand, L. Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.A.; Dotson, C.D.; Elson, A.E.; Voigt, A.; Boehm, U.; Meyerhof, W.; Steinle, N.I.; Munger, S.D. Tas2r bitter taste receptors regulate thyroid function. FASEB J. 2015, 29, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.L.; Hoon, M.A.; Erlenbach, I.; Chandrashekar, J.; Zuker, C.S.; Ryba, N.J.P. The receptors and coding logic for bitter taste. Nature 2005, 434, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Wölfle, U.; Elsholz, F.A.; Kersten, A.; Haarhaus, B.; Müller, W.E.; Schempp, C.M. Expression and functional activity of the bitter taste receptors TAS2R1 and TAS2R38 in human keratinocytes. Skin Pharmacol. Physiol. 2015, 28, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cao, J.; Iguchi, N.; Riethmacher, D.; Huang, L. Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol. Hum. Reprod. 2013, 19, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Investig. 2012, 122, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Ben-Shahar, Y.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile cilia of human airway epithelia are chemosensory. Science 2009, 325, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.A. The genetics of the bitter taste receptor T2R38 in upper airway innate immunity and implications for chronic rhinosinusitis. Laryngoscope 2016, 127, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Kofonow, J.M.; Rosen, P.L.; Siebert, A.P.; Chen, B.; Doghramji, L.; Xiong, G.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J. Clin. Investig. 2014, 124, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Spector, A.C.; Reed, D.R.; Coldwell, S.E. The bad taste of medicines: Overview of basic research on bitter taste. Clin. Ther. 2013, 35, 1225–1246. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ishimaru, Y. Oral and extra-oral taste perception. Semin. Cell Dev. Biol. 2013, 24, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Taruno, A.; Vingtdeux, V.; Ohmoto, M.; Ma, Z.; Dvoryanchikov, G.; Li, A.; Adrien, L.; Zhao, H.; Leung, S.; Abernethy, M.; et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 2013, 495, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Netea, M.G.; Holland, S.M. Mendelian genetics of human susceptibility to fungal infection. Cold Spring Harb. Perspect. Med. 2014, 4, a019638. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, C.; Bufe, B.; Batram, C.; Meyerhof, W. Oligomerization of TAS2R bitter taste receptors. Chem. Sens. 2010, 35, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Bufe, B.; Breslin, P.A.S.; Kuhn, C.; Reed, D.R.; Tharp, C.D.; Slack, J.P.; Kim, U.-K.; Drayna, D.; Meyerhof, W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr. Biol. 2005, 15, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Sens. 2010, 35, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Lipchock, S.V.; Mennella, J.A.; Spielman, A.I.; Reed, D.R. Human bitter perception correlates with bitter receptor messenger rna expression in taste cells. Am. J. Clin. Nutr. 2013, 98, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Adappa, N.D.; Zhang, Z.; Palmer, J.N.; Kennedy, D.W.; Doghramji, L.; Lysenko, A.; Reed, D.R.; Scott, T.; Zhao, N.W.; Owens, D.; et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int. Forum Allergy Rhinol. 2014, 4, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W.; Reed, D.R. The genetics of phenylthiocarbamide perception. Ann. Hum. Biol. 2001, 28, 111–142. [Google Scholar] [PubMed]
- Behrens, M.; Gunn, H.C.; Ramos, P.C.M.; Meyerhof, W.; Wooding, S.P. Genetic, functional, and phenotypic diversity in TAS2R38-mediated bitter taste perception. Chem. Senses 2013, 38, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.A. Sinonasal mucociliary clearance in health and disease. Ann. Otol. Rhinol. Laryngol. Suppl. 2006, 115, 20–26. [Google Scholar] [CrossRef]
