Antarctic Krill Oil Diet Protects against Lipopolysaccharide-Induced Oxidative Stress, Neuroinflammation and Cognitive Impairment
Abstract
:1. Introduction
2. Results
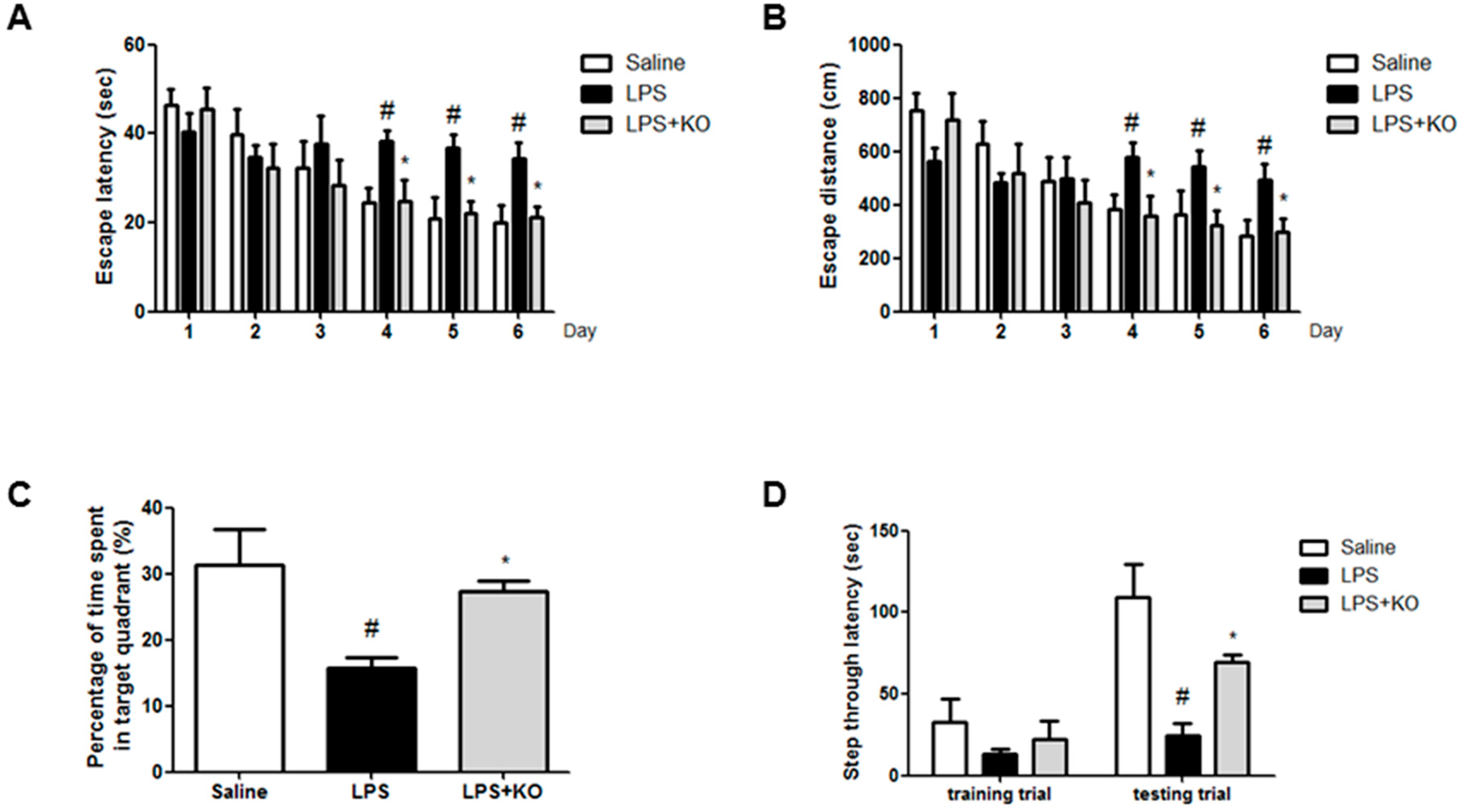
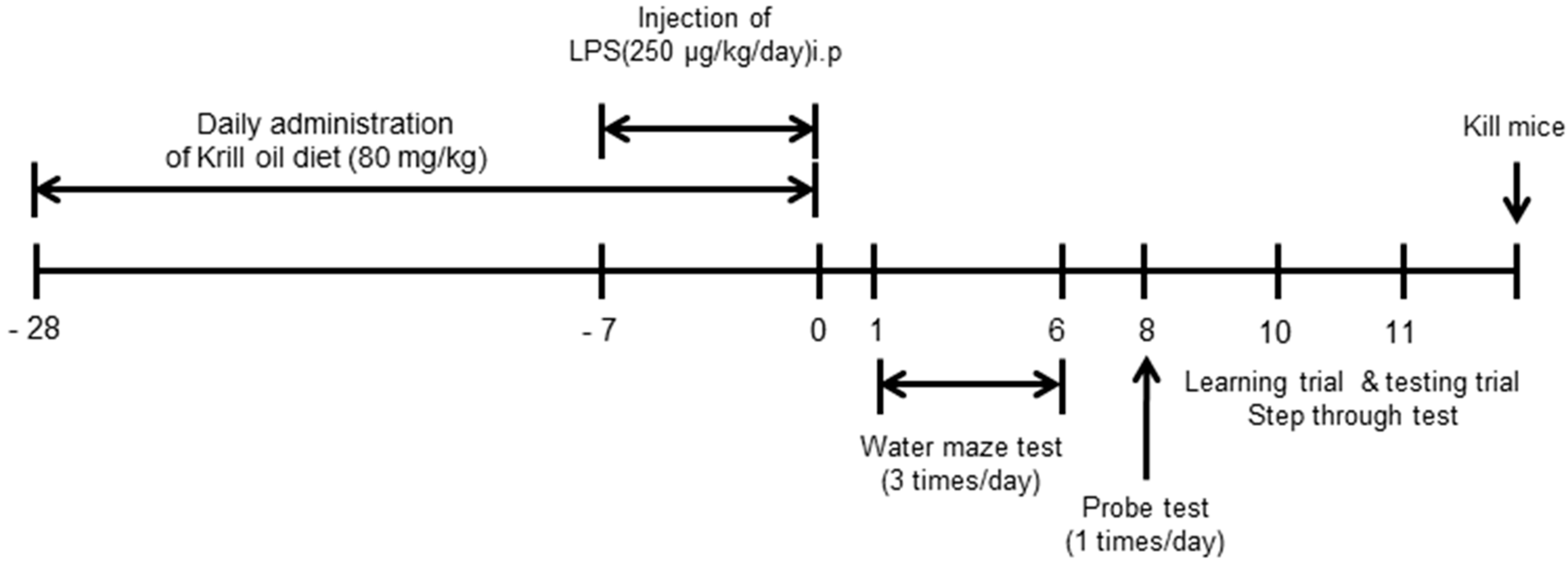
2.1. Krill Oil Treatment Attenuates LPS-Induced Cognitive Impairment
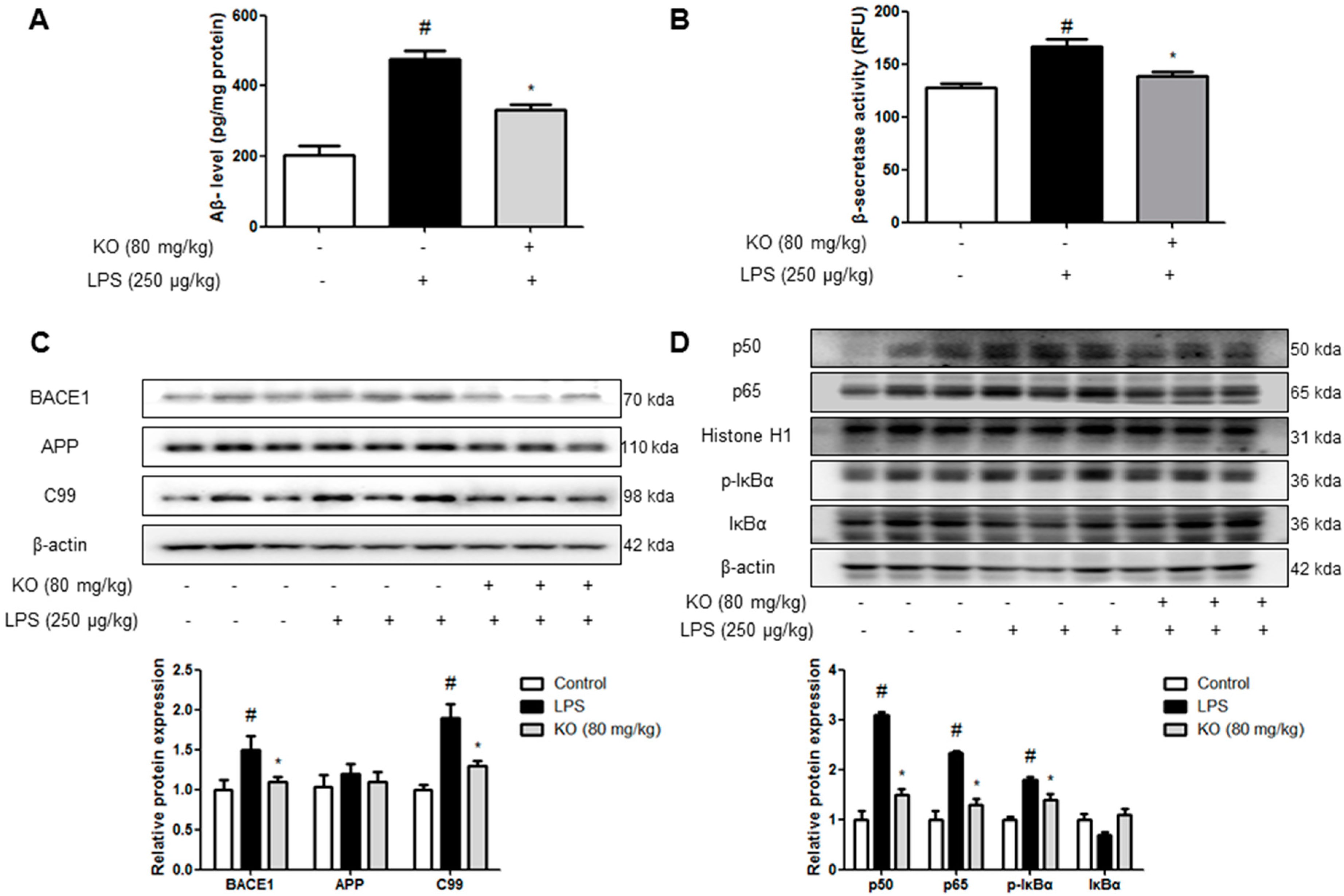
2.2. Krill Oil Treatment Inhibits the Accumulation of Aβ Peptides, Amyloidogenesis, and NF-κB Activation
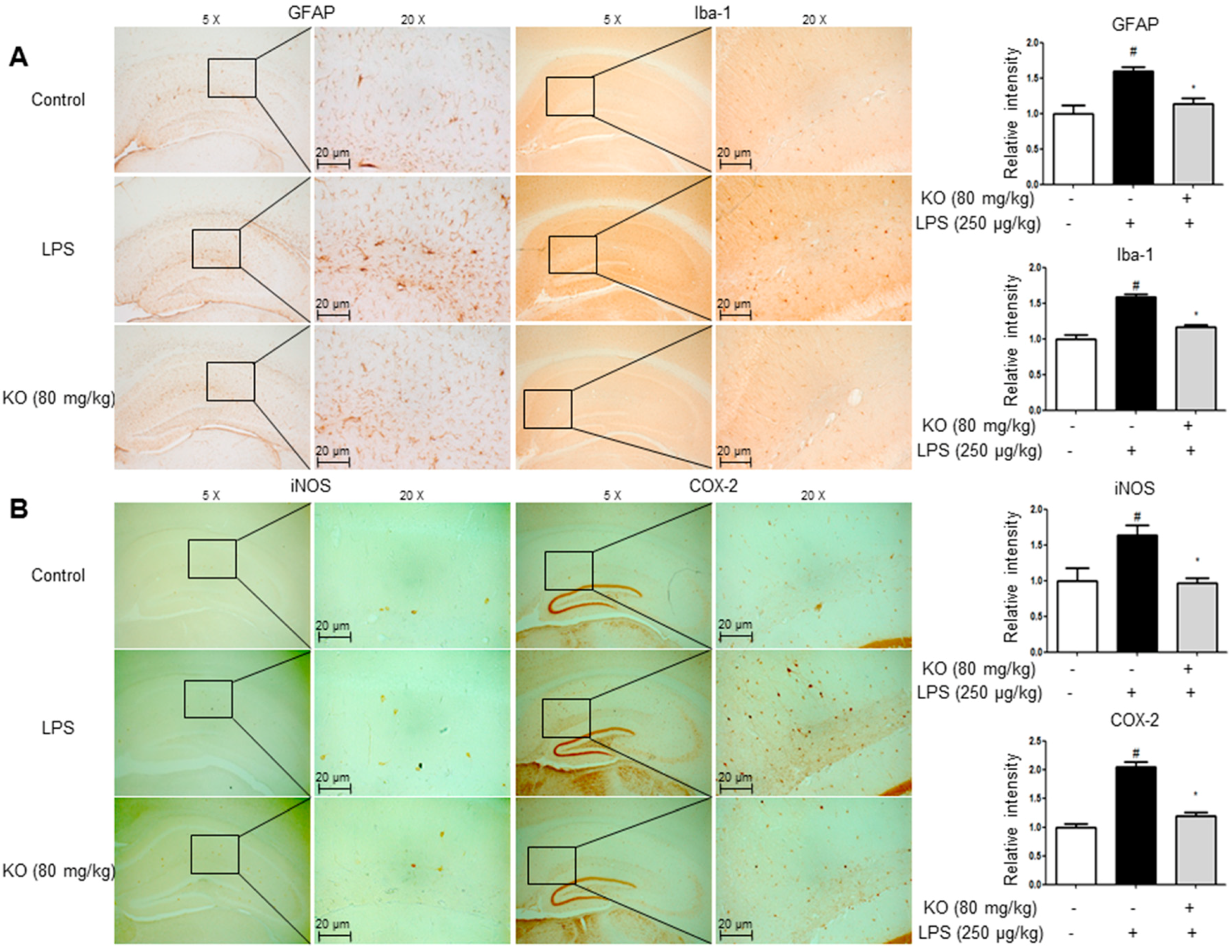
2.3. Krill Oil Treatment Inhibits Neuroinflammation
2.4. Krill Oil Inhibits LPS-Induced Oxidative Stress
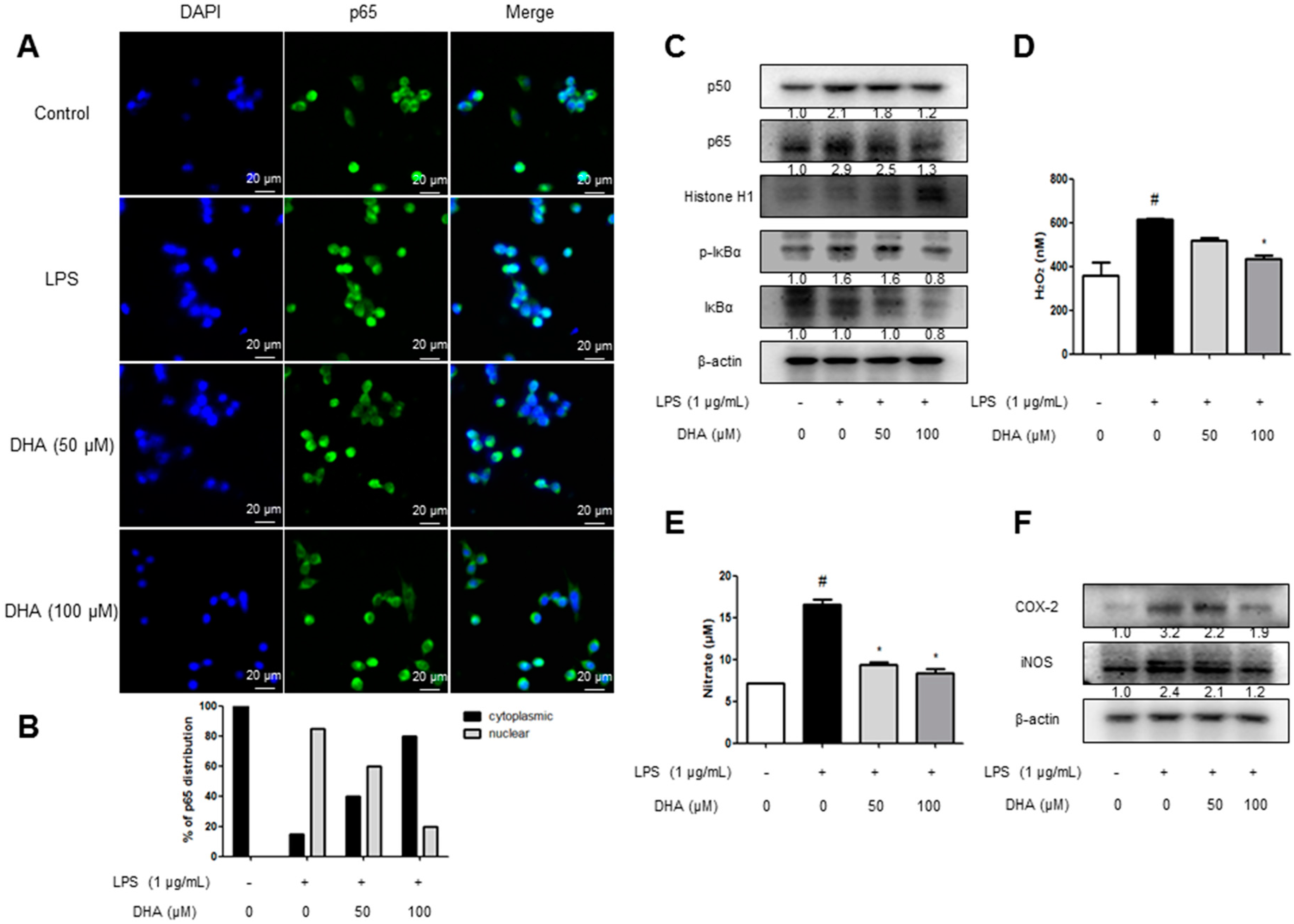
2.5. EPA and DHA Prevent LPS-Stimulated Nuclear Translocation of the NF-κB Complex
2.6. EPA and DHA Prevent LPS-Stimulated Oxidative Stress and Neuroinflammation
3. Discussion
4. Methods
4.1. Ethical Approval
4.2. Materials
4.2.1. Preparation of Enzymatically Decomposed Krill Oil
4.2.2. Animal Experiment
4.2.3. Behavior Tests
4.2.4. Brain Collection and Preservation
4.2.5. Immunohistochemical Staining
4.2.6. Immunofluorescence Staining
4.2.7. Western Blot Analysis
4.2.8. Nitrate Assay
4.2.9. RNA Isolation and Quantitative Real-Time RT-PCR
4.2.10. Measurement of Aβ1–42
4.2.11. Oxidative Stress Assay
4.2.12. Assay of β-Secretase Activities
4.2.13. Microglial BV-2 Cell Culture
4.2.14. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of interest
References
