Volasertib Enhances Sensitivity to TRAIL in Renal Carcinoma Caki Cells through Downregulation of c-FLIP Expression
Abstract
:1. Introduction
2. Results
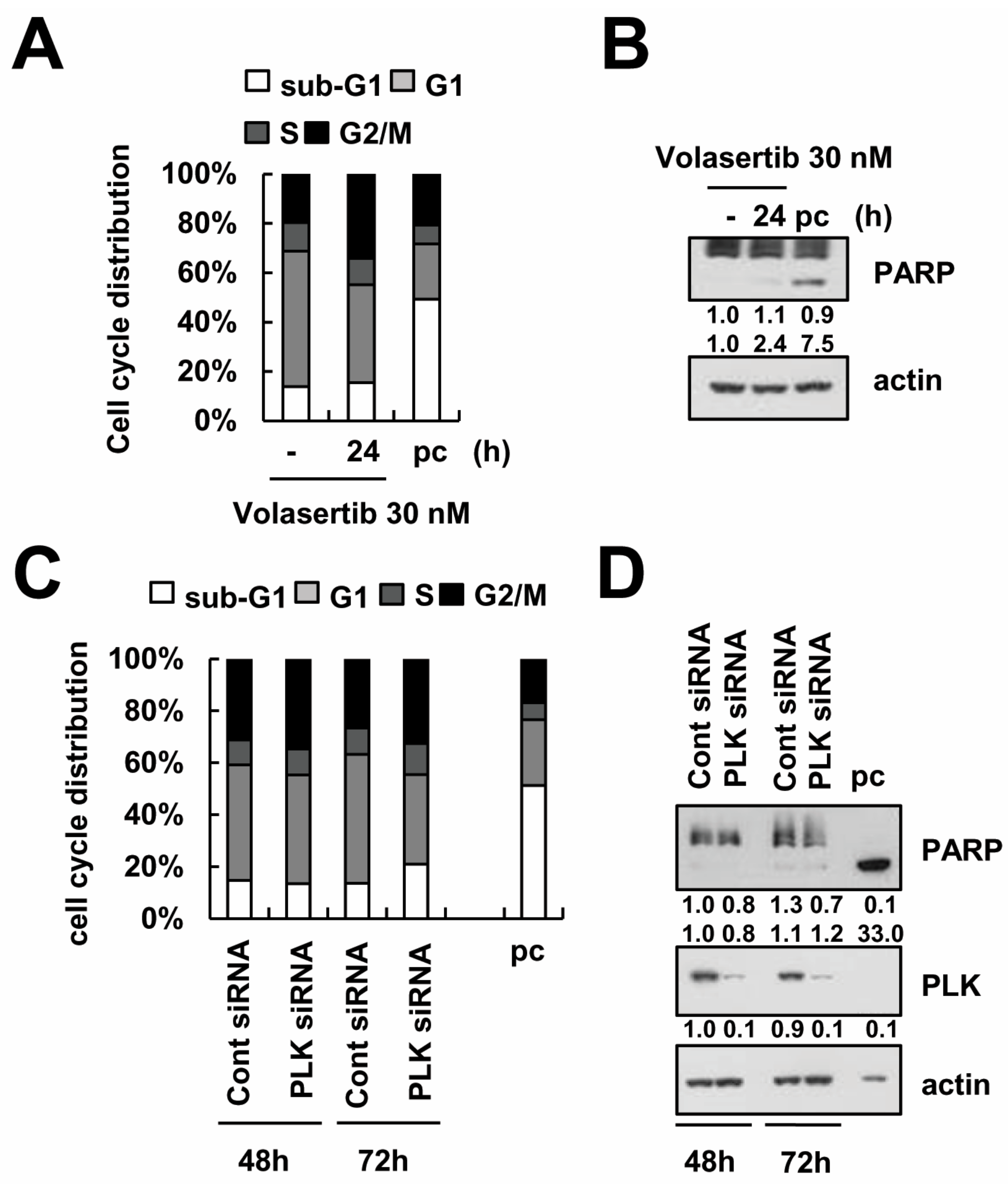
2.1. The Effect of Volasertib (BI 6727), a Polo-Like Kinase1 (PLK1) Inhibitor, on Apoptotic Cell Death in Human Renal Carcinoma Caki Cells
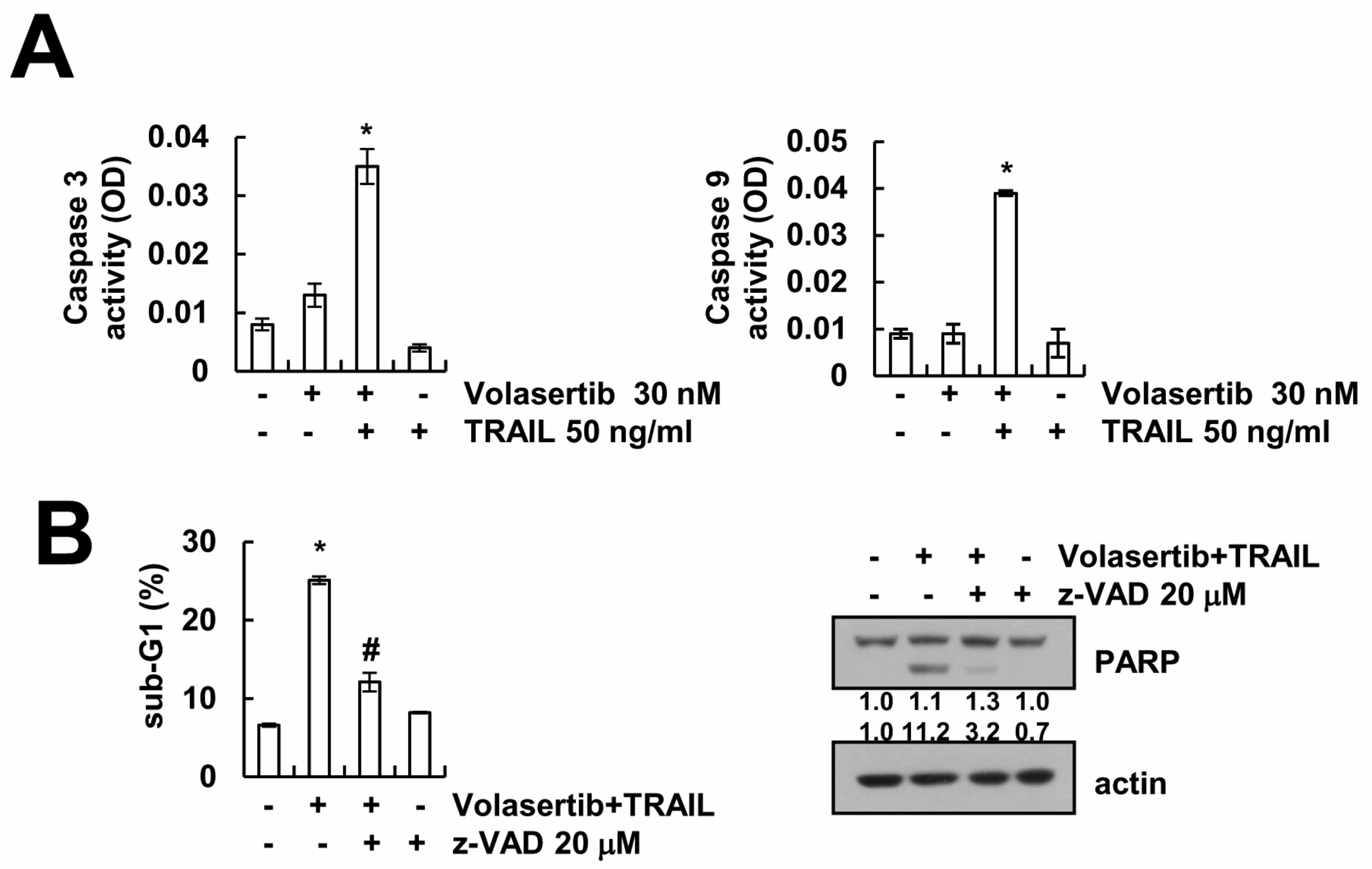
2.2. The Effect of Volasertib on TRAIL-Induced Apoptotic Cell Death in Human Renal Carcinoma Caki Cells
2.3. Volasertib-Induced Apoptosis Is Caspase-Dependent in Caki Cells
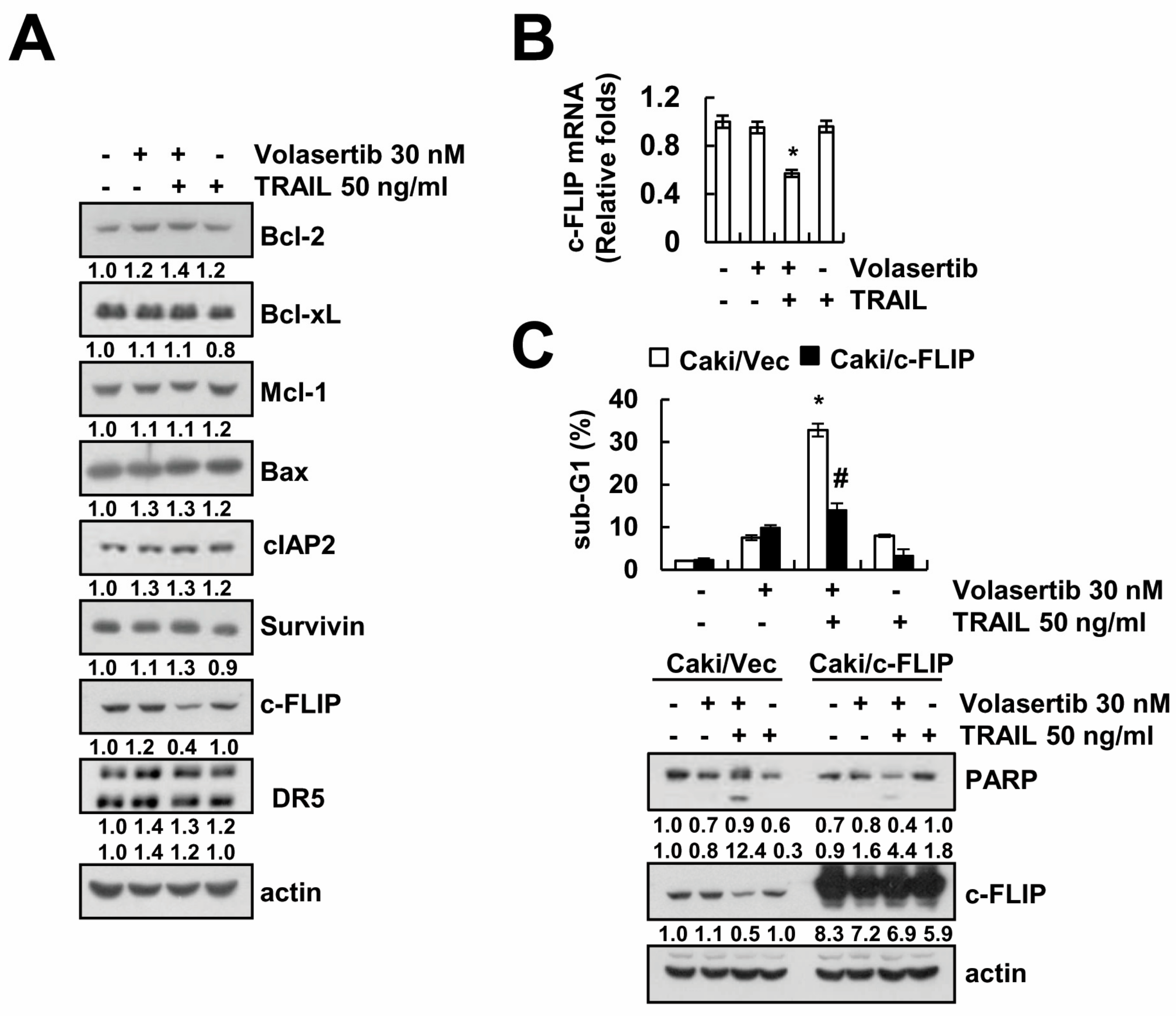
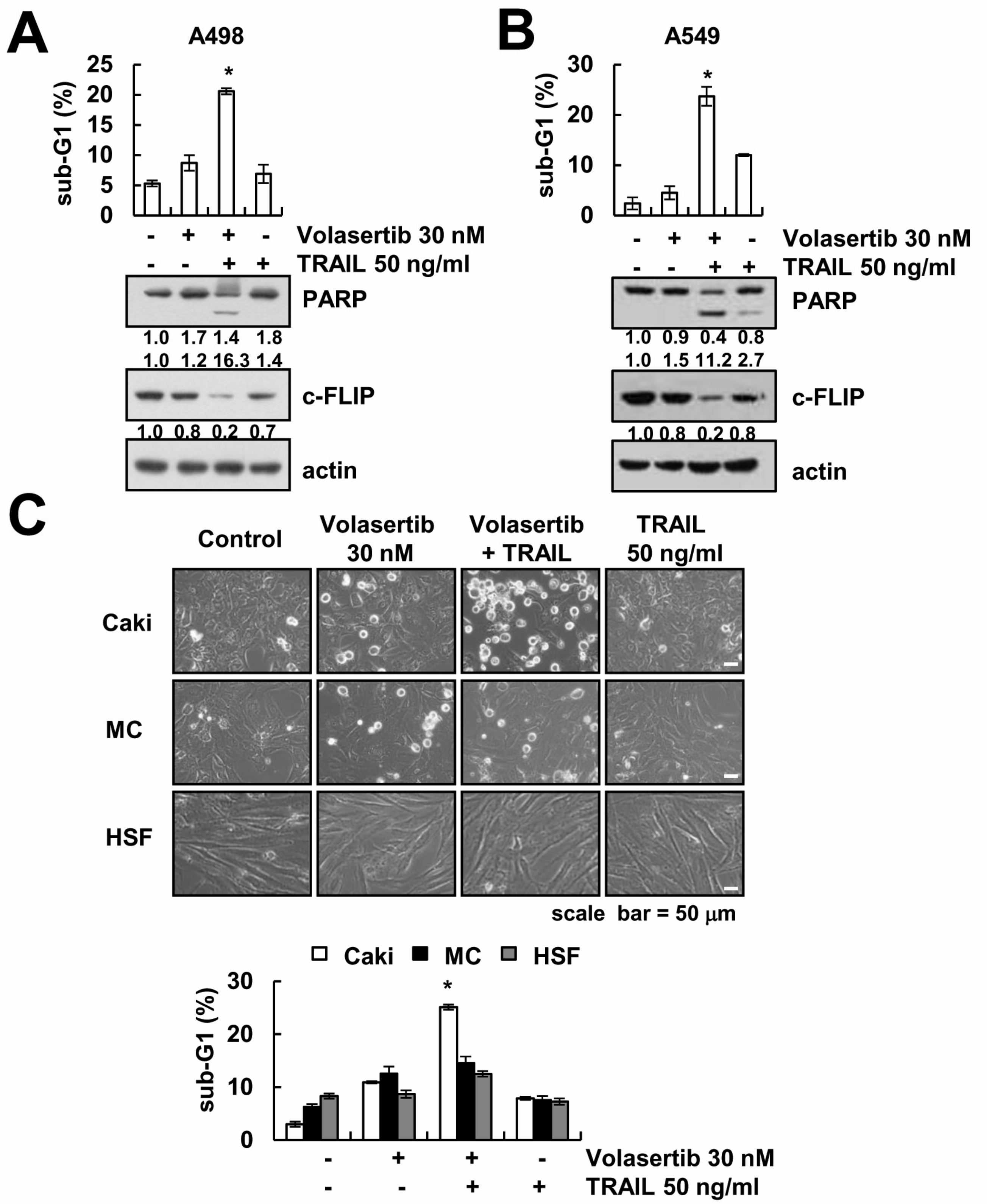
2.4. Combined Treatment Volasertib and TRAIL Induces the Downregulation of c-FLIP Expression
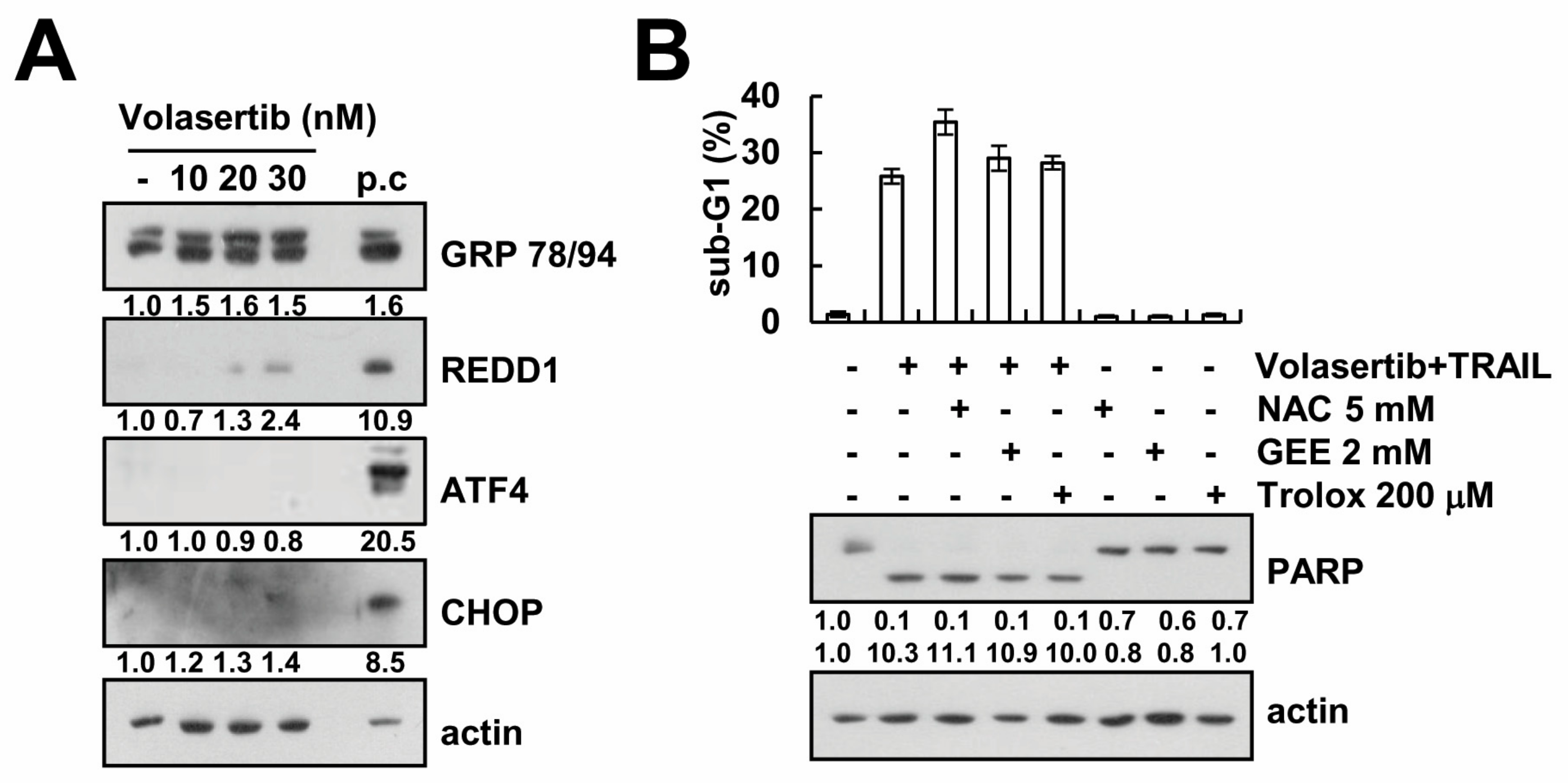
2.5. Volasertib-Mediated TRAIL Sensitization Is Not Associated with Induction of ER Stress and ROS Signaling Pathway
2.6. Knockdown of PLK1 Enhances Caki Cells to TRAIL-Mediated Apoptosis
2.7. The Combined Treatment of Volasertib and TRAIL Induces Apoptosis in Other Cancer Cells, but Not Normal Cells
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Materials
4.2. Flow Cytometry Analysis
4.3. Small Interfering RNA (SIRNA)
4.4. Western Blotting Analysis
4.5. Annexin V Staining
4.6. 4′,6′-Diamidino-2-Phenylindole Staining (DAPI) for Nuclei Condensation and Fragmentation
