1. Introduction
Arsenic trioxide (As
2O
3) has been widely used in traditional medicine for treatment of various diseases for more than two thousand years [
1]. Recently, it has been established as one of the most effective drugs for treatment of patient with acute promyelocytic leukemia (APL), as well as applied to other types of solid malignant tumors and diseases as clinical treatment [
2,
3].
APL is a subtype of acute myeloid leukemia (AML) with distinctive biological and clinical features that has recently been found to be highly curable by all-trans retinoic acid (ATRA) and As
2O
3 treatment [
4,
5]. Additionally, APL is characterized by the reciprocal chromosomal translocation t(15;17)(q22;q21), resulting in the fusion of
promyelocytic leukemia (
PML) gene to the
retinoic acid receptor α (
RARα) gene, and, finally, the expression of PML-RARα fusion protein to block cell differentiation. Fortunately, this specific genetic lesion determines the unique response to treatment with As
2O
3 or all-trans retinoic acid (ATRA), especially As
2O
3 has dual effects on inducing partial differentiation and apoptosis of APL cells, differently from ATRA [
6,
7,
8].
Indeed, As
2O
3-induced PML-RARα degradation is the basis of its clinical efficacy in APL [
9,
10]. Additionally, it has been found that As
2O
3 directly targeted PML moiety of PML-RARα fusion protein and causes its fusion protein degradation resulting in clinical remission [
9,
10]. In fact, PML protein contains cysteine-rich zinc binding domains, a RING (R), a B-box type 1 (B1) and a B-box type 2 (B2) motifs, followed by a Coiled-Coil (CC) region, namely (RBCC) [
11]. It has already been reported that rapid degradation of PML-RARα fusion protein was considered to be associated with direct interaction of As
2O
3 by targeting cysteine residues in RBCC domains of PML, and consequent induction of PML-RARα conformational changes, oligomerization and then modified by SUMOylation as well as ubiquitination, finally resulting in clinical remission by PML-RARα fusion protein degradation [
12,
13,
14]. Although As
2O
3 has significant clinical effectiveness in the treatment of APL, it has been reported that arsenic resistant patients are commonly found in relapsed APL after consolidation therapy, and their outcomes are extremely poor [
15]. Several studies have shown that point mutations in B-box2 of PML or PML-RARα fusion genes are frequently observed [
16], and all mutant PML proteins have exhibited strong resistance to As
2O
3 treatment in vitro [
17,
18].
To date, more than nine mutations have been found in PML, and most mutations were identified in B-box 2 motif, such as A216V, L218P, C212A, C213A, S214L, A216T, L217F, etc. [
19]. However, it still remains unknown why As
2O
3 loses its ability to induce degradation of these mutants PML protein. On the other hand, without PML mutations, APL patients have exhibited no resistance to arsenic trioxide [
17], indicating that the binding of As
2O
3 in the RBCC domain appears to be critical for the effect of arsenic on PML-RARα degradation; more specifically, Cys77/80 and Cys88/91 in the RING domain as well as Cys212 and Cys213 in the B2 domain are found to be arsenic targeting sites [
12].
In humans, at least seven PML isoforms (PML-I to PML-VII) have been identified, and all isoforms are generated by alternative splicing of the primary
PML gene [
11,
20,
21]. Additionally, these isoforms share a common N-terminal region, which includes the RBCC domain, but differ in their C-terminal regions due to alternative splicing of exons and account for isoform-specific functions [
22]. Interestingly, PML isoforms I–VI are localized in nucleus, and PML nuclear bodies (PML-NBs) are currently suggested to be formed by these six isoforms, while the remaining isoform, PML-VII, is localized in cytoplasm [
23]. Moreover, PML-IV and -V are commonly used to establish the effect of As
2O
3 on PML protein solubility changes or PML protein degradation [
12]. In fact, transfer of PML proteins from the Radio Immunoprecipitation Assay (RIPA) lysis buffer soluble fraction to the RIPA insoluble nuclear matrix (i.e., solubility changes) by As
2O
3 is considered one of the most important conditions for PML protein degradation [
17,
18]. For instance, if following exposure to As
2O
3 PML protein is not shifted from RIPA soluble fraction to the RIPA insoluble matrix, it indicates that PML protein might be resistant to the As
2O
3 treatment.
In the current study, we have tried to find some available arsenic compounds that can degrade PML or PML mutant proteins to overcome the limitations in treatment of arsenic resistant APL patients. Here, we used phenylarsine oxide (PAO) to determine whether the mutant PML could be degraded by exposure to PAO. To prove this, three different PML expression vectors (i.e., PML-IV, PML-V and A216V) were transfected to HEK293T cells and then exposed to iAsIII and PAO. Although iAsIII is capable of inducing the normal PML-IV and -V solubility changes (i.e., induce PML protein shifted from RIPA soluble fraction to the RIPA insoluble matrix), it failed to induce the mutant PML (A216V) protein solubility changes. As anticipated, PAO has shown potential effect on induction of both normal and abnormal PML proteins solubility changes.
