Antimicrobial Treatment of Polymeric Medical Devices by Silver Nanomaterials and Related Technology
Abstract
:1. Introduction
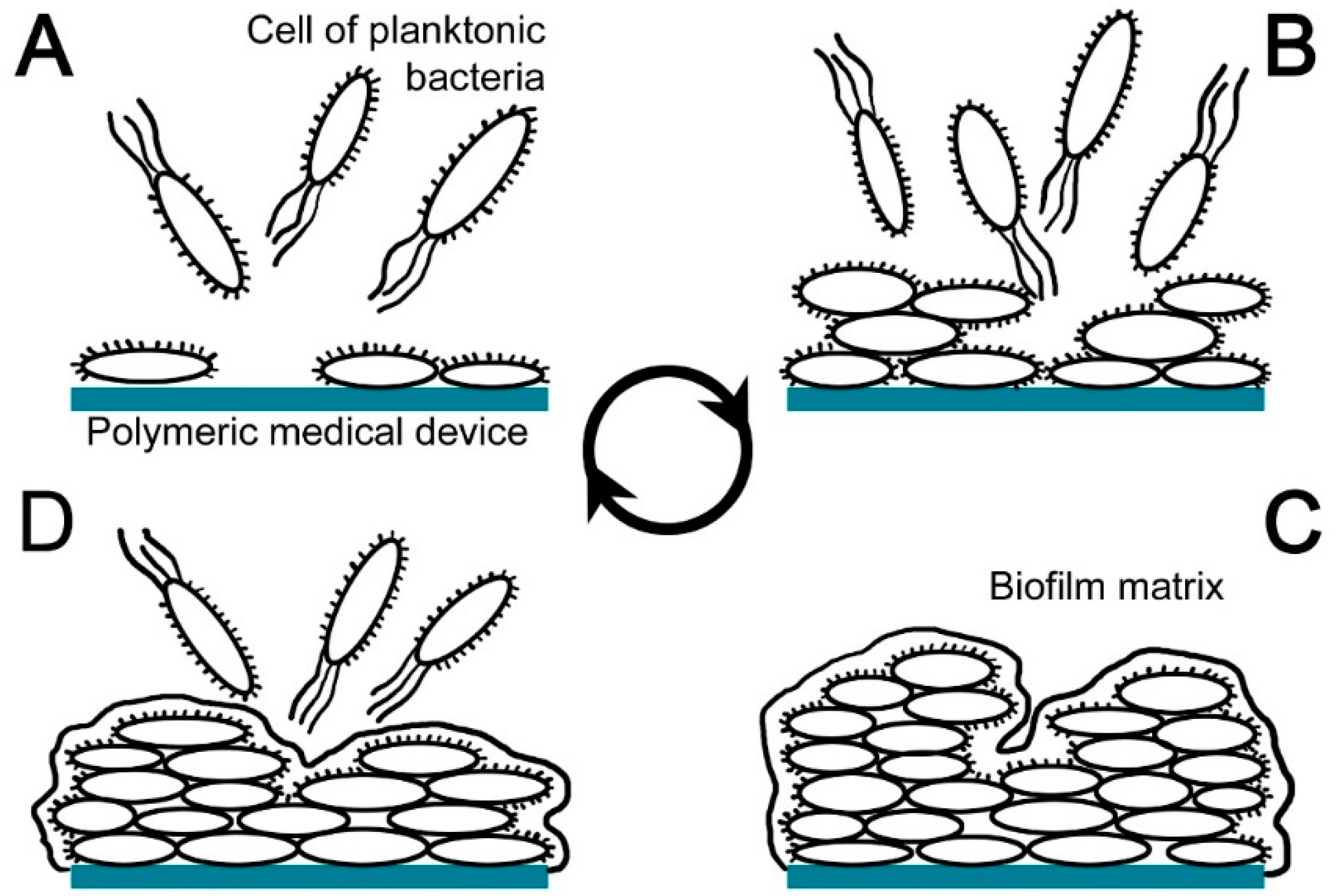
2. Polymeric Medical Devices Related to Nosocomial Infections
- coating of the material surface, masking of its surface properties and contaminating surrounding media (body fluids);
- leaching out of additives and monomers originating from polymer by microbial degradation;
- enzymatic and/or radical attack of polymer and additives, which causes embrittlement of the material and the loss of its mechanical stability;
- accumulation of water and penetration of microbial filaments into the polymer, which results in increased conductivity of material and formation of swelling;
- excretion of lipophilic microbial pigments, which leads to a colour change in the polymer.