4.7. Cell Death Assessment by DNA Fragmentation Assay
4.8. Asp-Glu-Val-Asp-Ase (DEVDase) and Leu-Glu-His-Asp-Ase (LEHDase) Activity Assays
4.9. Quantitative PCR (qPCR)
4.10. c-FLIP Constructs and Stable Cells
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| ROS | Reactive oxygen species |
| HSF | Human skin fibroblast |
| DR | Death receptor |
References
- Pan, G.; Ni, J.; Wei, Y.F.; Yu, G.; Gentz, R.; Dixit, V.M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 1997, 277, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.P.; Marsters, S.A.; Pitti, R.M.; Gurney, A.; Skubatch, M.; Baldwin, D.; Ramakrishnan, L.; Gray, C.L.; Baker, K.; Wood, W.I.; et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 1997, 277, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Smith, F.; Wang, Q.; Wang, X.; Evers, B.M. Assessment of differential gene expression patterns in human colon cancers. Ann. Surg. 2000, 232, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Ganten, T.M.; Koschny, R.; Haas, T.L.; Sykora, J.; Li-Weber, M.; Herzer, K.; Walczak, H. Proteasome inhibition sensitizes hepatocellular carcinoma cells, but not human hepatocytes, to TRAIL. Hepatology 2005, 42, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Chopra, P.; Sethi, G.; Dastidar, S.G.; Ray, A. Polo-like kinase inhibitors: An emerging opportunity for