- Gudis, D.; Zhao, K.Q.; Cohen, N.A. Acquired cilia dysfunction in chronic rhinosinusitis. Am. J. Rhinol. Allergy 2012, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.B.; Gudis, D.A.; Cohen, N.A. Epithelium, cilia, and mucus: Their importance in chronic rhinosinusitis. Immunol. Allergy Clin. N. Am. 2009, 29, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Eliezer, N.; Sade, J.; Silberberg, A.; Nevo, A.C. The role of mucus in transport by cilia. Am. Rev. Respir. Dis. 1970, 102, 48–52. [Google Scholar] [PubMed]
- Knowles, M.R.; Boucher, R.C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Investig. 2002, 109, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, P.N.; Koch, G.; Thompson, J.A.; Xavier, K.B.; Cool, R.H.; Quax, W.J. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 2012, 76, 46–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Workman, A.D.; Carey, R.M.; Chen, B.; Rosen, P.L.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Cohen, N.A. Fungal aflatoxins reduce respiratory mucosal ciliary function. Sci. Rep. 2016, 6, 33221. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, A.; Burlingame, A.L.; Eberhard, C.; Kenyon, G.L.; Nealson, K.H.; Oppenheimer, N.J. Structural identification of autoinducer of photobacterium fischeri luciferase. Biochemistry 1981, 20, 2444–2449. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.J.; Lee, S.K.; Kim, T.; Ghim, C.M. Microbial linguistics: Perspectives and applications of microbial cell-to-cell communication. BMB Rep. 2011, 44, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.J.; Stolz, D.B. Demonstration of biofilm in human bacterial chronic rhinosinusitis. Am. J. Rhinol. 2005, 19, 452–457. [Google Scholar] [PubMed]
- Bendouah, Z.; Barbeau, J.; Hamad, W.A.; Desrosiers, M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol. Head Neck Surg. 2006, 134, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Tizzano, M.; Gulbransen, B.D.; Vandenbeuch, A.; Clapp, T.R.; Herman, J.P.; Sibhatu, H.M.; Churchill, M.E.A.; Silver, W.L.; Kinnamon, S.C.; Finger, T.E. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc. Natl. Acad. Sci. USA 2010, 107, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, M., Jr.; Lane, A.P. A comparison of experimental methods in molecular chronic rhinosinusitis research. Am. J. Rhinol. 2007, 21, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Dimova, S.; Brewster, M.E.; Noppe, M.; Jorissen, M.; Augustijns, P. The use of human nasal in vitro cell systems during drug discovery and development. Toxicol. In Vitro 2005, 19, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Chen, B.; Redding, K.M.; Margolskee, R.F.; Cohen, N.A. Mouse nasal epithelial innate immune responses to Pseudomonas aeruginosa quorum-sensing molecules require taste signaling components. Innate Immun. 2013, 20, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C. Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 1997, 99, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J. Nitric oxide and antimicrobial activity of reactive oxygen intermediates. Immunopharmacology 1997, 37, 35–41. [Google Scholar] [CrossRef]
- Jones, M.L.; Ganopolsky, J.G.; Labbé, A.; Wahl, C.; Prakash, S. Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl. Microbiol. Biotechnol. 2010, 88, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Salathe, M. Regulation of mammalian ciliary beating. Annu. Rev. Physiol. 2007, 69, 401–422. [Google Scholar] [CrossRef] [PubMed]