3. Methods of Antimicrobial Treatment of Polymeric Medical Devices
3.1. Conventional Antibiotics and Antiseptics
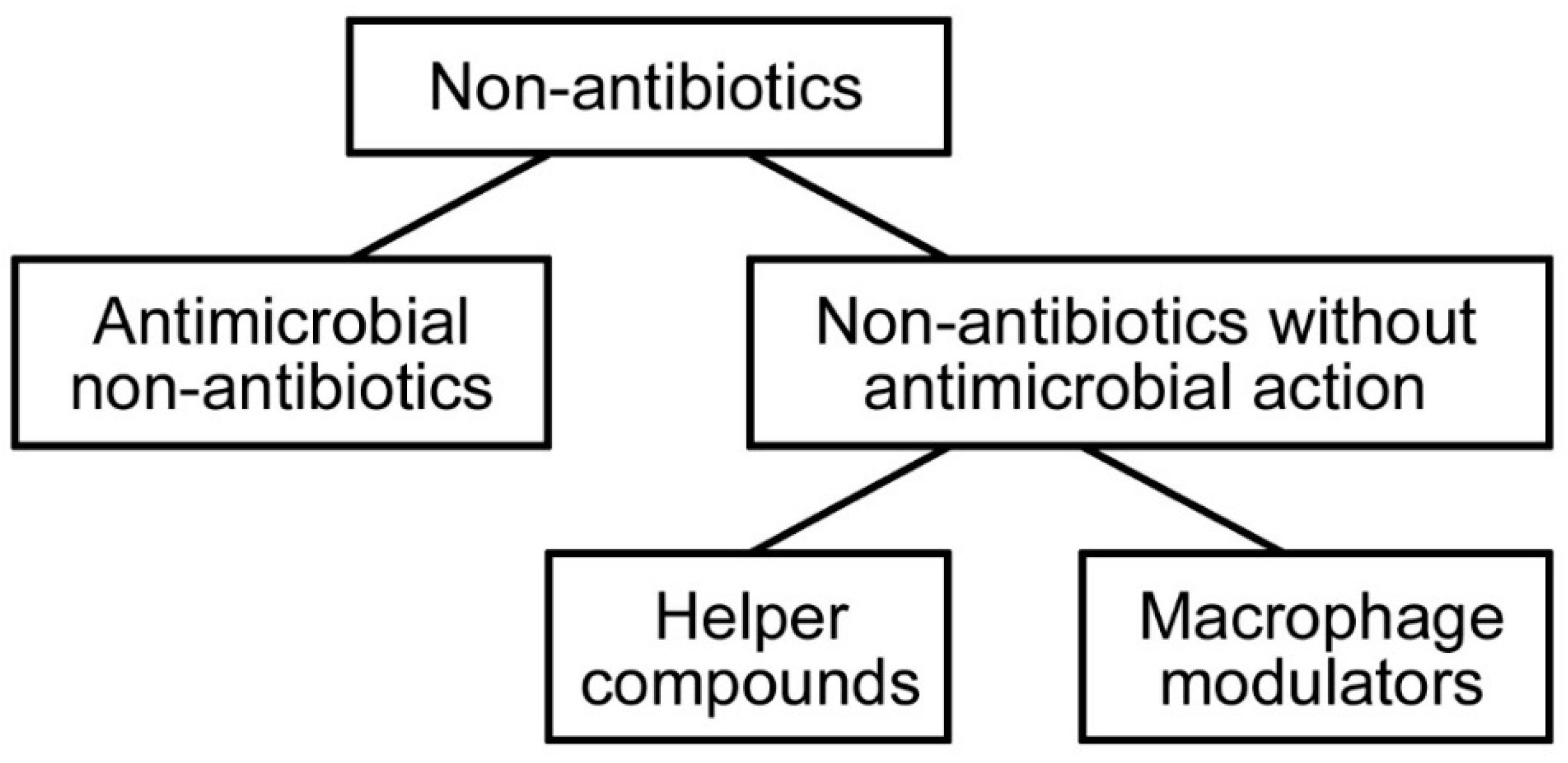
3.2. Non-Antibiotics
3.3. Noble Metals
4. Nanostructured Silver
4.1. Mechanism of Antimicrobial Action
4.2. Types of Silver Nanostructures
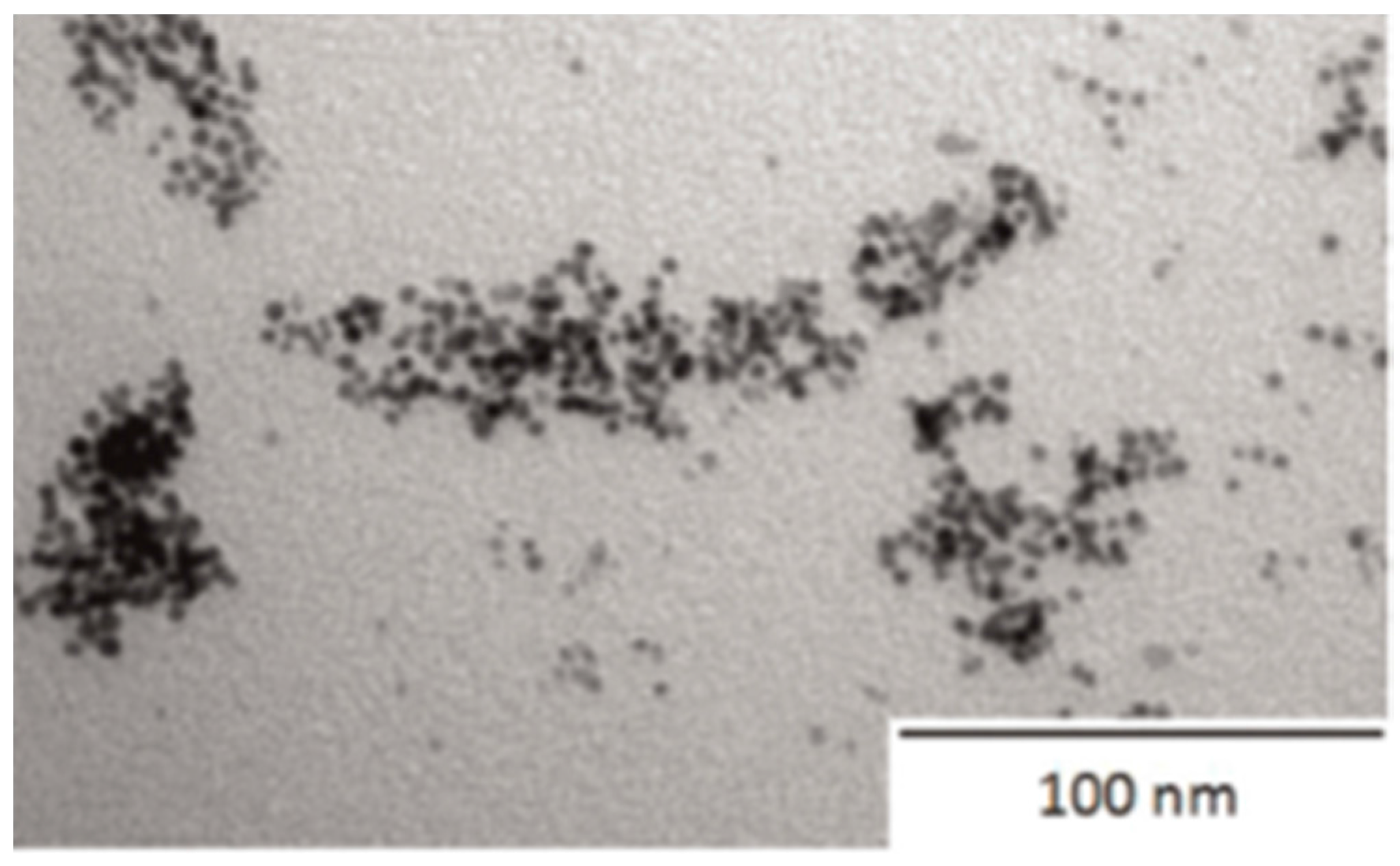
4.2.1. Silver Nanoparticles
4.2.2. Silver Nanowires
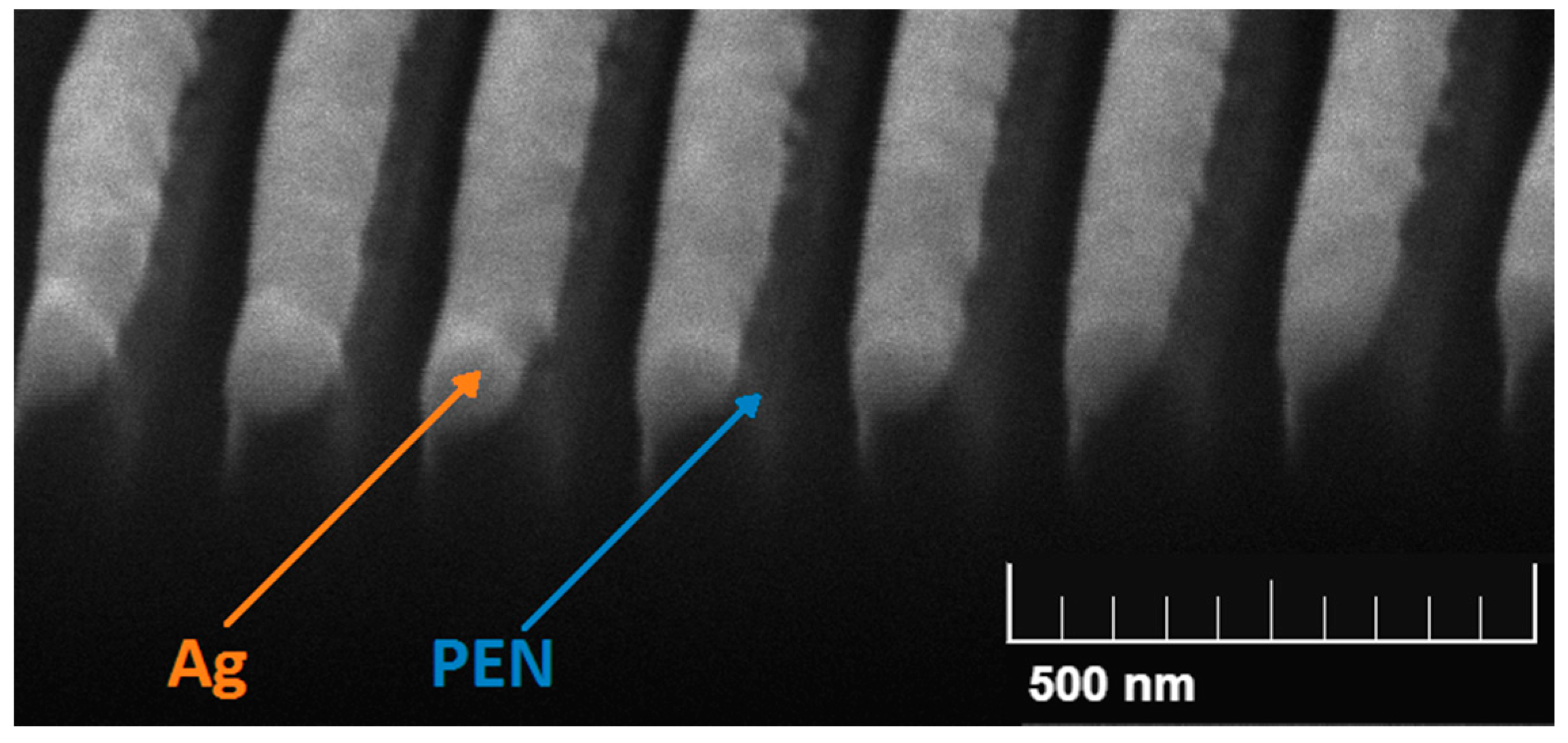
4.2.3. Silver Nanolayers
4.3. Overview of Preparation Techniques of Silver Nanostructures
4.3.1. Silver Nanoparticles
4.3.2. Silver Nanowires
4.3.3. Silver Nanolayers
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Samuel, U.; Guggenbichlerb, J.P. Prevention of catheter-related infections: The potential of a new nano-silver impregnated catheter. Int. J. Antimicrob. Agents 2004, 23, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for prevention of surgical site infection, 1999. Am. J. Infect. Control 1999, 27, 97–134. [Google Scholar] [CrossRef]
- Richards, M.J.; Edwards, J.R.; Culver, D.H.; Gaynes, R.P. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 2000, 21, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Maki, D.G.; Cobb, L.; Garman, J.K.; Shapiro, J.M.; Ringer, M.; Helgerson, R.B. An attachable silver-impregnated cuff for prevention of infection with central venous catheters: A prospective randomized multicenter trial. Am. J. Med. 1988, 85, 307–314. [Google Scholar] [PubMed]
- Weber, D.J.; Raasch, R.; Rutala, W.A. Nosocomial Infections in the ICU: The Growing Importance of Antibiotic-Resistant Pathogens. Chest 1999, 115, 34–41. [Google Scholar] [CrossRef]
- Weinstein, R.A. Nosocomial infection update. Emerg. Infect. Dis. 1998, 4, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Bonilla, A.; Fernandez-Garcia, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–792. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, T. Antibacterial and bacterium adsorbing macromolecules. Macromol. Mater. Eng. 2001, 286, 63–87. [Google Scholar] [CrossRef]
- Gabriel, G.J.; Som, A.; Madkour, A.E.; Eren, T.; Tew, G.N. Infectious disease: Connecting innate immunity to biocidal polymers. Mater. Sci. Eng. R. 2007, 57, 28–64. [Google Scholar] [CrossRef] [PubMed]
- Page, K.; Wilson, M.; Parkin, I.P. Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections. J. Mater. Chem. 2009, 19, 3819–3831. [Google Scholar] [CrossRef]
- Roe, D.; Karandikar, B.; Bonn-Savage, N.; Gibbins, B.; Roullet, J.B. Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother. 2008, 61, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ohko, Y.; Sekiguchi, Y.; Fujishima, A.; Kubota, Y. Self-sterilization using silicone catheters coated with Ag and TiO2 nanocomposite thin film. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85B, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.; Polivkova, M.; Kasalkova, N.S.; Kolska, Z.; Svorcik, V. Properties of silver nanostructure-coated PTFE and its biocompatibility. Nanoscale Res. Lett. 2013, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tamboli, M.S.; Kulkarni, M.V.; Patil, R.H.; Gade, W.N.; Navale, S.C.; Kale, B.B. Nanowires of silver–polyaniline nanocomposite synthesized via in situ polymerization and its novel functionality as an antibacterial agent. Colloids Surf. B Biointerfaces 2012, 92, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.; Kassel, M.; Cantrell, J.; Kaka, S.; Morar, R.; Mahomed, A.G.; Philips, J.I. The presence and sequence of endotracheal tube colonization in patients undergoing mechanical ventilation. Eur. Respir. J. 1999, 13, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A. Nosocomial Candidiasis: Emerging Species, Reservoirs, and Modes of Transmission. Clin. Infect. Dis. 1996, 22, 89–94. [Google Scholar] [CrossRef]
- Schierholz, J.M.; Beuth, J. Implant infections: A haven for opportunistic bacteria. J. Hosp. Infect. 2001, 49, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C. Relevance of biofilms for the biodeterioration of surfaces of polymeric materials. Polym. Degrad. Stable 1998, 59, 309–315. [Google Scholar] [CrossRef]