cancer therapeutics. Expert Opin. Investig. Drugs 2010, 19, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Schmit, T.L.; Ledesma, M.C.; Ahmad, N. Modulating polo-like kinase 1 as a means for cancer chemoprevention. Pharm. Res. 2010, 27, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Z.; Liu, Z. Polo-like kinase 1 is overexpressed in renal cancer and participates in the proliferation and invasion of renal cancer cells. Tumour Biol. 2013, 34, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Deeraksa, A.; Pan, J.; Sha, Y.; Liu, X.D.; Eissa, N.T.; Lin, S.H.; Yu-Lee, L.Y. PLK1 is upregulated in androgen-insensitive prostate cancer cells and its inhibition leads to necroptosis. Oncogene 2013, 32, 2973–2983. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, D.; Steegmaier, M.; Hoffmann, M.; Grauert, M.; Baum, A.; Quant, J.; Haslinger, C.; Garin-Chesa, P.; Adolf, G.R. BI 6727, a Polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin. Cancer Res. 2009, 15, 3094–3102. [Google Scholar] [CrossRef] [PubMed]
- Nonomiya, Y.; Noguchi, K.; Tanaka, N.; Kasagaki, T.; Katayama, K.; Sugimoto, Y. Effect of AKT3 expression on MYC- and caspase-8-dependent apoptosis caused by polo-like kinase inhibitors in HCT 116 cells. Cancer Sci. 2016, 107, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.W.; Xue, Y.Q.; Li, Y.; Di, J.M.; Qiu, J.G.; Zhang, W.J.; Jiang, Q.W.; Yang, Y.; Chen, Y.; Wei, M.N.; et