2. Results
In the current study, we tried to establish whether arsenite could induce the PML-IV, PML-V or mutant PML-IV (A216V) solubility changes. In this regard, three different PML plasmids were transfected into HEK293T cell and then exposed to iAs
III in time- and concentration-dependent manners. As expected, iAs
III is capable of inducing the normal PML-IV and -V proteins solubility changes, as shown in
Figure 1A,B. Both PML proteins’ solubility can be shifted from RIPA soluble fraction (S) to the RIPA insoluble matrix (P) after 12-h exposure and modified or degraded in pellet fractions, suggesting that arsenic (iAs
III) can induce the normal PML protein solubility changes (
Figure 1A,B).
Additionally, mutant PML-IV (A216V) transfected HEK293T cells were exposed to 4, 10 and 20 µM of iAs
III for 6 h (
Figure 1C). Our data showed that iAs
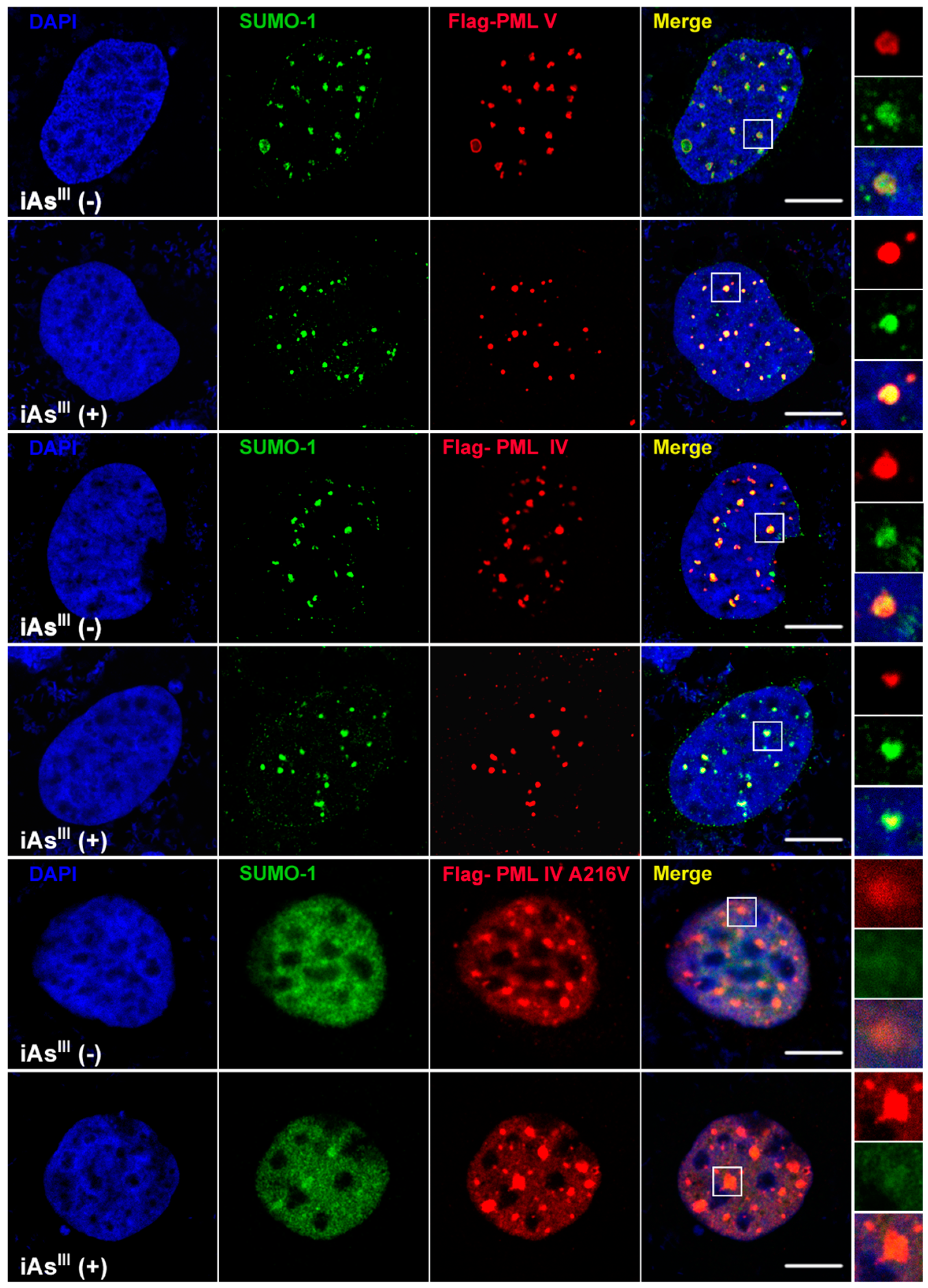
III cannot induce or degrade the mutated PML protein (A216V) solubility changes at high levels of arsenic (i.e., 20 µM). Likewise, formation of PML-nuclear bodies (NBs) was also determined by confocal microscopy after transfection of PML-IV, PML-V and A216V mutant in 293T cells (
Figure 2). Interestingly, it was found that PML-NBs can be clearly formed by mutants PML-IV (A216V) and is similar to normal PML-IV or -V. After exposure to iAs
III, there were no significant differences between the PML-NBs morphology changes as compared to corresponding without arsenic treatment group, indicating the PML protein (e.g., mutant) resistance to iAs
III is probably dependence on its protein solubility changes, but not on PML-NBs formation.