- Gao, G.Z.; Lange, D.; Hilpert, K.; Kindrachuk, J.; Zou, Y.Q.; Cheng, J.T.J.; Kazemzadeh-Narbat, M.; Yu, K.; Wang, R.Z.; Straus, S.K.; et al. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides. Biomaterials 2011, 32, 3899–3909. [Google Scholar] [CrossRef] [PubMed]
- Bergogne-Bérézin, E.; Decreé, D.; Joly-Guillou, M.L. Opportunistic nosocomial multiply resistant bacterial infections—their treatment and prevention. J. Antimicrob. Chemother. A 1993, 32, 39–47. [Google Scholar] [CrossRef]
- Handwerger, S.; Raucher, B.; Altarac, D.; Monka, J.; Marchione, S.; Singh, K.V.; Murray, B.E.; Wolff, J.; Walters, B. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin, and gentamicin. Clin. Infect. Dis. 1993, 16, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.C.; Hill, B.C.; O’Hara, C.M.; Steingrimsson, O.; Cooksey, R.C. Epidemiologic typing of Enterobacter sakazakii in two neonatal nosocomial outbreaks. Diagn. Microbiol. Infect. Dis. 1990, 13, 467–472. [Google Scholar] [CrossRef]
- Jarvis, W.R. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin. Infect. Dis. 1995, 20, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.K.C.; Ip, M.; Poon, W.S.; Mak, C.W.K.; Ng, R.Y.T. Antibiotics-impregnated ventricular catheter versus systemic antibiotics for prevention of nosocomial CSF and non-CSF infections: A prospective randomised clinical trial. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1064–1067. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, J.E.; Amaral, L. The potential management of resistant infections with non-antibiotics. J. Antimicrob. Chemother. 1997, 40, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Toracchio, S.; Marzio, L. Primary and secondary antibiotic resistance of Helicobacter pylori strains isolated in central Italy during the years 1998–2002. Dig. Liver Dis. 2003, 35, 541–545. [Google Scholar] [CrossRef]
- Chemaly, R.F.; Sharma, P.S.; Youssef, S.; Gerber, D.; Hwu, P.; Hanmod, S.S.; Jiang, Y.; Hachem, R.Y.; Raad, I.I. The efficacy of catheters coated with minocycline and rifampin in the prevention of catheter-related bacteremia in cancer patients receiving high-dose interleukin-2. Int. J. Infect. Dis. 2010, 14, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Jose, B.; Antoci, V.; Zeiger, A.R.; Wickstrom, E.; Hickok, N.J. Vancomycin covalently bonded to titanium beads kills Staphylococcus aureus. Chem. Biol. 2005, 12, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.L.; Modak, S.M. Mechanism of silver sulfadiazine action on burn wound infections. Antimicrob. Agents Chemother. 1974, 5, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Elsner, J.J.; Berdicevsky, I.; Zilberman, M. In vitro microbial inhibition and cellular response to novel biodegradable composite wound dressings with controlled release of antibiotics. Acta Biomater. 2011, 7, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.H.; Wang, J.T.; Chang, S.C.; Hsueh, P.R.; Luh, K.T. Evaluation of antiseptic-impregnated central venous catheters for prevention of catheter-related infection in intensive care unit patients. Diagn. Microbiol. Infect. Dis. 2000, 38, 1–5. [Google Scholar] [CrossRef]
- Wainwright, M.; Phoenix, D.A.; Gaskell, M.; Marshall, B. Photobactericidal activity of methylene blue derivatives against vancomycin-resistant Enterococcus spp. J. Antimicrob. Chemother. 1999, 44, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, M.; Mehrgan, H.; Hadji-Nejad, S. Enhancement of Vancomycin Activity by phenothiazines against vancomycin-resistant Enterococcus faecium in vitro. Basic Clin. Pharmacol. Toxicol. 2010, 107, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Balazs, D.J.; Triandafillu, K.; Wood, P.; Chevolot, Y.; van Delden, C.; Harms, H.; Hollenstein, C.; Mathieu, H.J. Inhibition of bacterial adhesion on PVC endotracheal tubes by RF-oxygen glow discharge, sodium hydroxide and silver nitrate treatments. Biomaterials 2004, 25, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.O.; Spadaro, J.A. Treatment of orthopaedic infections with electrically generated silver ions: A preliminary report. J. Bone Jt. Surg. Am. 1978, 60, 871–881. [Google Scholar] [CrossRef]