al. Volasertib suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Am. J. Cancer Res. 2016, 6, 2476–2488. [Google Scholar] [PubMed]
- Cholewa, B.D.; Ndiaye, M.A.; Huang, W.; Liu, X.; Ahmad, N. Small molecule inhibition of polo-like kinase 1 by volasertib (BI 6727) causes significant melanoma growth delay and regression in vivo. Cancer Lett. 2017, 385, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, H.; Takada, Y.; Shishodia, S.; Jayaprakasam, B.; Nair, M.G.; Aggarwal, B.B. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-κB) activation and NF-κB-regulated gene expression. Mol. Cancer Ther. 2006, 5, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O.; Lens, S.; Gaide, O.; Alevizopoulos, K.; Tschopp, J. NF-κB signals induce the expression of c-FLIP. Mol. Cell. Biol. 2001, 21, 5299–5305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Yang, H.; Huang, X.; Otu, H.; Libermann, T.A.; DeWolf, W.C.; Khosravi-Far, R.; Olumi, A.F. c-FOS as a proapoptotic agent in TRAIL-induced apoptosis in prostate cancer cells. Cancer Res. 2007, 67, 9425–9434. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Yerbes, R.; Lopez-Rivas, A. Flavopiridol induces cellular FLICE-inhibitory protein degradation by the proteasome and promotes TRAIL-induced early signaling and apoptosis in breast tumor cells. Cancer Res. 2006, 66, 8858–8869. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, T.; Fujiwara, T.; Uno, F.; Teraishi, F.; Kadowaki, Y.; Itoshima, T.; Takata, Y.; Kagawa, S.; Roth, J.A.; Tschopp, J.; et al. Accelerated degradation of cellular FLIP protein through the ubiquitin-proteasome pathway in p53-mediated apoptosis of human cancer cells. Oncogene 2001, 20, 5225–5231. [Google Scholar] [CrossRef] [PubMed]
- Krueger, A.; Schmitz, I.; Baumann, S.; Krammer, P.H.; Kirchhoff, S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J. Biol. Chem. 2001, 276, 20633–20640. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Lee, J.T.; Park, J.W.; Kwon, T.K. Acquired TRAIL resistance in human breast cancer cells are caused by the sustained cFLIP(L) and XIAP protein levels and ERK activation. Biochem. Biophys. Res. Commun. 2006, 351, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.U.; Kim, T.H.; Kim, D.E.; Min, K.J.; Kwon, T.K. NOX4-mediated ROS production induces apoptotic cell death via down-regulation of c-FLIP and Mcl-1 expression in combined treatment with thioridazine and curcumin. Redox Biol. 2017, 13, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.R.; Min, K.J.; Woo, S.M.; Choe, M.; Choi, K.S.; Lee, Y.K.; Yoon, G.; Kwon, T.K. Inhibition of cathepsin S induces mitochondrial ROS that sensitizes TRAIL-mediated apoptosis through p53-mediated downregulation of Bcl-2 and c-FLIP. Antioxid. Redox Signal. 