- Jain, B.; Rubinstein, I.; Robbins, R.A.; Leise, K.L.; Sisson, J.H. Modulation of airway epithelial cell ciliary beat frequency by nitric oxide. Biochem. Biophys. Res. Commun. 1993, 191, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.A.; Margolskee, R.F.; Kinnamon, S.C.; Ogura, T. Making sense with trp channels: Store-operated calcium entry and the ion channel TRPM5 in taste receptor cells. Cell Calcium 2003, 33, 541–549. [Google Scholar] [CrossRef]
- Tizzano, M.; Finger, T.E. Chemosensors in the nose: Guardians of the airways. Physiology 2013, 28, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.J.; Christensen, M.; Finger, T.E.; Tizzano, M. Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc. Natl. Acad. Sci. USA 2014, 111, 6075–6080. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, K.K.; Baker, E.H.; Fraser, O.; Chung, Y.L.; Mace, O.J.; Tarelli, E.; Philips, B.J.; Baines, D.L. Glucose homeostasis across human airway epithelial cell monolayers: Role of diffusion, transport and metabolism. Pflug. Arch. 2009, 457, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Cui, M.; Zhao, B.; Liu, Z.; Snyder, L.A.; Benard, L.M.; Osman, R.; Margolskee, R.F.; Max, M. Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J. Biol. Chem. 2005, 280, 15238–15246. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Cui, M.; Zhao, B.; Snyder, L.A.; Benard, L.M.; Osman, R.; Max, M.; Margolskee, R.F. Identification of the cyclamate interaction site within the transmembrane domain of the human sweet taste receptor subunit T1R3. J. Biol. Chem. 2005, 280, 34296–34305. [Google Scholar] [CrossRef] [PubMed]
- Garnett, J.P.; Baker, E.H.; Baines, D.L. Sweet talk: Insights into the nature and importance of glucose transport in lung epithelium. Eur. Respir. J. 2012, 40, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Koziel, H.; Koziel, M.J. Pulmonary complications of diabetes mellitus. Pneumonia. Infect. Dis. Clin. N. Am. 1995, 9, 65–96. [Google Scholar]
- Zhang, Z.; Adappa, N.D.; Lautenbach, E.; Chiu, A.G.; Doghramji, L.; Howland, T.J.; Cohen, N.A.; Palmer, J.N. The effect of diabetes mellitus on chronic rhinosinusitis and sinus surgery outcome. Int. Forum Allergy Rhinol. 2014, 4, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Gliklich, R.E.; Metson, R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol. Head Neck Surg. 1995, 113, 104–109. [Google Scholar] [CrossRef]
- Hopkins, C.; Gillett, S.; Slack, R.; Lund, V.J.; Browne, J.P. Psychometric validity of the 22-item sinonasal outcome test. Clin. Otolaryngol. 2009, 34, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Adappa, N.D.; Howland, T.J.; Palmer, J.N.; Kennedy, D.W.; Doghramji, L.; Lysenko, A.; Reed, D.R.; Lee, R.J.; Cohen, N.A. Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int. Forum Allergy Rhinol. 2013, 3, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Adappa, N.D.; Farquhar, D.; Palmer, J.N.; Kennedy, D.W.; Doghramji, L.; Morris, S.A.; Owens, D.; Mansfield, C.; Lysenko, A.; Lee, R.J.; et al. TAS2R38 genotype predicts surgical outcome in nonpolypoid chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2016, 6, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Mfuna Endam, L.; Filali-Mouhim, A.; Boisvert, P.; Boulet, L.P.; Bosse, Y.; Desrosiers, M. Genetic variations in taste receptors are associated with chronic rhinosinusitis: A replication study. Int. Forum Allergy Rhinol. 2014, 4, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Dżaman, K.; Zagor, M.; Sarnowska, E.; Krzeski, A.; Kantor, I. The correlation of TAS2R38 gene variants with higher risk for chronic rhinosinusitis in polish patients. Otolaryngol. Pol. 2016, 70, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Grossi, S.; Montrasio, G.; Binelli, G.; Cinquetti, R.; Simmen, D.; Castelnuovo, P.; Campomenosi, P. TAS2R38 taste receptor gene and chronic rhinosinusitis: New data from an Italian population. BMC Med. Genet. 2016, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Ferril, G.R.; Nick, J.A.; Getz, A.E.; Barham, H.P.; Saavedra, M.T.; Taylor-Cousar, J.L.; Nichols, D.P.; Curran-Everett, D.; Kingdom, T.T.; Ramakrishnan, V.R. Comparison of radiographic and clinical characteristics of low-risk and high-risk cystic fibrosis genotypes. Int. Forum Allergy Rhinol. 2014, 4, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Johansen, H.K.; Nir, M.; Høiby, N.; Koch, C.; Schwartz, M. Severity of cystic fibrosis in patients homozygous and heterozygous for ΔF508 mutation. Lancet 1991, 337, 631–634. [Google Scholar] [CrossRef]