- Villa, F.A.; Gerwick, L. Marine natural product drug discovery: Leads for treatment of inflammation, cancer, infections, and neurological disorders. Immunopharmacol. Immunotoxicol. 2010, 32, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.P.; Poppe, S.C.; Bondan, E.F. Neuroprotective properties of the marine carotenoid astaxanthin and omega-3 fatty acids, and perspectives for the natural combination of both in krill oil. Nutrients 2014, 6, 1293–1317. [Google Scholar] [CrossRef] [PubMed]
- Ulven, S.M.; Holven, K.B. Comparison of bioavailability of krill oil versus fish oil and health effect. Vasc. Health Risk Manag. 2015, 11, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Tou, J.C.; Jaczynski, J. Amino acid and mineral composition of protein and other components and their recovery yields from whole Antarctic krill (Euphausia superba) using isoelectric solubilization/precipitation. J. Food Sci. 2009, 74. [Google Scholar] [CrossRef] [PubMed]
- Ierna, M.; Kerr, A.; Scales, H.; Berge, K.; Griinari, M. Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskelet. Disord. 2010, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Ramirez, V.; Macias-Islas, M.A.; Ortiz, G.G.; Pacheco-Moises, F.; Torres-Sanchez, E.D.; Sorto-Gomez, T.E.; Cruz-Ramos, J.A.; Orozco-Avina, G.; Celis de la Rosa, A.J. Efficacy of fish oil on serum of TNF-α, IL-1β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon β-1b. Oxidative Med. Cell. Longev. 2013, 2013, 709493. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Vigerust, N.F.; Bjorndal, B.; Bohov, P.; Brattelid, T.; Svardal, A.; Berge, R.K. Krill oil versus fish oil in modulation of inflammation and lipid metabolism in mice transgenic for TNF-α. Eur. J. Nutr. 2013, 52, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Gemma, C.; Vila, J.; Bachstetter, A.; Bickford, P.C. Oxidative Stress and the