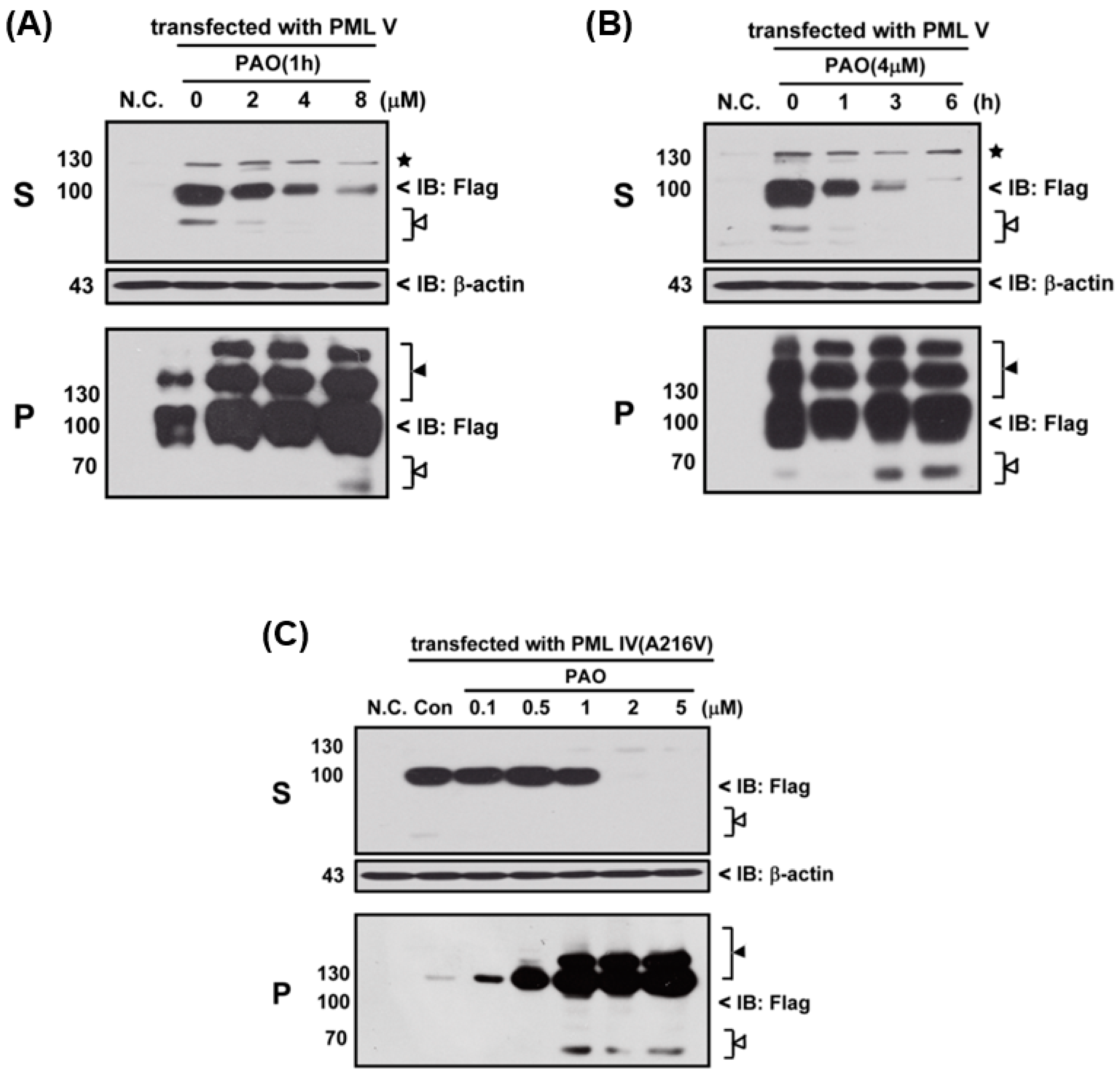
Next, we determined the effects of PAO on PML protein degradation, thereby three different variants of PML protein (i.e., normal and abnormal PML), PML-IV, PML-V and mutant PML-IV (A216V)-transfected 293T cells, were exposed to PAO in time- and concentration-dependent manners, as shown in
Figure 3. In fact, PAO is capable of inducing PML protein solubility changes from supernatant (S) to pellet fractions (P) in both dose- and time-dependent manners (
Figure 3A,B), suggesting that PAO have similar effect on normal PML degradation as like iAs
III. However, iAs
III was also shown to be unable to induce the PML mutant (A216V) protein degradation [
2,
3]. Therefore, we attempt to determine the solubility changes of PML (A216V) protein after exposure to different concentrations of PAO, and interestingly more than 2 µM of PAO were found to be sufficient for inducing mutant PML (A216V) protein solubility changes as well as PML protein modification in pellet fractions after 6 h exposure (
Figure 3C), indicating that PAO have strong dual effects on both normal or abnormal PML protein solubility changes along with PML proteins degradation.
