The Mechanisms and Biomedical Applications of an NIR BODIPY-Based Switchable Fluorescent Probe
Abstract
:1. Introduction
2. Results and Discussion
2.1. Switching Mechanisms of ADP(CA)2
2.1.1. Fluorescence Response to Polarity
2.1.2. Fluorescence Response to Viscosity
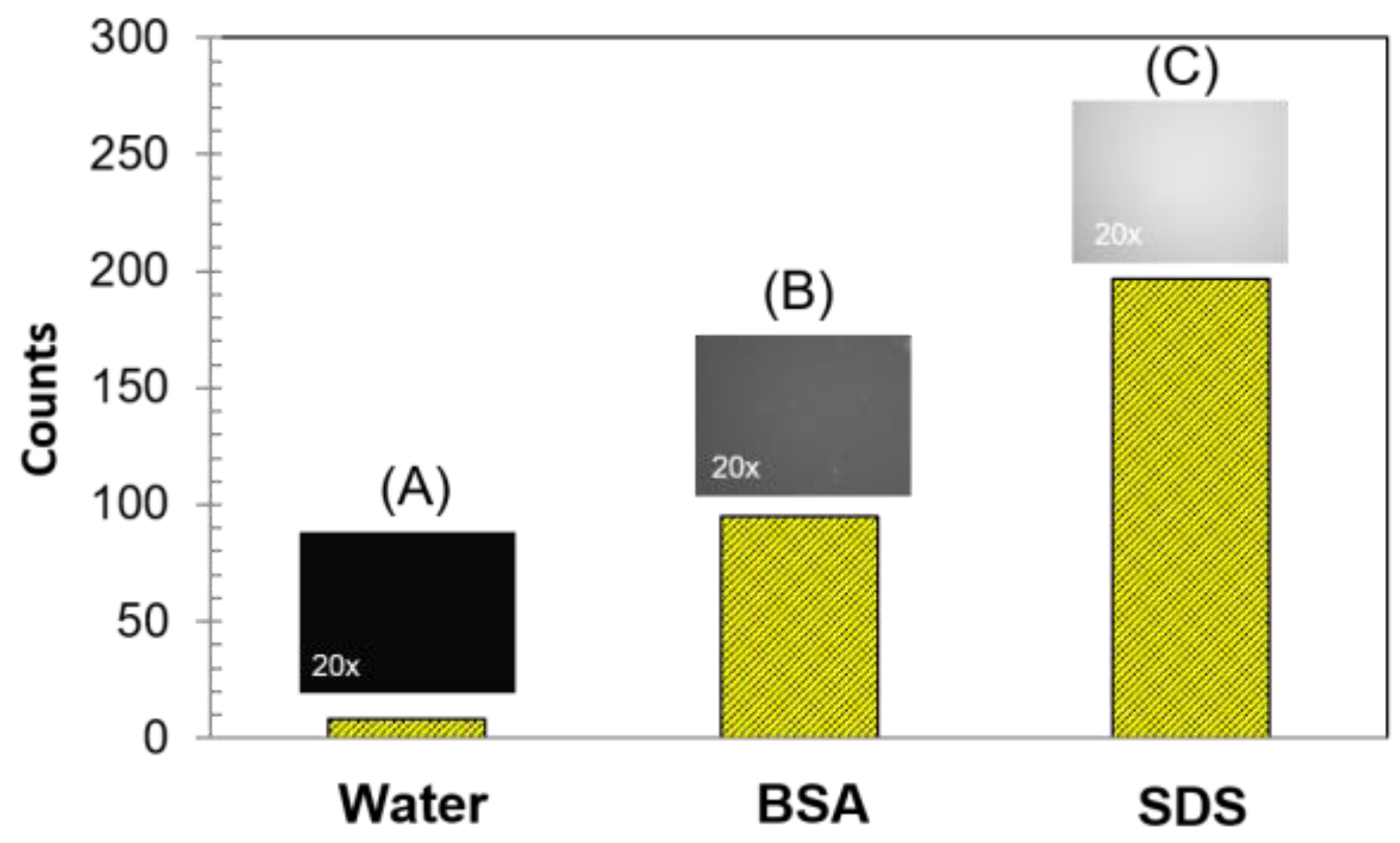
2.1.3. Fluorescence Response to Biological Macromolecules and Surfactant
2.2. Applications of ADP(CA)2 as a Switchable Fluorescent Probe (SFP)
2.2.1. Wash-Free Live-Cell Imaging
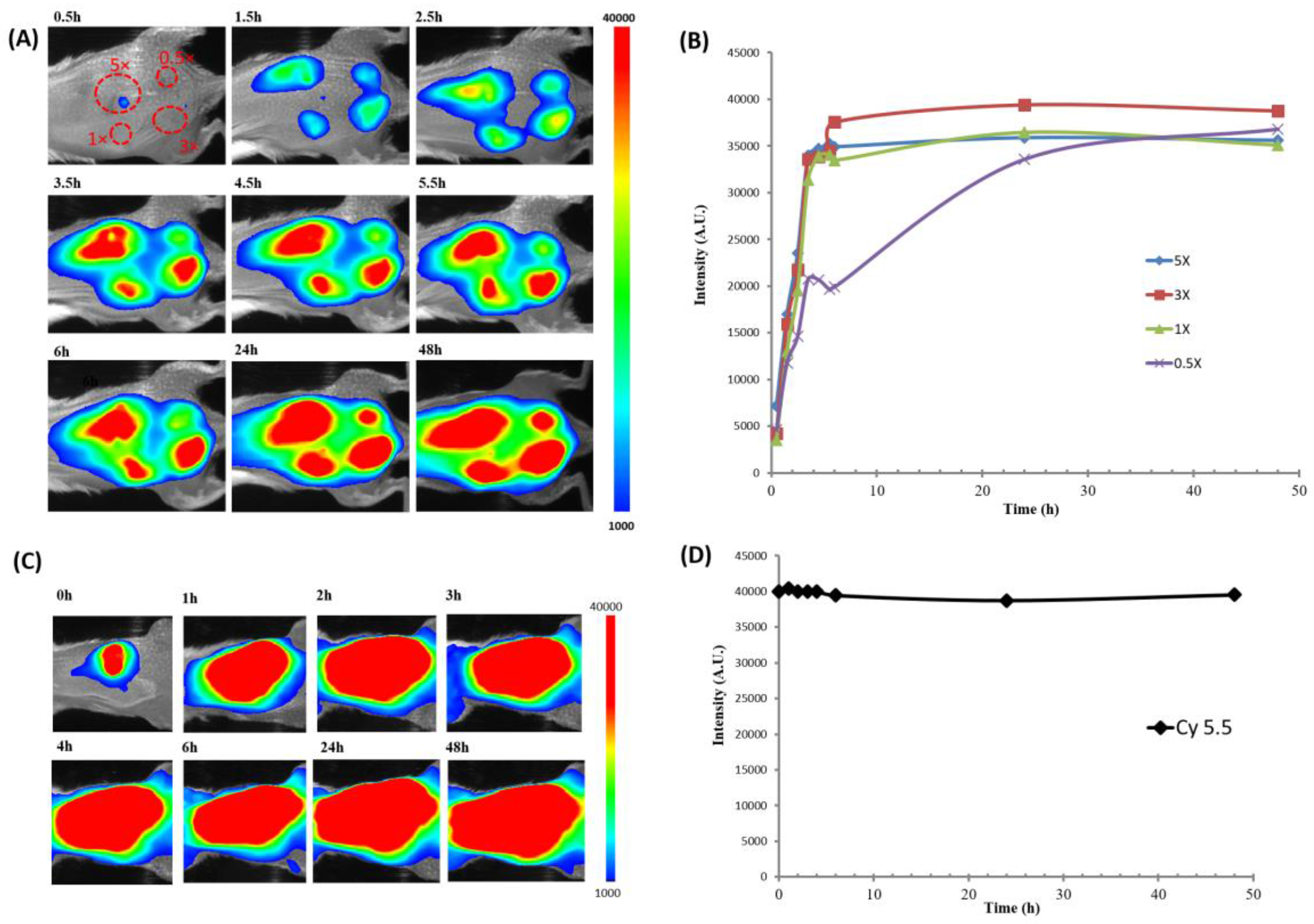
2.2.2. Fluorescence Imaging in Live Animals
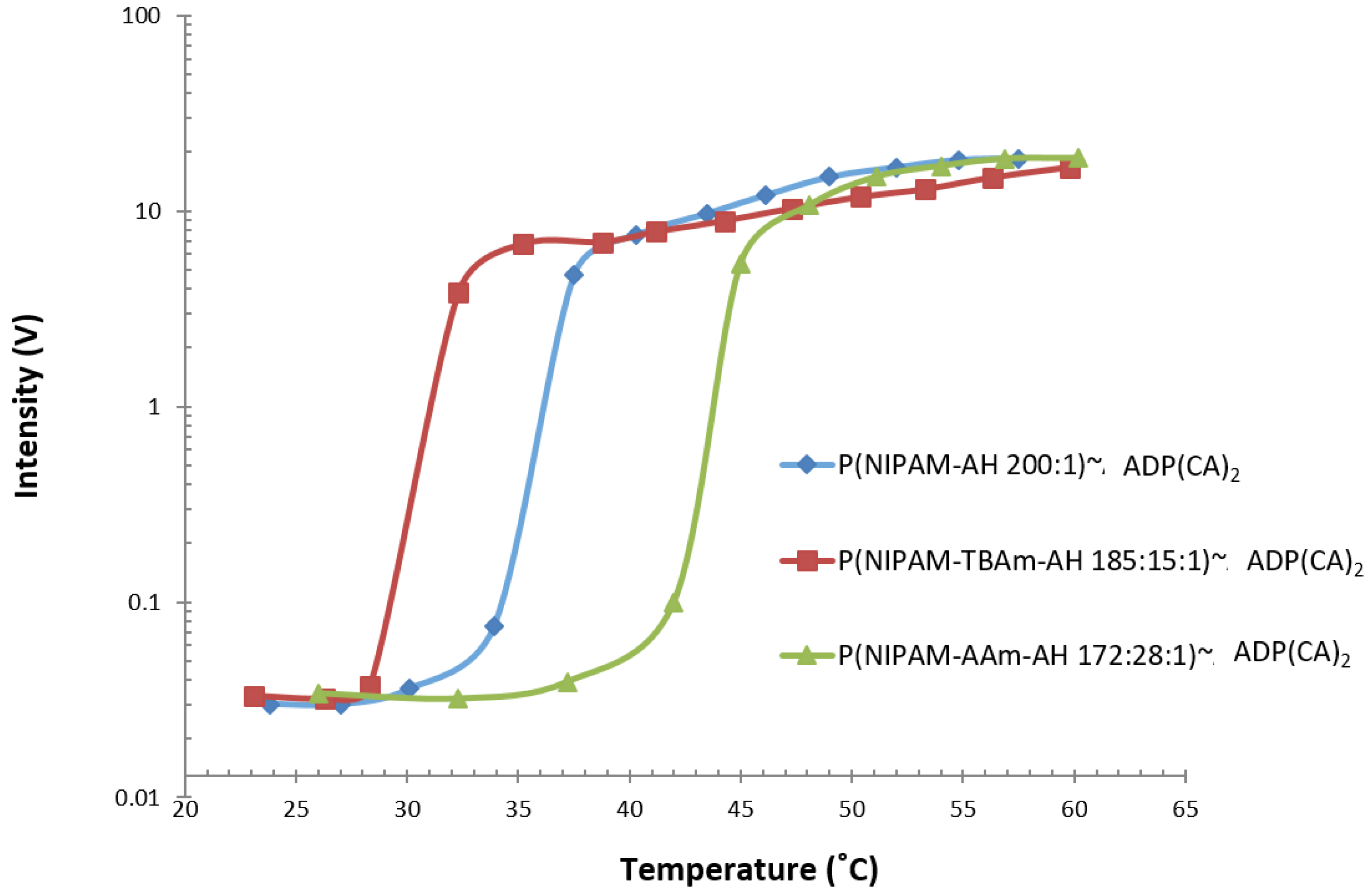
2.2.3. Temperature Sensing
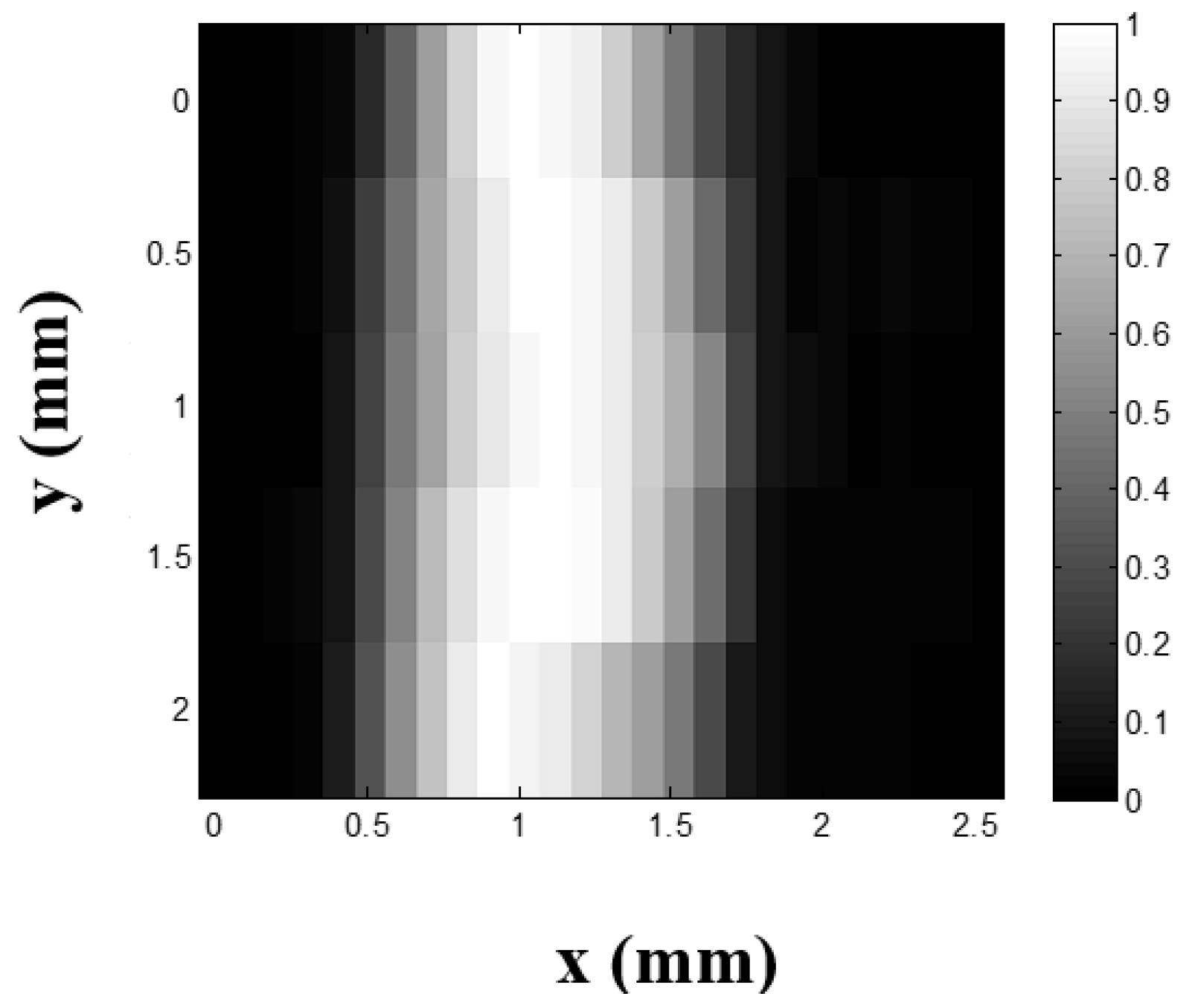
2.2.4. Ultrasound-Switchable Fluorescence (USF) Imaging in Tissue-Mimicking Phantoms
3. Conclusions
4. Materials and Methods
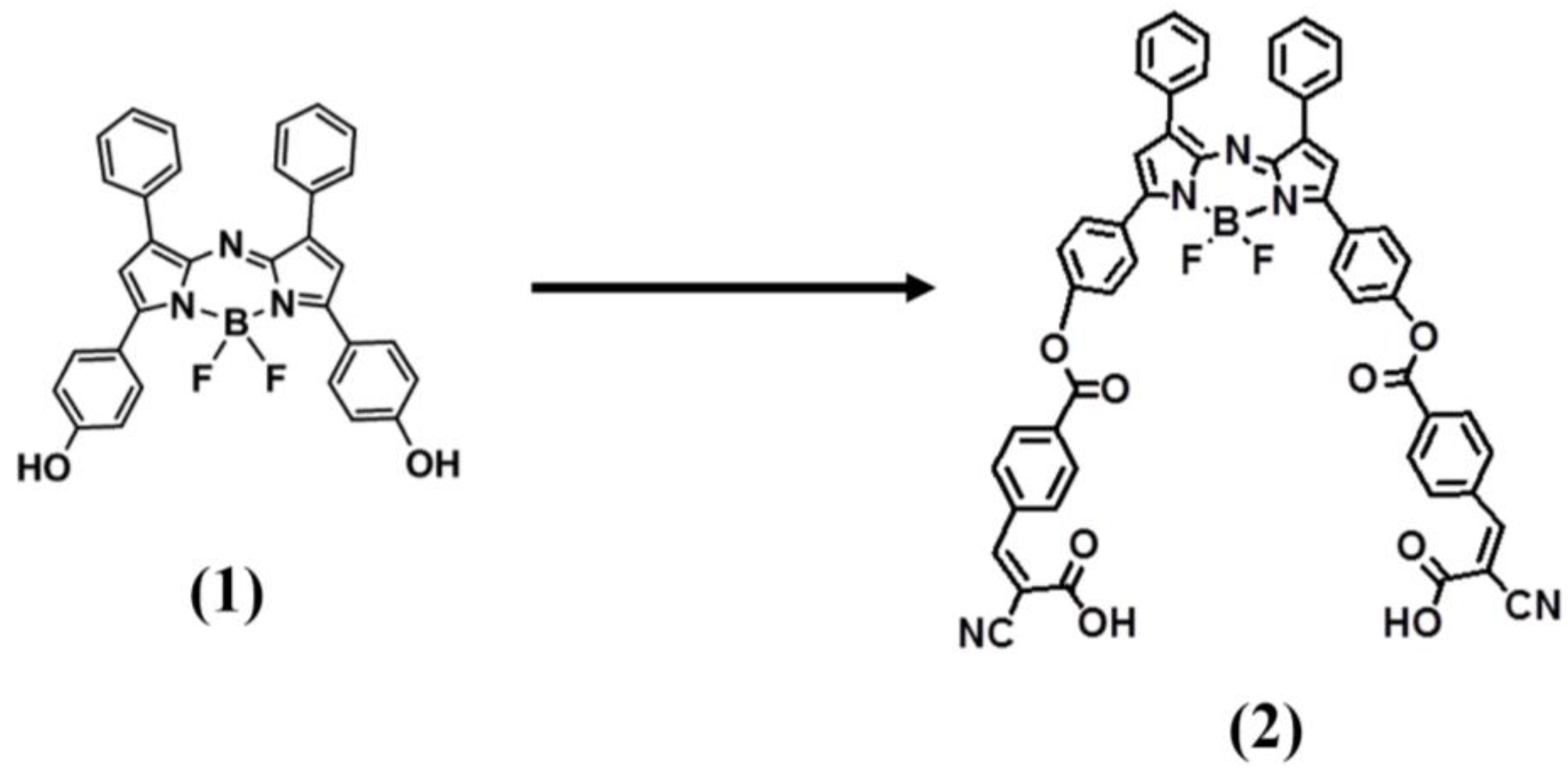
4.1. Conjugates of ADP(CA)2 and NIPAM Polymer
4.2. Fluorescence Measurement of ADP(CA)2 under Varying Micro-Environment Conditions
4.2.1. Polarity
4.2.2. Viscosity
4.2.3. Interaction with Biological Macromolecules and Surfactant
4.3. Wash-free live-cell Imaging
4.4. Fluorescence Imaging in Live Animals
4.5. Temperature Sensing
4.6. Ultrasound-Switchable Fluorescence (USF) Imaging in Tissue-Mimicking Phantoms