Aging Brain: From Theory to Prevention. In Brain Aging: Models, Methods, and Mechanisms; Riddle, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative stress and β-amyloid protein in Alzheimer’s disease. Neuromol. Med. 2011, 13, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Hemmelgarn, B.T.; Chuang, C.C.; Best, T.M. The Role of Oxidative Stress-Induced Epigenetic Alterations in Amyloid-β Production in Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2015, 2015, 604658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxidative Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxidative Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-peptide (1–42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Schott, J.M.; Revesz, T. Inflammation in Alzheimer’s disease: Insights from immunotherapy. Brain 2013, 136, 2654–2656. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012, 756357. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, Z.G.; Ding, J.; Liu, N.; Wang, D.M.; Ding, L.C.; Yang, C. Chronic lipopolysaccharide exposure induces cognitive dysfunction without affecting BDNF expression in the rat hippocampus. Exp. Ther. Med. 2014, 7, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.H.; Lee, Y.J.; Choi, D.Y.; Han, S.B.; Jung, J.K.; Hwang, B.Y.; Moon, D.C.; Kim, Y.; Lee, M.K.; Oh, K.W.; et al. Anti-amyloidogenic effect of thiacremonone through anti-inflamation in vitro and in vivo models. J. Alzheimers Dis. 2012, 29, 659–676. [Google Scholar] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Chung, J.H.; Yoon, J.S.; Ha, Y.M.; Bae, S.; Lee, E.K.; Jung, K.J.; Kim, M.S.; Kim, Y.J.; Kim, M.K.; et