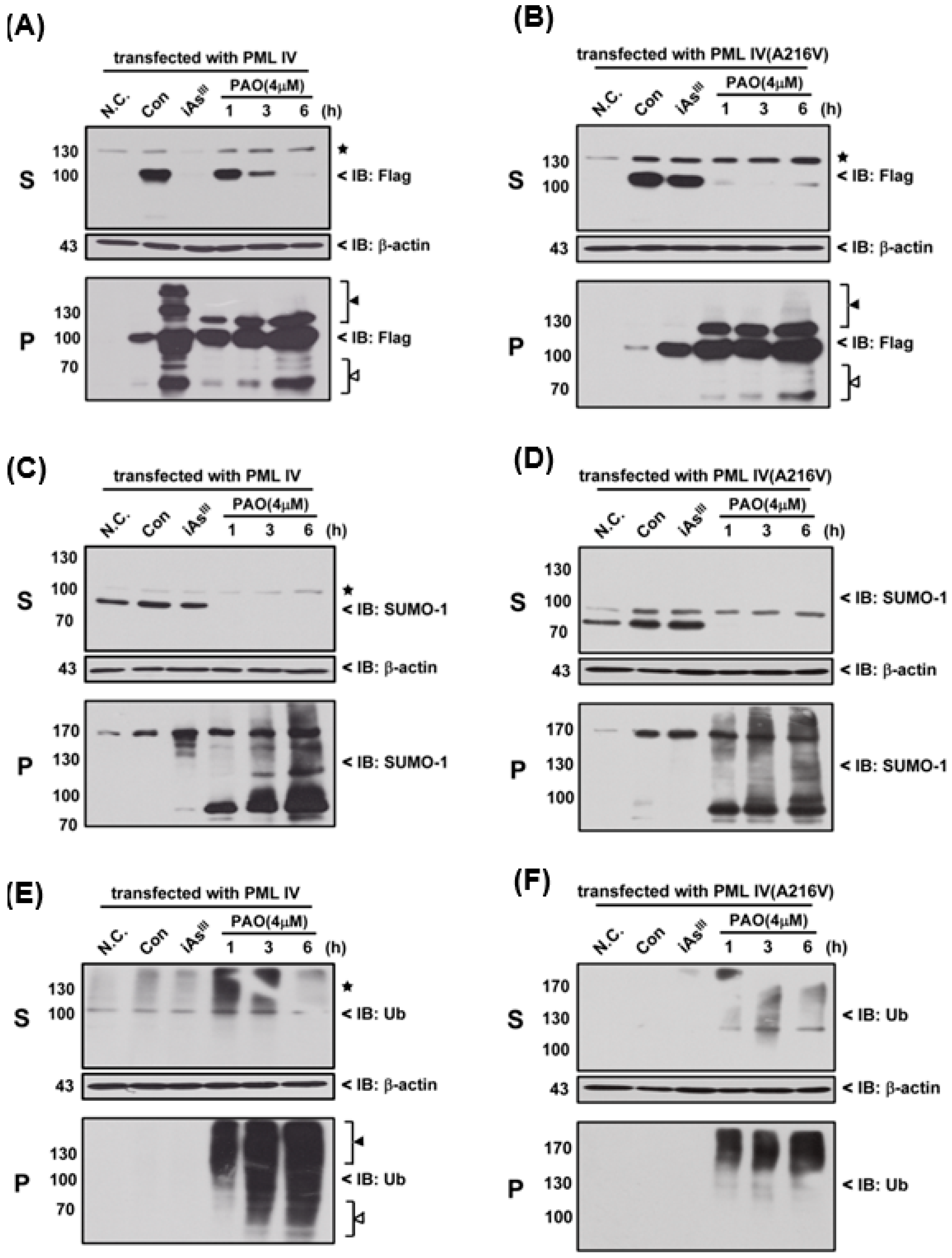
Moreover, we used 4 µM of PAO to compare the differences (e.g., SUMOylation and ubiquitination) between the normal PML and mutant PML (A216V) at different time periods, as shown in
Figure 4. Interestingly, it seems that mutant PML protein was more sensitive to PAO treatment than normal PML-IV at 1 h exposure (
Figure 4A,B).