- Panzner, M.J.; Deeraksa, A.; Smith, A.; Wright, B.D.; Hindi, K.M.; Kascatan-Nebioglu, A.; Torres, A.G.; Judy, B.M.; Hovis, C.E.; Hilliard, J.K.; et al. Synthesis and in vitro efficacy studies of silver carbene complexes on biosafety level 3 bacteria. Eur. J. Inorg. Chem. 2009, 13, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.W.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, M.; Zodrow, K.R.; Qi, G.G.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Surface functionalization of thin-film composite membranes with copper nanoparticles for antimicrobial surface properties. Environ. Sci. Technol. 2014, 48, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.P.; Walker, K.A.; Obare, S.O.; Docherty, K.M. Size-dependent antimicrobial effects of novel palladium nanoparticles. PLoS ONE 2014, 9, e85981. [Google Scholar] [CrossRef] [PubMed]
- Maki, D.G.; Stolz, S.M.; Wheeler, S.; Mermel, L.A. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter: A randomized, controlled trial. Ann. Intern. Med. 1997, 127, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Dastidar, S.G.; Fanning, S.; Kristiansen, J.E.; Molnar, J.; Pages, J.M.; Schelz, Z.; Spengler, G.; Viveiros, M.; Amaral, L. Potential role of non-antibiotics (helper compounds) in the treatment of multidrug-resistant Gram-negative infections: Mechanisms for their direct and indirect activities. Int. J. Antimicrob. Agents 2008, 31, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar] [PubMed]
- Mijnendonckx, K.; Leys, N.; Mahillon, J.; Silver, S.; van Houdt, R. Antimicrobial silver: Uses, toxicity and potential for resistance. Biomaterials 2013, 26, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Aflori, M.; Miron, C.; Dobromir, M.; Drobota, M. Bactericidal effect on Foley catheters obtained by plasma and silver nitrate treatments. High Perform. Polym. 2015, 27, 655–660. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. N-Heterocyclic carbene-silver complexes: A new class of antibiotics. Coord. Chem. Rev. 2007, 251, 884–895. [Google Scholar] [CrossRef]
- Siegel, J.; Staszek, M.; Polivkova, M.; Reznickova, A.; Rimpelova, S.; Svorcik, V. Green synthesized noble metals for biological applications. Mater. Today Proc. 2016, 3, 608–616. [Google Scholar] [CrossRef]
- Siegel, J.; Polivkova, M.; Staszek, M.; Kolarova, K.; Rimpelova, S.; Svorcik, V. Nanostructured silver coatings on polyimide and their antibacterial response. Mater. Lett. 2015, 145, 89–90. [Google Scholar] [CrossRef]
- Polivkova, M.; Štrublová, V.; Hubáček, T.; Rimpelová, S.; Švorčík, V.; Siegel, J. Surface characterization and antibacterial response of silver nanowire arrays supported on laser-treated polyethylene naphthalate. Mater. Sci. Eng. C 2016. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Pratsinis, S.E. Engineering nanosilver as an antibacterial, biosensor and bioimaging material. Curr. Opin. Chem. Eng. 2011, 1, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Polivkova, M.; Valova, M.; Siegel, J.; Rimpelova, S.; Hubacek, T.; Lyutakov, O.; Svorcik, V. Antibacterial properties of palladium nanostructures sputtered on polyethylene naphthalate. RSC Adv. 2015, 5, 73767–73774. [Google Scholar] [CrossRef]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, M.; Hara, K.; Kudo, J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005, 71, 7589–7593. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Tanagawa, M.; Atsuta, M. Characterization and inhibitory effect of antibacterial dental resin composites incorporating silver-supported materials. J. Biomed. Mater. Res. A 1999, 47, 516–522. [Google Scholar] [CrossRef]
- Kittler, S.; Greulich, C.; Diendorf, J.; Koller, M.; Epple, M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microb. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Schierholz, J.M.; Wachol-Drewek, Z.; Lucas, L.J.; Pulverer, G. Activity of silver ions in different media. Zent. Bl. Bakteriol. 1998, 287, 411–420. [Google Scholar] [CrossRef]