al. Ginsenoside Rd inhibits the expressions of iNOS and COX-2 by suppressing NF-κB in LPS-stimulated RAW264.7 cells and mouse liver. J. Ginseng Res. 2013, 37, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Meng, S.; Wang, J.; Li, S.; Liu, J.; Li, H.; Li, T.; Song, W.; Zhou, W. Two novel DNA motifs are essential for BACE1 gene transcription. Sci. Rep. 2014, 4, 6864. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxidative Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [PubMed]
- Kaur, U.; Banerjee, P.; Bir, A.; Sinha, M.; Biswas, A.; Chakrabarti, S. Reactive oxygen species, redox signaling and neuroinflammation in Alzheimer’s disease: The NF-κB connection. Curr. Top. Med. Chem. 2015, 15, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.; Lue, L.F. Anti-inflammatory and immune therapy for Alzheimer’s disease: Current status and future directions. Curr. Neuropharmacol. 2007, 5, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Verdile, G.; Keane, K.N.; Cruzat, V.F.; Medic, S.; Sabale, M.; Rowles, J.; Wijesekara, N.; Martins, R.N.; Fraser, P.E.; Newsholme, P. Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer’s Disease. Mediat. Inflamm. 2015, 2015, 105828. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, T.; Chang, L.; Song, Y.; Wu, Y.; Ma, D. Systemic inflammation induces a profound long term brain cell injury in rats. Acta Neurobiol. Exp. 2014, 74, 298–306. [Google Scholar]
- Noworyta-Sokolowska, K.; Gorska, A.; Golembiowska, K. LPS-induced oxidative stress and inflammatory reaction in the rat striatum. Pharmacol. Rep. 2013, 65, 863–869. [Google Scholar] [CrossRef]