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of Near-Infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Nagano, T. Development of fluorescent probes for bioimaging applications. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Terai, T.; Nagano, T. Fluorescent probes for bioimaging applications. Curr. Opin. Chem. Biol. 2008, 12, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Lee, M.H.; Kim, H.J.; Kim, J.S.; Yoon, J. A new trend in rhodamine-based chemosensors: Application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 2008, 37, 1465. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yuan, Z.; Simmons, J.T.; Sreenath, K. Zn(II)-coordination modulated ligand photophysical processes—The development of fluorescent indicators for imaging biological Zn(II) ions. RSC Adv. 2014, 4, 20398. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.M.; Lippard, S.J. Small-molecule fluorescent sensors for investigating zinc metalloneurochemistry. Acc. Chem. Res. 2009, 42, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bai, Y.; Han, Z.; He, W.; Guo, Z. Photoluminescence imaging of Zn 2+ in living systems. Chem. Soc. Rev. 2015, 44, 4517–4546. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef] [PubMed]
- Haidekker, M.A.; Theodorakis, E.A. Environment-sensitive behavior of fluorescent molecular rotors. J. Biol. Eng. 2010, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yuan, L.; Yuan, Z.; Doyle, N.K.; Dilbeck, T.; Bahadur, D.; Ramakrishnan, S.; Dearden, A.; Huang, C.; Ma, B. Phosphorescent molecular butterflies with controlled potential-energy surfaces and their application as luminescent viscosity sensor. Inorg. Chem. 2016, 55, 8564–8569. [Google Scholar] [CrossRef] [PubMed]
- Hosny, N.A.; Mohamedi, G.; Rademeyer, P.; Owen, J.; Wu, Y.; Tang, M.-X.; Eckersley, R.J.; Stride, E.; Kuimova, M.K. Mapping microbubble viscosity using fluorescence lifetime imaging of molecular rotors. Proc. Natl. Acad. Sci. USA 2013, 110, 9225–9230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tian, Y.; Yuan, Z.; Han, M.; Wang, J.; Zhu, L.; Tameh, M.S.; Huang, C.; Ma, B. Precise design of phosphorescent molecular butterflies with tunable photoinduced structural change and dual emission. Angew. Chemie Int. Ed. 2015, 54, 9591–9595. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Tian, Y.; Yuan, Z.; Zhu, L.; Ma, B. A Phosphorescent molecular ‘butterfly’ that undergoes a photoinduced structural change allowing temperature sensing and white emission. Angew. Chemie Int. Ed. 2014, 53, 10908–10912. [Google Scholar] [CrossRef] [PubMed]