2017, 27, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, X.; Zhang, H.; Lei, D.; Liu, D.; Xu, F.; Luan, X. Overexpression of cFLIP in head and neck squamous cell carcinoma and its clinicopathologic correlations. J. Cancer Res. Clin. Oncol. 2008, 134, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Oyarzo, M.P.; Medeiros, L.J.; Atwell, C.; Feretzaki, M.; Leventaki, V.; Drakos, E.; Amin, H.M.; Rassidakis, G.Z. c-FLIP confers resistance to FAS-mediated apoptosis in anaplastic large-cell lymphoma. Blood 2006, 107, 2544–2547. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.K.; Lee, M.G.; Chi, S.G.; Kim, Y.W.; Park, J.H. Increased expression of cFLIP(L) in colonic adenocarcinoma. J. Pathol. 2001, 194, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.D.; Yu, J.P.; Liu, J.; Luo, H.S.; Chen, H.X.; Yu, H.G. Overexpression of cellular FLICE-inhibitory protein (FLIP) in gastric adenocarcinoma. Clin. Sci. 2004, 106, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Kwon, Y.J.; Chun, Y.J. CYP1B1 Activates Wnt/β-catenin signaling through suppression of HERC5-mediated ISGylation for protein degradation on β-catenin in HeLa cells. Toxicol. Res. 2017, 33, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Shin, D.Y. Repression of the F-box protein Skp2 is essential for actin damage-induced tetraploid G1 arrest. BMB Rep. 2017, 50, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, T.J.; Leem, J.; Choi, K.S.; Park, J.W.; Kwon, T.K. Sanguinarine-induced apoptosis: Generation of ROS, down-regulation of Bcl-2, c-FLIP, and synergy with TRAIL. J. Cell. Biochem. 2008, 104, 895–907. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, M.-Y.; Min, K.-j.; Woo, S.M.; Seo, S.U.; Kim, S.; Park, J.-W.; Kwon, T.K. Volasertib Enhances Sensitivity to TRAIL in Renal Carcinoma Caki Cells through Downregulation of c-FLIP Expression. Int. J. Mol. Sci. 2017, 18, 2568. https://doi.org/10.3390/ijms18122568
Jeon M-Y, Min K-j, Woo SM, Seo SU, Kim S, Park J-W, Kwon TK. Volasertib Enhances Sensitivity to TRAIL in Renal Carcinoma Caki Cells through Downregulation of c-FLIP Expression. International Journal of Molecular Sciences. 2017; 18(12):2568. https://doi.org/10.3390/ijms18122568
Chicago/Turabian StyleJeon, Mi-Yeon, Kyoung-jin Min, Seon Min Woo, Seung Un Seo, Shin Kim, Jong-Wook Park, and Taeg Kyu Kwon. 2017. "Volasertib Enhances Sensitivity to TRAIL in Renal Carcinoma Caki Cells through Downregulation of c-FLIP Expression" International Journal of Molecular Sciences 18, no. 12: 2568. https://doi.org/10.3390/ijms18122568