- Bardou, I.; Kaercher, R.M.; Brothers, H.M.; Hopp, S.C.; Royer, S.; Wenk, G.L. Age and duration of inflammatory environment differentially affect the neuroimmune response and catecholaminergic neurons in the midbrain and brainstem. Neurobiol. Aging 2014, 35, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Grudzien, A.; Shaw, P.; Weintraub, S.; Bigio, E.; Mash, D.C.; Mesulam, M.M. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol. Aging 2007, 28, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Nazem, A.; Sankowski, R.; Bacher, M.; Al-Abed, Y. Rodent models of neuroinflammation for Alzheimer’s disease. J. Neuroinflamm. 2015, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, Y.K.; Yuk, D.Y.; Choi, D.Y.; Ban, S.B.; Oh, K.W.; Hong, J.T. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of β-amyloid generation. J. Neuroinflamm. 2008, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Glutathione and mitochondria. Front. Pharmacol. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Eder, C. Amyloid-β-induced reactive oxygen species production and priming are differentially regulated by ion channels in microglia. J. Cell. Physiol. 2011, 226, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Yuk, D.Y.; Lee, J.W.; Lee, S.Y.; Ha, T.Y.; Oh, K.W.; Yun, Y.P.; Hong, J.T. (−)-Epigallocatechin-3-gallate prevents lipopolysaccharide-induced elevation of β-amyloid generation and memory deficiency. Brain Res. 2009, 1250, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.I.; Lee, Y.K.; Park, S.G.; Choi, I.S.; Ban, J.O.; Park, H.K.; Nam, S.Y.; Yun, Y.W.; Han, S.B.; Oh, K.W.; et al. l-Theanine, an amino acid in green tea, attenuates