- Jansen, B.; Rinck, M.; Wolbring, P.; Strohmeier, A.; Jahns, T. In vitro evaluation of the antimicrobial efficacy and biocompatibility of a silver-coated central venous catheter. J. Biomater. Appl. 1994, 9, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Osińska-Jaroszuk, M.; Ginalska, G.; Belcarz, A.; Uryniak, A. Vascular prostheses with covalently bound gentamicin and amikacin reveal superior antibacterial properties than silver-impregnated ones: An in vitro study. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Guggenbichler, J.P.; Boswald, M.; Lugauer, S.; Krall, T. A new technology of microdispersed silver in polyurethane induces antimicrobial activity in central venous catheters. Infection 1999, 27, 16–23. [Google Scholar] [CrossRef]
- Trooskin, S.Z.; Donetz, A.P.; Baxter, J.; Harvey, R.A.; Greco, R.S. Infection-resistant continuous peritoneal dialysis catheters. Nephron 1989, 46, 263–267. [Google Scholar] [CrossRef]
- Jansen, B.; Jansen, S.; Peters, G.; Pulverer, G. In Vitro efficacy of a central venous catheter (‘Hydrocath’) loaded with teicoplanin to prevent bacterial colonization. J. Hosp. Infect. 1992, 22, 93–107. [Google Scholar] [CrossRef]
- Raad, I.; Darouiche, R.; Hachem, R.; Mansouri, M.; Bodey, G.P. The broad-spectrum activity and efficacy of catheters coated with minocycline and rifampin. J. Infect. Dis. 1996, 173, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Hampl, J.; Schierholz, J.; Jansen, B.; Aschoff, A. In vitro and in vivo efficacy of a rifampin-loaded silicone catheter for the prevention of CSF shunt infections. Acta Neurochir. 1995, 133, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Groeger, J.S.; Lucas, A.B.; Coit, D.; Laquaglia, M.; Brown, A.E.; Turnbull, A.; Exelby, P. A prospective, randomized evaluation of the effect of silver impregnated subcutaneous cuffs for preventing tunneled chronic venous access catheter infections in cancer patients. Ann. Surg. 1993, 218, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, S.; Hu, J.; d’Agostino, R.B.; Sherertz, R.J. Prolonged antimicrobial activity of a catheter containing chlorhexidine-silver sulfadiazine extends protection against catheter infections in vivo. Antimicrob. Agents Chemother. 2001, 45, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Braydich-Stolle, L.; Hussain, S.; Schlager, J.J.; Hofmann, M.C. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol. Sci. 2005, 88, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Diakowska, D.; Lewandowski, A.; Kopec, W.; Diakowski, W.; Chrzanowska, T. Oxidative DNA damage and total antioxidant status in serum of patients with esophageal squamous cell carcinoma. Hepatogastroenterology 2007, 54, 1701–1704. [Google Scholar] [PubMed]
- Ahamed, M.; Karns, M.; Goodson, M.; Rowe, J.; Hussain, S.M.; Schlager, J.J.; Hong, Y.L. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 2008, 233, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Duan, X.F.; Wei, Q.Q.; Lieber, C.M. Directed assembly of one-dimensional nanostructures into functional networks. Science 2001, 291, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.N.; Yang, P.D.; Sun, Y.G.; Wu, Y.Y.; Mayers, B.; Gates, B.; Yin, Y.D.; Kim, F.; Yan, Y.Q. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Staszek, M.; Siegel, J.; Rimpelova, S.; Lyutakov, O.; Svorcik, V. Cytotoxicity of noble metal nanoparticles sputtered into glycerol. Mater. Lett. 2015, 158, 351–354. [Google Scholar] [CrossRef]
- Sun, Y.G.; Xia, Y.N. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2014, 10, 1119. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Cohen, M.S.; Stern, J.M.; Vanni, A.J.; Kelley, R.S.; Baumgart, E.; Field, D.; Libertino, J.A.; Summerhayes, I.C. In vitro analysis of a nanocrystalline silver-coated surgical mesh. Surg. Infect. 2007, 8, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.Y.; Young, P.M.; Lee, W.H.; Cavaliere, R.; Whitchurch, C.B.; Rohanizadeh, R. Non-cytotoxic silver nanoparticle-polyvinyl alcohol hydrogels with anti-biofilm activity: Designed as coatings for endotracheal tube materials. Biofouling 2014, 30, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.G.; Gates, B.; Mayers, B.; Xia, Y.N. Crystalline silver nanowires by soft solution processing. Nano Lett. 2002, 2, 165–168. [Google Scholar] [CrossRef]