- Maçanita, A.L.; Zachariasse, K.A. Viscosity dependence of intramolecular excimer formation with 1,5-bis(1-pyrenylcarboxy)pentane in alkane solvents as a function of temperature. J. Phys. Chem. A 2011, 115, 3183–3195. [Google Scholar] [CrossRef] [PubMed]
- Gota, C.; Uchiyama, S.; Yoshihara, T.; Tobita, S.; Ohwada, T. Temperature-dependent fluorescence lifetime of a fluorescent polymeric thermometer, poly(N -isopropylacrylamide), labeled by polarity and hydrogen bonding sensitive 4-sulfamoyl-7-aminobenzofurazan. J. Phys. Chem. B 2008, 112, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Wei, M.Y.; Liu, Y.; Pitta, H.; Xie, Z.; Hong, Y.; Nguyen, K.T.; Yuan, B. Development of ultrasound-switchable fluorescence imaging contrast agents based on thermosensitive polymers and nanoparticles. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 6801214. [Google Scholar] [PubMed]
- Pei, Y.; Wei, M.-Y.; Cheng, B.; Liu, Y.; Xie, Z.; Nguyen, K.; Yuan, B. High resolution imaging beyond the acoustic diffraction limit in deep tissue via ultrasound-switchable NIR fluorescence. Sci. Rep. 2014, 4, 4690. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Bandi, V.; Wei, M.-Y.; Pei, Y.; D’Souza, F.; Nguyen, K.T.; Hong, Y.; Tang, L.; Yuan, B. Centimeter-deep tissue fluorescence microscopic imaging with high signal-to-noise ratio and picomole sensitivity. arXiv, 2015; arXiv:1510.02112. [Google Scholar]
- Cheng, B.; Bandi, V.; Wei, M.-Y.; Pei, Y.; D’Souza, F.; Nguyen, K.T.; Hong, Y.; Yuan, B. High-resolution ultrasound-switchable fluorescence imaging in centimeter-deep tissue phantoms with high signal-to-noise ratio and high sensitivity via novel contrast agents. PLoS ONE 2016, 11, 0165963. [Google Scholar] [CrossRef] [PubMed]
- Kuimova, M.K.; Botchway, S.W.; Parker, A.W.; Balaz, M.; Collins, H.A.; Anderson, H.L.; Suhling, K.; Ogilby, P.R. Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat. Chem. 2009, 1, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, R.D.; Martin, A.; Adamson, K.; O’Reilly, E.; Mollard, P.; Forster, R.J.; Keyes, T.E. The application of water soluble, mega-Stokes-shifted BODIPY fluorophores to cell and tissue imaging. J. Microsc. 