β-amyloid-induced cognitive dysfunction and neurotoxicity: Reduction in oxidative damage and inactivation of ERK/p38 kinase and NF-κB pathways. Free Radic. Biol. Med. 2009, 47, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Hwang, C.J.; Lee, H.P.; Kim, C.S.; Son, D.J.; Ham, Y.W.; Hellstrom, M.; Han, S.B.; Kim, H.S.; Park, E.K.; et al. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-κB. Neuropharmacology 2017, 117, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Thomas, C.J.; Radcliffe, J.; Itsiopoulos, C. Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. BioMed Res. Int. 2015, 2015, 172801. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of omega-3 fatty acids in the treatment of depressive disorders: A comprehensive meta-analysis of randomized clinical trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Tsao, Y.Y.; Leung, Y.M.; Su, K.P. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: Implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010, 35, 2238–2248. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Guenard, F.; Barbier, O.; Vohl, M.C. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis. 2016, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Mullen, A.; Loscher, C.E.; Roche, H.M. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J. Nutr. Biochem. 2010, 21, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Hwang, C.J.; Yun, H.-M.; Park, K.-R.; Song, J.K.; Seo, H.O.; Hyun, B.K.; Choi, D.Y.; Yoo, H.-S.; Oh, K.-W.; Hwang, D.Y. Memory Impairment in Estrogen Receptor α Knockout Mice Through Accumulation of Amyloid-β Peptides. Mol. Neurobiol. 