- Sun, Y.G.; Xia, Y.N. Large-scale synthesis of uniform silver nanowires through a soft, self-seeding, polyol process. Adv. Mater. 2002, 14, 833–837. [Google Scholar] [CrossRef]
- Choi, S.; Park, J.; Hyun, W.; Kim, J.; Kim, J.; Lee, Y.B.; Song, C.; Hwang, H.J.; Kim, J.H.; Hyeon, T.; et al. Stretchable heater using ligand-exchanged silver nanowire nanocomposite for wearable articular thermotherapy. ACS Nano 2015, 9, 6626–6633. [Google Scholar] [CrossRef] [PubMed]
- Rebollar, E.; Frischauf, I.; Olbrich, M.; Peterbauer, T.; Hering, S.; Preiner, J.; Hinterdorfer, P.; Romanin, C.; Heitz, J. Proliferation of aligned mammalian cells on laser-nanostructured polystyrene. Biomaterials 2008, 29, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, H.; Dadsetan, M. Influence of laser surface modifying of polyethylene terephthalate on fibroblast cell adhesion. Radiat. Phys. Chem. 2003, 67, 381–385. [Google Scholar] [CrossRef]
- Xu, C.Y.; Yang, F.; Wang, S.; Ramakrishna, S. In vitro study of human vascular endothelial cell function on materials with various surface roughness. J. Biomed. Mater. Res. 2004, 71, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Cavalcanti-Adam, E.A.; Glass, R.; Blümmel, J.; Eck, W.; Kantlehner, M.; Kessler, H.; Spatz, J.P. Activation of integrin function by nanopatterned adhesive interfaces. Chemphyschem. 2004, 5, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Bollen, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Rimondini, L.; Faré, S.; Brambilla, E.; Felloni, A.; Consonni, C.; Brossa, F.; Carrassi, A. The effect of surface roughness on early in vivo plaque colonization on titanium. J. Periodontol. 1997, 68, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.H.; Liu, Y.L. Preparation of graphene oxide with silver nanowires to enhance antibacterial properties and cell compatibility. RSC Adv. 2015, 5, 85748–85755. [Google Scholar] [CrossRef]
- Tang, C.L.; Sun, W.; Lu, J.M.; Yan, W. Role of the anions in the hydrothermally formed silver nanowires and their antibacterial property. J. Colloid Interface Sci. 2014, 416, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, B.; Chen, G.C.; Lei, B.; Hua, H.; Peng, H.L.; Yan, Z.M. Large-area chemical vapor deposition-grown monolayer graphene-wrapped silver nanowires for broad-spectrum and robust antimicrobial coating. Nano Res. 2016, 9, 963–973. [Google Scholar] [CrossRef]
- Stoehr, L.C.; Gonzalez, E.; Stampfl, A.; Casals, E.; Duschl, A.; Puntes, V.; Oostingh, G. J. Shape matters: Effects of silver nanospheres and wires on human alveolar epithelial cells. Part. Fibre Toxicol. 2011, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic potential of silver nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, M. J.; Shin, S. Toxic effects of silver nanoparticles and nanowires on erythrocyte rheology. Food Chem. Toxicol. 2014, 67, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.; Jurik, P.; Kolska, Z.; Svorcik, V. Annealing of silver nanolayers sputtered on polytetrafluoroethylene. Surf. Interface Anal. 2013, 45, 1063–1066. [Google Scholar] [CrossRef]
- Chinnasamy, R.; Krishnamoorthy, R.; Shamugam, R.K.; Thangavelu, R.R. Synthesis and antibacterial studies of nanostructured Ag thin films. Adv. Mater. Res. 2013, 678, 291–296. [Google Scholar]
- Aleksandrova, T.P.; Vais, A.A.; Masliy, A.I.; Burmistrov, V.A.; Gusev, A.A.; Bagavieva, S.K. Synthetic fibers with silver-containing coatings and their antimicrobial properties. Mater. Manuf. Process 2015, 30, 798–803. [Google Scholar] [CrossRef]