- Jorissen, M.B.; de Boeck, K.; Cuppens, H. Genotype-phenotype correlations for the paranasal sinuses in cystic fibrosis. Am. J. Respir. Crit. Care Med. 1999, 159, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, B.; Richardson, M.A. Impact of sinusitis in cystic fibrosis. J. Allergy Clin. Immunol. 1992, 90, 547–552. [Google Scholar] [CrossRef]
- Marks, S.C.; Kissner, D.G. Management of sinusitis in adult cystic fibrosis. Am. J. Rhinol. 1997, 11, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Mainz, J.G.; Koitschev, A. Management of chronic rhinosinusitis in CF. J. Cyst. Fibros. 2009, 8, S10–S14. [Google Scholar] [CrossRef]
- Adappa, N.D.; Workman, A.D.; Hadjiliadis, D.; Dorgan, D.J.; Frame, D.; Brooks, S.; Doghramji, L.; Palmer, J.N.; Mansfield, C.; Reed, D.R.; et al. T2R38 genotype is correlated with sinonasal quality of life in homozygous ΔF508 cystic fibrosis patients. Int. Forum Allergy Rhinol. 2016, 6, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Ciofu, O.; Bjarnsholt, T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010, 5, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Adappa, N.D.; Truesdale, C.M.; Workman, A.D.; Doghramji, L.; Mansfield, C.; Kennedy, D.W.; Palmer, J.N.; Cowart, B.J.; Cohen, N.A. Correlation of T2R38 taste phenotype and in vitro biofilm formation from nonpolypoid chronic rhinosinusitis patients. Int. Forum Allergy Rhinol. 2016, 6, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Tyler, M.A.; Russell, C.B.; Smith, D.E.; Rottman, J.B.; Padro Dietz, C.J.; Hu, X.; Citardi, M.J.; Fakhri, S.; Assassi, S.; Luong, A. Large scale gene expression profiling reveals distinct type 2 inflammatory patterns in chronic rhinosinusitis subtypes. J. Allergy Clin. Immunol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Genoway, K.A.; Philpott, C.M.; Javer, A.R. Pathogen yield and antimicrobial resistance patterns of chronic rhinosinusitis patients presenting to a tertiary rhinology centre. J. Otolaryngol. Head Neck Surg. 2011, 40, 232–237. [Google Scholar] [PubMed]
- Carey, R.M.; Workman, A.D.; Chen, B.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; Lee, R.J.; Cohen, N.A. Staphylococcus aureus triggers nitric oxide production in human upper airway epithelium. Int. Forum Allergy Rhinol. 2015, 5, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Roudnitzky, N.; Behrens, M.; Engel, A.; Kohl, S.; Thalmann, S.; Hübner, S.; Lossow, K.; Wooding, S.P.; Meyerhof, W. Receptor polymorphism and genomic structure interact to shape bitter taste perception. PLoS Genet. 2015, 11, e1005530. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douglas, J.E.; Cohen, N.A. Taste Receptors Mediate Sinonasal Immunity and Respiratory Disease. Int. J. Mol. Sci. 2017, 18, 437. https://doi.org/10.3390/ijms18020437
Douglas JE, Cohen NA. Taste Receptors Mediate Sinonasal Immunity and Respiratory Disease. International Journal of Molecular Sciences. 2017; 18(2):437. https://doi.org/10.3390/ijms18020437
Chicago/Turabian StyleDouglas, Jennifer E., and Noam A. Cohen. 2017. "Taste Receptors Mediate Sinonasal Immunity and Respiratory Disease" International Journal of Molecular Sciences 18, no. 2: 437. https://doi.org/10.3390/ijms18020437