2014, 253, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Uchiyama, S.; Liu, Y.; Nguyen, K.T.; Alexandrakis, G. High-resolution imaging in a deep turbid medium based on an ultrasound-switchable fluorescence technique. Appl. Phys. Lett. 2012, 101, 33703. [Google Scholar] [CrossRef] [PubMed]
- Loving, G.S.; Sainlos, M.; Imperiali, B. Monitoring protein interactions and dynamics with solvatochromic fluorophores. Trends Biotechnol. 2010, 28, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erkkila, K.E.; Odom, D.T.; Barton, J.K. Recognition and reaction of metallointercalators with DNA. Chem. Rev. 1999, 99, 2777–2796. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.N.; El-Khouly, M.E.; Subbaiyan, N.K.; Zandler, M.E.; Supur, M.; Fukuzumi, S.; D’Souza, F. Syntheses, electrochemistry, and photodynamics of ferrocene–azadipyrromethane donor–acceptor dyads and triads. J. Phys. Chem. A 2011, 115, 9810–9819. [Google Scholar] [CrossRef] [PubMed]
- Baruah, M.; Qin, W.; Flors, C.; Hofkens, J.; Vallée, R.A.L.; Beljonne, D.; van der Auweraer, M.; de Borggraeve, W.M.; Boens, N. Solvent and pH dependent fluorescent properties of a dimethylaminostyryl borondipyrromethene dye in solution. J. Phys. Chem. A 2006, 110, 5998–6009. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Sunahara, H.; Urano, Y.; Kojima, H.; Nagano, T. Design and synthesis of a library of BODIPY-based environmental polarity sensors utilizing photoinduced electron-transfer-controlled fluorescence ON/OFF switching. J. Am. Chem. Soc. 2007, 129, 5597–5604. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Miyamoto, R.; Shiraishi, Y.; Hirai, T. BODIPY-conjugated thermoresponsive copolymer as a fluorescent thermometer based on polymer microviscosity. Langmuir 2009, 25, 13176–13182. [Google Scholar] [CrossRef] [PubMed]
- Elert, G. The Physics Hypertextbook. Available online: http://physics.info/ (accessed on 6 December 2016).
- Segur, J.B.; Oberstar, H.E. Viscosity of Glycerol and Its Aqueous Solutions. Ind. Eng. Chem. 1951, 43, 2117–2120. [Google Scholar] [CrossRef]
- Rodríguez, A.; Muñoz, M.; del Mar Graciani, M.; Fernández Chacón, S.; Moyá, M.L. Kinetic study in water-ethylene glycol cationic, zwitterionic, nonionic, and anionic micellar solutions. Langmuir 2004, 20, 945–952. [Google Scholar] [CrossRef] [PubMed]



| Probe | S/B Ratio | Fluorescence Intensity Counts | Loading Time |
|---|---|---|---|
| ADP(CA)2 | 5.93 | 172 (light source: 50%) | 120 min |
| ADP(CA)2~Polymer | 3.75 | 255 *(light source: 50%) | 120 min |