On the other hand, PAO (but not of iAs
III) has completely induced SUMO-1 protein solubility changes from S to P fractions in two different PML transfected cells (
Figure 4 C,D). Likewise, PAO also dramatically induced PML protein ubiquitination in supernatant and pellet fractions when compared to iAs
III (
Figure 4E,F), indicating that induction of PML protein SUMOylation and ubiquitination by PAO are much stronger than iAs
III.
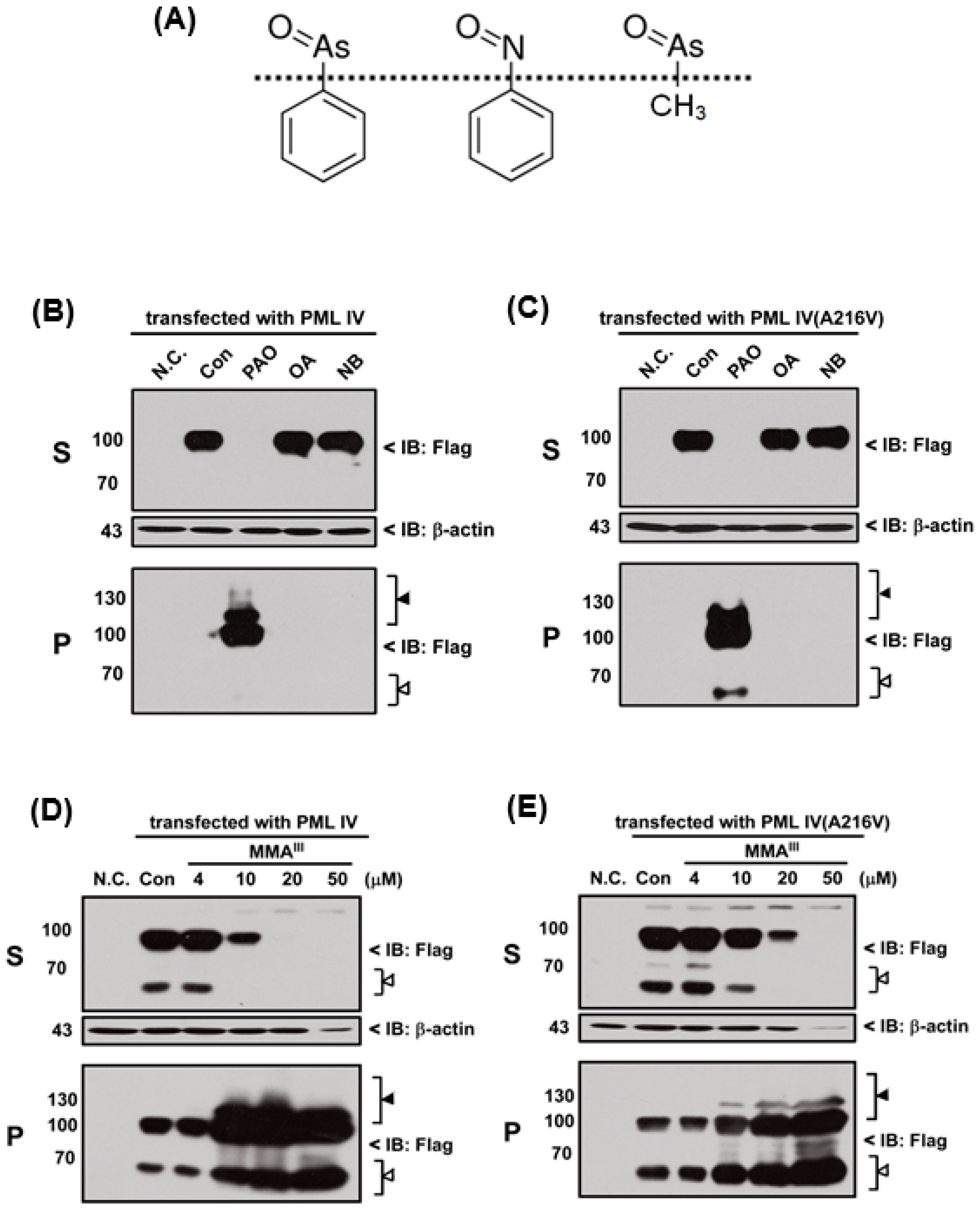
In fact, PAO is a protein phosphatase inhibitor [
24] and contains a phenyl group. Thus, we hypothesized that inhibition of protein phosphatase activity or phenyl group of PAO may be involved in PML protein solubility changes. Therefore, three different chemical compounds such as phenylarsine oxide (PAO), okadaic acid (OA; inhibitor of protein phosphatase) and nitrosobenzene (NB; contain phenyl group) were used to compare the PML-IV or mutant PML (A216V) protein solubility