- Dubas, S.T.; Kumlangdudsana, P.; Potiyaraj, P. Layer-by-layer deposition of antimicrobial silver nanoparticles on textile fibers. Colloids Surf. A 2006, 289, 105–109. [Google Scholar] [CrossRef]
- Carvalho, D.; Sousa, T.; Morais, P.V.; Piedade, A.P. Polymer/metal nanocomposite coating with antimicrobial activity against hospital isolated pathogen. Appl. Surf. Sci. 2016, 379, 489–496. [Google Scholar] [CrossRef]
- Siegel, J.; Krajcar, R.; Kolska, Z.; Hnatowicz, V.; Svorcik, V. Annealing of gold nanostructures sputtered on polytetrafluoroethylene. Nanoscale Res. Lett. 2011, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Xia, Y.N. Bottom-up and top-down approaches to the synthesis of monodispersed spherical colloids of low melting-point metals. Nano Lett. 2004, 4, 2047–2050. [Google Scholar] [CrossRef]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top-down and bottom-up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [PubMed]
- Xu, C.A.; van Zalinge, H.; Pearson, J.L.; Glidle, A.; Cooper, J.M.; Cumming, D.R.S.; Haiss, W.; Yao, J.L.; Schiffrin, D.J.; Proupin-Perez, M.; et al. A combined top-down bottom-up approach for introducing nanoparticle networks into nanoelectrode gaps. Nanotechnology 2006, 17, 3333–3339. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Gudikandula, K.; Maringanti, S.C. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Garcia-Barrasa, J.; Lopez-de-Luzuriaga, J.M.; Monge, M. Silver nanoparticles: Synthesis through chemical methods in solution and biomedical applications. Cent. Eur. J. Chem. 2011, 9, 7–19. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Leach, A.M.; McDowell, M.; Gall, K. Deformation of top-down and bottom-up silver nanowires. Adv. Funct. Mater. 2007, 17, 43–51. [Google Scholar] [CrossRef]
- Tak, Y.; Hong, S.J.; Lee, J.S.; Yong, K. Solution-based synthesis of a cds nanoparticle/zno nanowire heterostructure array. Cryst. Growth Des. 2009, 9, 2627–2632. [Google Scholar] [CrossRef]
- Heurlin, M.; Magnusson, M.H.; Lindgren, D.; Ek, M.; Wallenberg, L.R.; Deppert, K.; Samuelson, L. Continuous gas-phase synthesis of nanowires with tunable properties. Nature 2012, 492, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Z.; Huang, X.; Liu, Q.; Cao, X.H.; Huo, F.W.; Zhang, H.; Gan, C.L. Vapor-liquid-solid growth of endotaxial semiconductor nanowires. Nano Lett. 2012, 12, 5565–5570. [Google Scholar] [CrossRef] [PubMed]
- Crowell, J.E. Chemical methods of thin film deposition: Chemical vapor deposition, atomic layer deposition, and related technologies. J. Vac. Sci. Technol. A 2003, 21, 88–95. [Google Scholar] [CrossRef]
- Reichelt, K.; Jiang, X. The preparation of thin films by physical vapour deposition methods. Thin Solid Films 1990, 191, 91–126. [Google Scholar] [CrossRef]
- Humphreys, R.G.; Satchell, J.S.; Chew, N.G.; Edwards, J.A.; Goodyear, S.W.; Blenkinsop, S.E.; Dosser, O.D.; Cullis, A.G. Physical vapour deposition techniques for the growth of YBa2Cu3O7 thin films. Supercond. Sci. Technol. 1990, 3, 38–52. [Google Scholar] [CrossRef]
- Mane, R.S.; Lokhande, C.D. Chemical deposition method for metal chalcogenide thin films. Mater. Chem. Phys. 2000, 65, 1–31. [Google Scholar] [CrossRef]
- Slepička, P.; Slepičková Kasálková, N.; Siegel, J.; Kolská, Z.; Bačáková, L.; Švorčík, V. Nano-structured and functionalized surfaces for cytocompatibility improvement and bactericidal action. Biotechnol. Adv. 2015, 33, 1120–1129. [Google Scholar] [CrossRef] [PubMed]



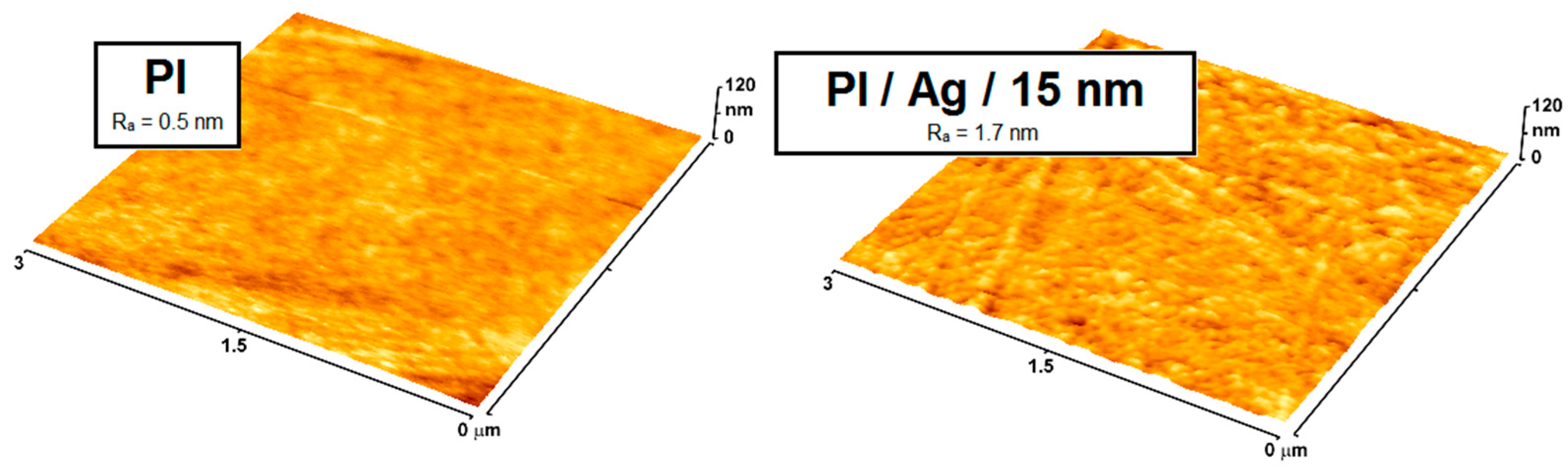
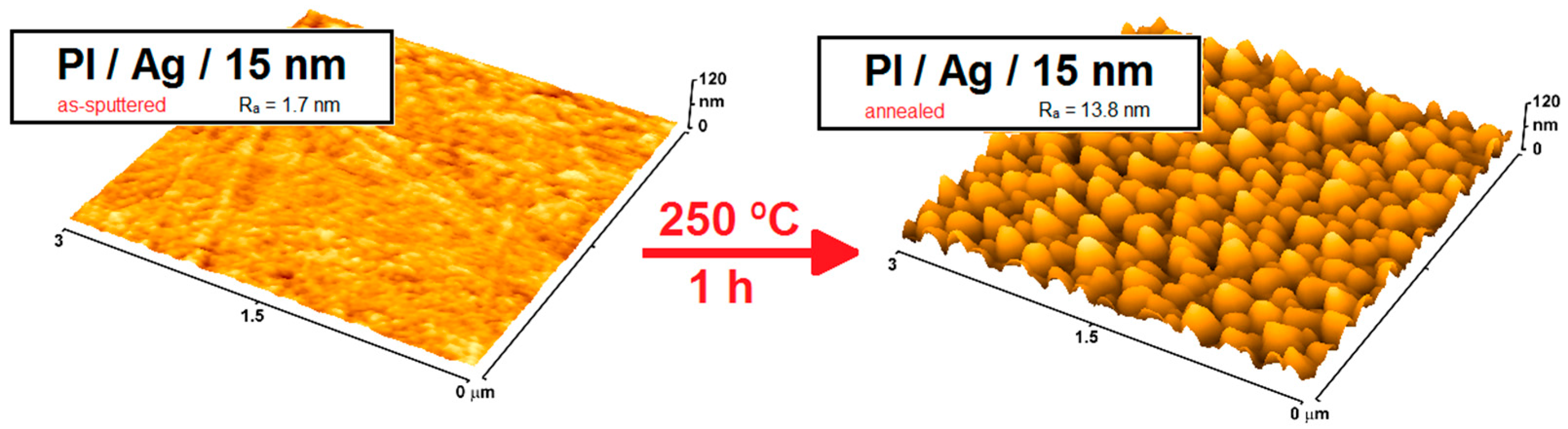
| Microbial Species | Medical Devices | Examples |
|---|---|---|
| Staphylococci, Enterobacteriaceae, Enterococci and Candida species | Catheters | Blood vessel catheter, CAPD catheters |
| Tubes | Cerebrospinal fluid shunts, endotracheal tubes | |
| Cardiological implants | Arterial grafts, cardiac valves, pacemaker electrodes, total artificial hearts | |
| Prostheses | Total joint replacements (endoprostheses), ocular and penile prostheses | |
| Enterobacteriaceae and Enterococci | Urinary catheters | Transurethral, suprapubic, and nephrostomy catheters, |