2014, 52, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Hartlage-Rubsamen, M.; Zeitschel, U.; Apelt, J.; Gartner, U.; Franke, H.; Stahl, T.; Gunther, A.; Schliebs, R.; Penkowa, M.; Bigl, V.; et al. Astrocytic expression of the Alzheimer’s disease beta-secretase (BACE1) is stimulus-dependent. Glia 2003, 41, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.M.; Park, M.H.; Hwang, C.J.; Song, H.S.; Lee, U.S.; Han, S.B.; Oh, K.W.; Ham, Y.W.; Song, M.J.; Son, D.J.; et al. Bee venom ameliorates lipopolysaccharide-induced memory loss by preventing NF-κB pathway. J. Neuroinflamm. 2015, 12, 124. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.Y.; Jang, J.S.; Son, D.J.; Im, H.-S.; Kim, J.Y.; Park, J.E.; Choi, W.R.; Han, S.-B.; Hong, J.T. Antarctic Krill Oil Diet Protects against Lipopolysaccharide-Induced Oxidative Stress, Neuroinflammation and Cognitive Impairment. Int. J. Mol. Sci. 2017, 18, 2554. https://doi.org/10.3390/ijms18122554
Choi JY, Jang JS, Son DJ, Im H-S, Kim JY, Park JE, Choi WR, Han S-B, Hong JT. Antarctic Krill Oil Diet Protects against Lipopolysaccharide-Induced Oxidative Stress, Neuroinflammation and Cognitive Impairment. International Journal of Molecular Sciences. 2017; 18(12):2554. https://doi.org/10.3390/ijms18122554
Chicago/Turabian StyleChoi, Ji Yeon, Jun Sung Jang, Dong Ju Son, Hyung-Sik Im, Ji Yeong Kim, Joung Eun Park, Won Rak Choi, Sang-Bae Han, and Jin Tae Hong. 2017. "Antarctic Krill Oil Diet Protects against Lipopolysaccharide-Induced Oxidative Stress, Neuroinflammation and Cognitive Impairment" International Journal of Molecular Sciences 18, no. 12: 2554. https://doi.org/10.3390/ijms18122554