change, as shown in Fgiure 5A. Unexpectedly, OA and NB have no considerable effects on the induction of either PML protein solubility changes when compared to PAO (
Figure 5B,C). Surprisingly, it was found that trivalent methylated MMA
III at high dose (i.e., more than 20 µM) is capable of inducing both PML proteins solubility changes (
Figure 5D,E), because there are structural similarities between MMA
III and PAO. To understand whether PAO and MMA
III induced PML protein solubility changes are specific, normal and abnormal
PML genes transfected cells were exposed to DMA
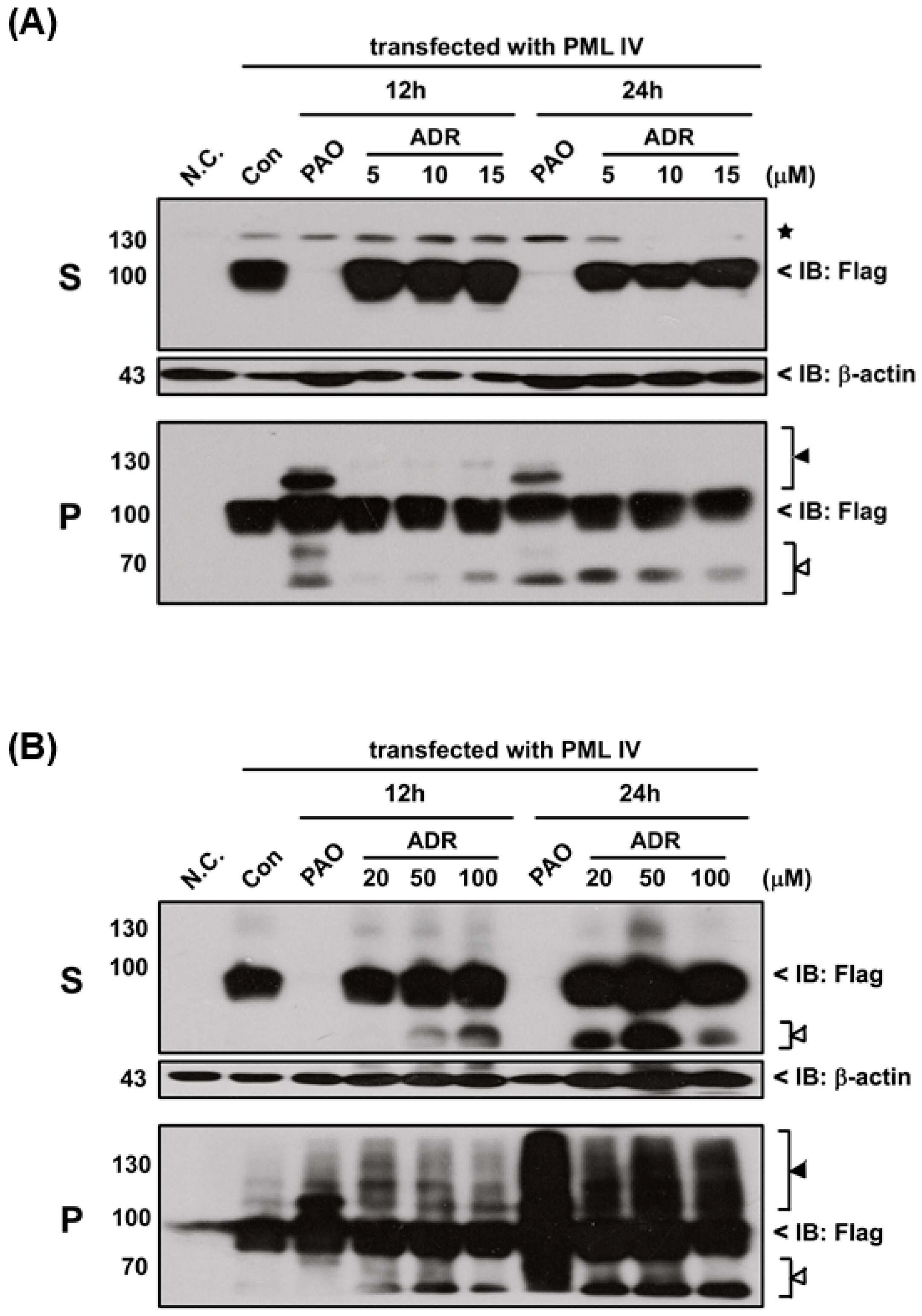
III or adriamycin (ADR; a chemotherapeutic agent). However, PML proteins solubility changes were not found after exposure to either DMA
III (
Figure S1) or ADR (
Figure 6) even though they cause cell death (
Figure S2), indicating that the PAO induced PML protein solubility changes are different from other chemical compounds.