| Urinary stents | Double-J stents |
| Type of Treatment | Treatment Method | Example |
|---|---|---|
| Antibiotics | Coating | Mynocycline, rifampin [28] |
| Covalent bond | Vancomycin [29] | |
| Ionic bond | Silver sulfadiazine [30] | |
| Matrix | Ceftazidime and gentamicin implants [31] | |
| Antiseptics | Impregnation | Chlorhexidine [32] |
| Non-antibiotics | Coating Synergism with antibiotics | Methylene blue and its derivatives [33] Phenothiazine and its derivatives [34] |
| Noble metals | Incorporation | Silver salts, ions and complexes [35,36,37] |
| Incorporation, Coating | Nanostructured silver, gold, copper and palladium [12,38,39,40] |
| Prevention | Therapy |
|---|---|
| Bone cements | Wound dressings |
| Neurosurgical shunts | Creams and ointments |
| Venous catheters | Epistaxis |
| Cardiovascular implants | Gonococcal eye infection |
| Contact lenses | Pleurodesis |
| Dental and surgical instruments | Granulomas |
| Bottom-Up | Top-Down |
|---|---|
| Supercritical fluid synthesis | Mechanical milling |
| Spinning | Etching |
| Chemical vapour deposition | Electro-explosion |
| Plasma spraying and flame spraying | Sputtering |
| Molecular beam epitaxy | Laser ablation |
| Sol and Sol-Gel process | Dry grinding system |
| Laser pyrolysis | Wet grinding system |
| Aerosol based process | Ultrasonic wave |
| Atomic or molecular condensation | Mechanical alloying method |
| Using of templates | Lithography |
| Chemical reduction | Cutting |
| Physical | Chemical | Biological |
|---|---|---|
| Laser ablation | Electro-explosion | Microorganism |
| Irradiation | Sol-gel synthesis | Bio-templates |
| Pulse vapour deposition | Chemical reduction | Plant-extract-assisted biogenesis |
| Ultrasonication | Microemulsion method | |
| Microwave method | Hydrothermal synthesis | |
| Electro chemical | Polyol synthesis | |
| Thermal evaporation | Microwave-assisted synthesis | |
| Lithography | Indirect reduction | |
| Melt mixing | UV-initiated photoreduction | |
| Electrospraying | .Photoinduced reduction | |
| Inert gas condensation | Electrochemical synthesis |
| Solution-Based | Gas-Phase |
|---|---|
| Solution–liquid–solid method | Vapour–liquid–solid growth |
| Solvothermal method | Physical vapour deposition |
| Template-based method | Chemical vapour deposition |
| Fluid–liquid–solid method | Vapour–solid growth |
| Sol-gel synthesis | Oxide-assisted growth |
| Chemical bath deposition | Laser ablation |
| Electrochemical deposition | Aerotaxy-based growth |
| Hydrothermal method | Electrodeless etching |
| Aqueous chemical growth | Thermal evaporation |
| Electrospraying | Carbothermal reduction |
| Hot-injection method | Flame-based synthesis |
| Microemulsion | Lithography |
| Physical | Chemical | ||
|---|---|---|---|
| Evaporation | Ion plating, laser ablation, molecular beam exipaxy, electron beam, thermal and vacuum evaporation | Plating Sol-Gel | Electroplating Electrolysis |
| Sputtering | Ion beam, reactive, magnetron, high target utilization, ion-assisted and gas flow sputtering | Chemical vapour deposition | Metalorganic vapour phase epitaxy, plasma-enhanced and thermal deposition |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polívková, M.; Hubáček, T.; Staszek, M.; Švorčík, V.; Siegel, J. Antimicrobial Treatment of Polymeric Medical Devices by Silver Nanomaterials and Related Technology. Int. J. Mol. Sci. 2017, 18, 419. https://doi.org/10.3390/ijms18020419
Polívková M, Hubáček T, Staszek M, Švorčík V, Siegel J. Antimicrobial Treatment of Polymeric Medical Devices by Silver Nanomaterials and Related Technology. International Journal of Molecular Sciences. 2017; 18(2):419. https://doi.org/10.3390/ijms18020419
Chicago/Turabian StylePolívková, Markéta, Tomáš Hubáček, Marek Staszek, Václav Švorčík, and Jakub Siegel. 2017. "Antimicrobial Treatment of Polymeric Medical Devices by Silver Nanomaterials and Related Technology" International Journal of Molecular Sciences 18, no. 2: 419. https://doi.org/10.3390/ijms18020419