| ADP(CA)2~Polymer | 4.5 | 143 (light source: 25%) | 120 min |
| Probe | λex,λem (nm) | IOn/Ioff | Tth (°C) | TBW (°C) |
|---|---|---|---|---|
| P(NIPAM-AH 200:1)~ADP(CA)2 | 655 & 711 | 304 | 34 | 4 |
| P(NIPAM-TBAm-AH 185:15:1)~ADP(CA)2 | 655 & 711 | 318 | 28.3 | 4.5 |
| P(NIPAM-AAm-AH 172:28:1)~ADP(CA)2 | 655 & 711 | 284 | 42 | 4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, B.; Bandi, V.; Yu, S.; D’Souza, F.; Nguyen, K.T.; Hong, Y.; Tang, L.; Yuan, B. The Mechanisms and Biomedical Applications of an NIR BODIPY-Based Switchable Fluorescent Probe. Int. J. Mol. Sci. 2017, 18, 384. https://doi.org/10.3390/ijms18020384
Cheng B, Bandi V, Yu S, D’Souza F, Nguyen KT, Hong Y, Tang L, Yuan B. The Mechanisms and Biomedical Applications of an NIR BODIPY-Based Switchable Fluorescent Probe. International Journal of Molecular Sciences. 2017; 18(2):384. https://doi.org/10.3390/ijms18020384
Chicago/Turabian StyleCheng, Bingbing, Venugopal Bandi, Shuai Yu, Francis D’Souza, Kytai T. Nguyen, Yi Hong, Liping Tang, and Baohong Yuan. 2017. "The Mechanisms and Biomedical Applications of an NIR BODIPY-Based Switchable Fluorescent Probe" International Journal of Molecular Sciences 18, no. 2: 384. https://doi.org/10.3390/ijms18020384