3. Discussion
PML or PML-RARα protein solubility changes, hyper-SUMOylation and degradation in response to As
2O
3 (i.e., iAs
III) constitute the molecular mechanisms of arsenic-induced treatment of APL [
14,
25]. Unfortunately, some patients still develop arsenic resistance during their therapy and are often found in relapsed APL. Moreover, their outcomes are also extremely poor. Recent studies have clearly revealed that genetic mutations in B-box2 moiety of
PML gene rendered resistance to As
2O
3 [
26]. Thus, new arsenic compounds are needed to investigate which will be more effective than As
2O
3, and can induce the mutated PML protein solubility changes and degradation.
In fact, PML protein solubility changes by As
2O
3 are suggested to be the most important and critical step for PML protein degradation in response to As
2O
3 treatment [
11]. For instance, if the mutant PML protein is resistant to As
2O
3 in vitro (i.e., no solubility changes), their corresponding PML mutants in APL patients also exhibit resistance to As
2O
3 [
12,
17]. In this study, we found that iAs
III can induce normal PML-IV and -V solubility changes, and PML-IV seems to be less sensitive to iAs
III treatment than PML-V (
Figure 1). Additionally, iAs
III had no appreciable effects on induction of solubility changes of mutant PML (A216V), which is consistent with other published reports [
16,
17,
18]. On the other hand, mutant PML (A216V) protein can form PML-NBs but it cannot be degraded by iAs
III, suggesting that formation of the PML-NBs is not a sufficient condition for mutant PML protein degradation (
Figure 2).
Phenylarsine oxide (PAO) is a membrane-permeable protein-tyrosine phosphatase (PTPase) inhibitor [
24]. An report has indicated that PAO is capable of inhibiting proliferation or inducing apoptosis of AML cells in vitro [
27]. This efficacy of PAO against AML cells increased interest in using it for the treatment of As
2O
3 resistant APL. Therefore, we attempted to examine whether PAO could induce mutant PML protein solubility changes; thereby, normal and abnormal PML overexpressed 293T cells were exposed to PAO in dose- and time-dependent manners (
Figure 3). Interestingly, we found that PAO can induce both normal and abnormal PML proteins solubility changes, especially PAO significantly increased PML protein modification in pellet fractions. Notably, low dose of PAO (i.e., 0.5–1 µM) had no appreciable effect on induction of mutant PML (A216V) protein solubility changes, while 2 µM of PAO were found to be sufficient to induce mutant PML protein solubility changes (S to P), indicating that PAO may have good effects on mutant PML protein degradation. Particularly, we also observed that mutant PML (A216V) protein is more sensitive to PAO than normal PML (
Figure 3).
PML or PML-RARα protein degradation by As
2O
3 was identified to be mainly through SUMOylation and ubiquitination dependent proteasome pathways [
13,
14]. Additionally, small ubiquitin-related modifier-1 (SUMO-1) is a well-characterized member of the family of ubiquitin-related proteins, and PML protein is modified by SUMOylation at three important lysine residues (K65, K160 and K490 present in corresponding regions as RING, B-box 1 and carboxy-terminal region) after exposure to As
2O
3 [
28]. In the current study, we found that PAO can dramatically induced PML protein SUMOylation and ubiquitination (
Figure 4), suggesting that normal or mutant PML might be degraded through SUMOylation and ubiquitination proteasome pathways after PML protein solubility changes.
Moreover, we hypothesized that inhibition of protein phosphatase activity or phenyl group of PAO may be involved in PML protein solubility changes, therefore inhibitor of protein phosphatase OA and nitrosobenzene (NB) were used to examine the normal and mutant PML protein solubility changes. However, neither NB nor OA could induce the PML protein solubility changes (
Figure 5). On the other hand, in our previous study, we reported that low dose exposure of methylated arsenicals (i.e., MMA
III and DMA
III) has no appreciable effect on the PML or PML-RARα fusion protein solubility changes [
29]. In this study, high concentrations of iAs
III, MMA
III and DMA
III were used to establish whether these arsenicals could induce the mutant PML protein degradation similarly to PAO. Unexpectedly, 50 µM of iAs
III or 50 µM of DMA
III failed to induce the mutant PML protein solubility changes (
Figure S1). Surprisingly, high concentrations of MMA
III induced PML proteins solubility changes similarly to PAO (
Figure 5).
Here, in order to confirm where PAO (or MMA
III) induced PML protein solubility changes are dependent on cell death by cytotoxicity, high concentrations (e.g., 100 µM) of adriamycin, a chemotherapeutic agent, was used to establish PML protein solubility changes (
Figure 6). Interestingly, there is no PML protein solubility changes observed by exposure to adriamycin even though it showed high toxicity (
Figure S2), suggesting that PAO induced PML protein solubility changes may depend on another mechanism.
4. Materials and Methods
4.1. Reagents
All reagents were of analytical grade. Milli-Q water (Millipore, Bedford, MA, USA) was used throughout the experiment. Trizma® HCl and Trizma® Base, phenazene methosulfate, decylubiquinone, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and phenylarsine oxide (PAO) were purchased from Sigma (St. Louis, MO, USA). Sodium arsenite (iAsIII), dimethylarsinic acid [(CH3)2AsO(OH)] (DMAV) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Monomethylarsonic acid (MMAV) was obtained from Tri Chemicals (Yamanashi, Japan). The arsenic standard solutions were stored in the dark at 4 °C. Diluted standard solutions for experiments were prepared daily prior to use.
4.2. Preparation of Trivalent Monomethylarsonous Acid (MMAIII) and Dimethylarsinous Acid (DMAIII)
MMAIII and DMAIII were prepared by reducing MMAV and DMAV, respectively, with 5 molar equivalents of l-cysteine in distilled water at 90 °C for 1 h. The trivalent forms were confirmed by comparison of the respective retention times on a GS 220 gel filtration column by HPLC-ICP MS (Showa Denko, Tokyo),with those prepared from their iodide forms in distilled water under nitrogen atmosphere. Purity of MMAIII (98%, with 2% of MMAV) and DMAIII (95%, and with 5% of DMAV) was confirmed by HPLC-ICP MS, and then used.
4.3. Confirmation of Arsenic Species by HPLC-ICP MS
The HPLC system consisted of a liquid chromatograph solvent delivery PU-610 pump and a DG 660B-2 degasser (GL Sciences Co., Tokyo, Japan). A strong anion exchange column (Hamilton PRPX-100, 5 μm 4.1 mm × 150 mm) was used to detect the arsenic species. A 20-µL aliquot of a sample solution was applied to the PRPX-100 column, and then the column was eluted with a mobile phase of 35 mM ammonium bicarbonate, pH adjusted to 8.2, with a flow rate of 0.8 mL/min (PRP X-100). Arsenic in the eluate was monitored with an Agilent HP7500cs ICP MS (Yokogawa, Hachiouji, Japan) equipped with an octopole reaction system (ORS) with a He flow of 3.0 mL per min to prevent molecular interference by 40Ar35Cl (signal at m/z 75).
4.4. Cell Culture
HEK293T cells were purchased from Cell Bank of China Science. Following receipt, cells were grown and frozen as a seed stock as they were available. Cells were passage for a maximum of 3 months, after which new seed stocks were thawed. Two cell lines were authenticated using DNA fingerprinting (variable number of tandem repeats), confirming that no cross-contamination occurred during this study. Two cell lines were tested for mycoplasma contamination at least two times per year.
Cells (1.0 × 106) were cultured in T25 flask, and were maintained in logarithmic growth phase using RPMI-1640 or Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin, at 37 °C in 5% CO2 atmosphere. After 24 h of seeding, cultures were washed with PBS, fresh medium was added, and the cells were treated with indicated doses of arsenicals and all-trans retinoic acid for indicated time.
4.5. Western Blot Analysis
HEK 293T cells were lysed in RIPA buffer without 8 mole (M) urea, and then incubated on ice and centrifuged as mentioned above to obtain the supernatant and pellet fractions. The supernatant was transferred and the pellet was washed with PBS, lysed in SDS buffer [1× tris-buffered saline ((TBS), 10% glycerol, 0.015% ethylenediaminetetraacetic acid (EDTA), 50 mM dithiothreitol (DTT), and 2% sodium dodecyl sulfate (SDS)], boiled for 10 min at 95 °C. Twenty-five microgram of each protein sample was resolved by 10%–12% SDS-PAGE and electroblotted onto nitrocellulose membranes. The membranes were blocked with non-fat milk and incubated overnight with different primary antibodies at 4 °C, followed by incubation with horseradish peroxidase (HRP)-linked secondary antibodies for 1 h at room temperature and then the proteins were visualized by enhanced chemiluminescence.
4.6. Immunofluorescence Microscopy
FLAG-PML transfected HEK293T cells were cultured on Chamber slides and then exposed to arsenicals. After washing with phosphate-buffered saline (PBS) twice, the slides were fixed in 4% paraformaldehyde and permeablized with 0.1% Triton X-100, blocked with PBST with 10% fetal bovine serum. Further, the cells were then blocked with 1% bovine serum albumin (BSA) in PBS, followed by incubation with primary antibodies overnight at 4 °C. Later, they were tagged with fluorescent secondary antibodies for 2 h. Slides were mounted using Vectashield mounting liquid (Vector Labs, Burlingame, CA, USA), sealed, and stored in dark at 4 °C. The cells were examined under a Zeiss (Göttingen, Germany) 510 confocal microscope.