Paraneoplastic Pemphigus: Insight into the Autoimmune Pathogenesis, Clinical Features and Therapy
Abstract
:1. Introduction
2. Epidemiology
3. Etiology
4. Genetic
5. Pathogenesis
6. Clinical Features
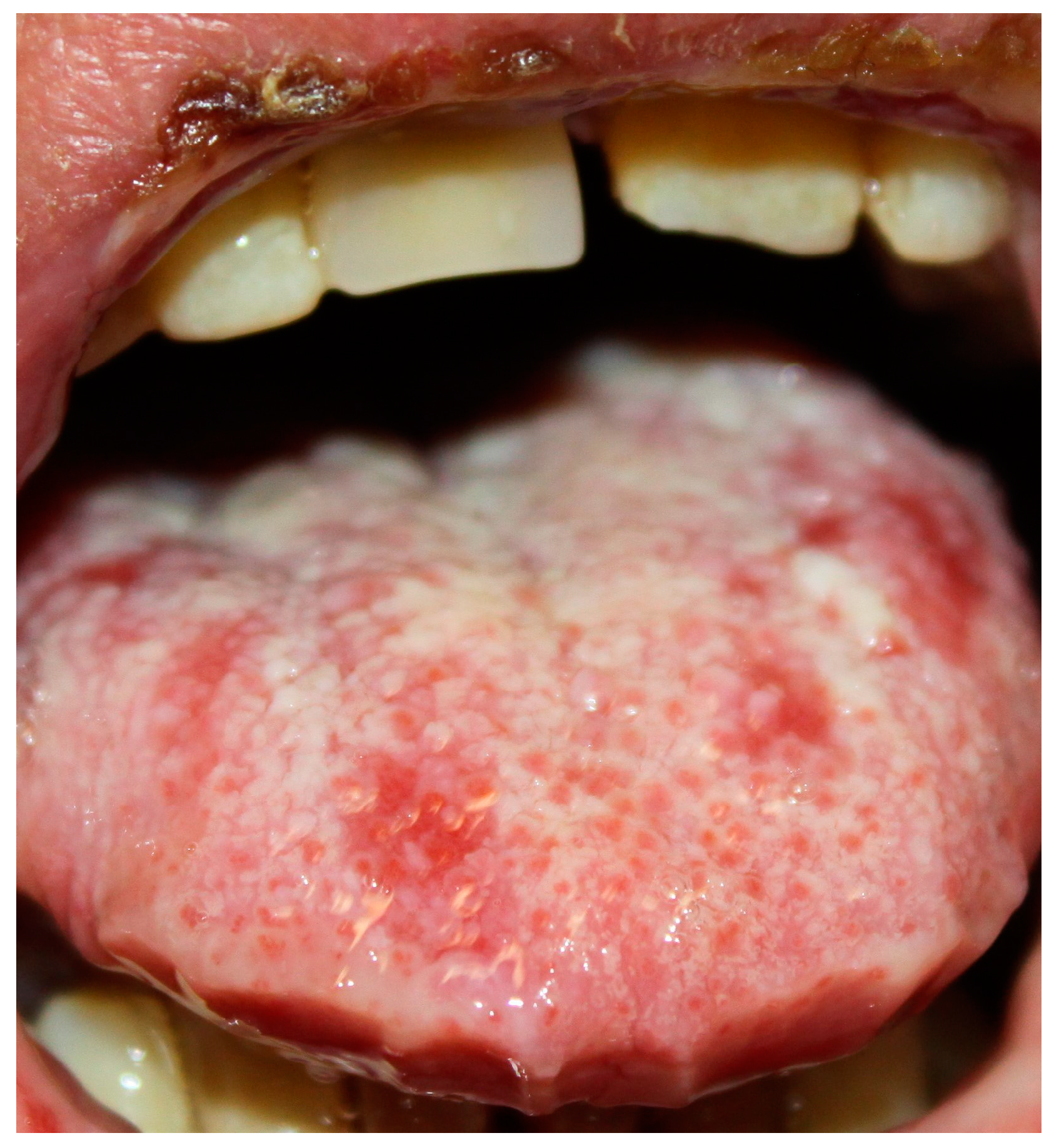
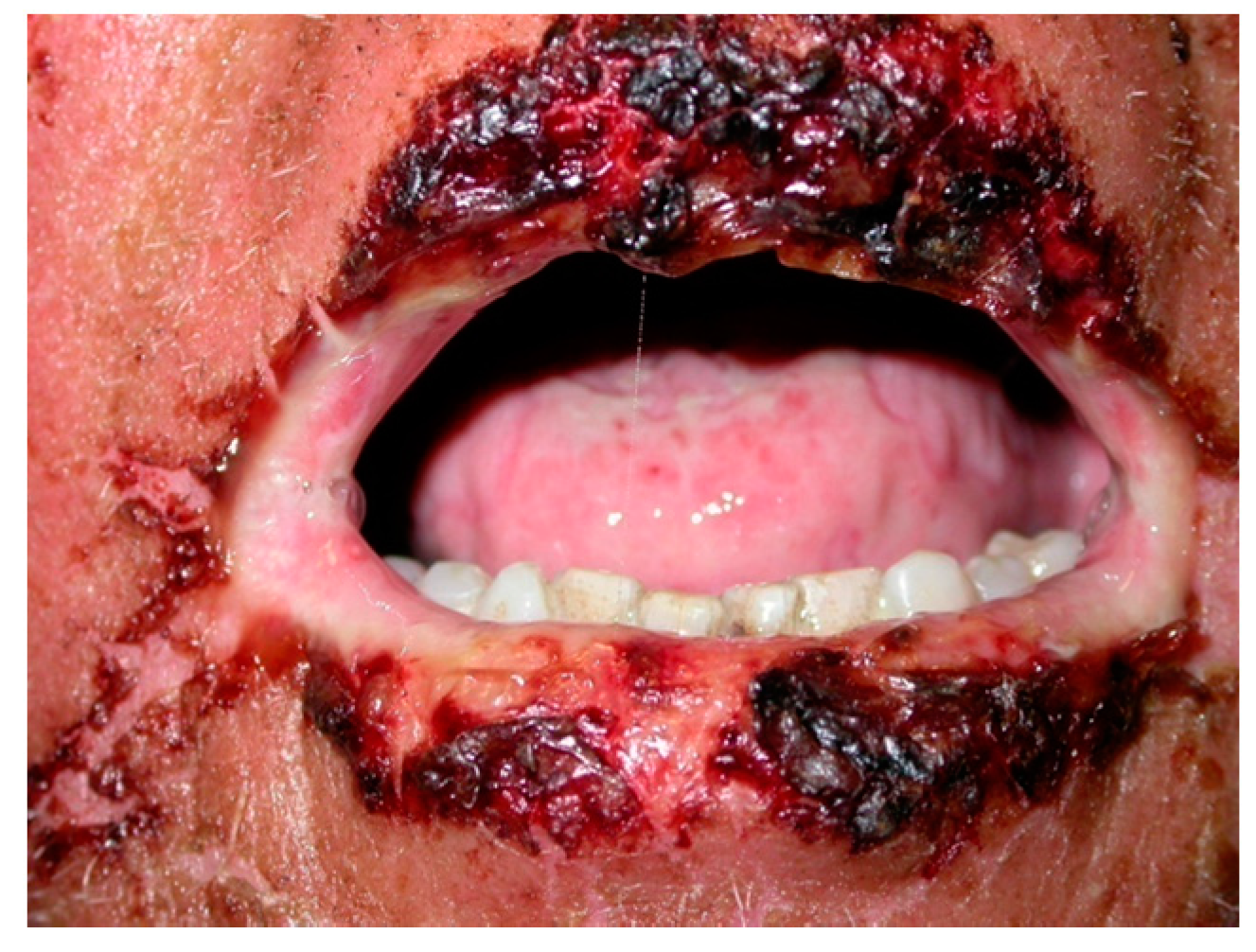
6.1. Oral Lesions
6.2. Secondary Mucosal Lesions
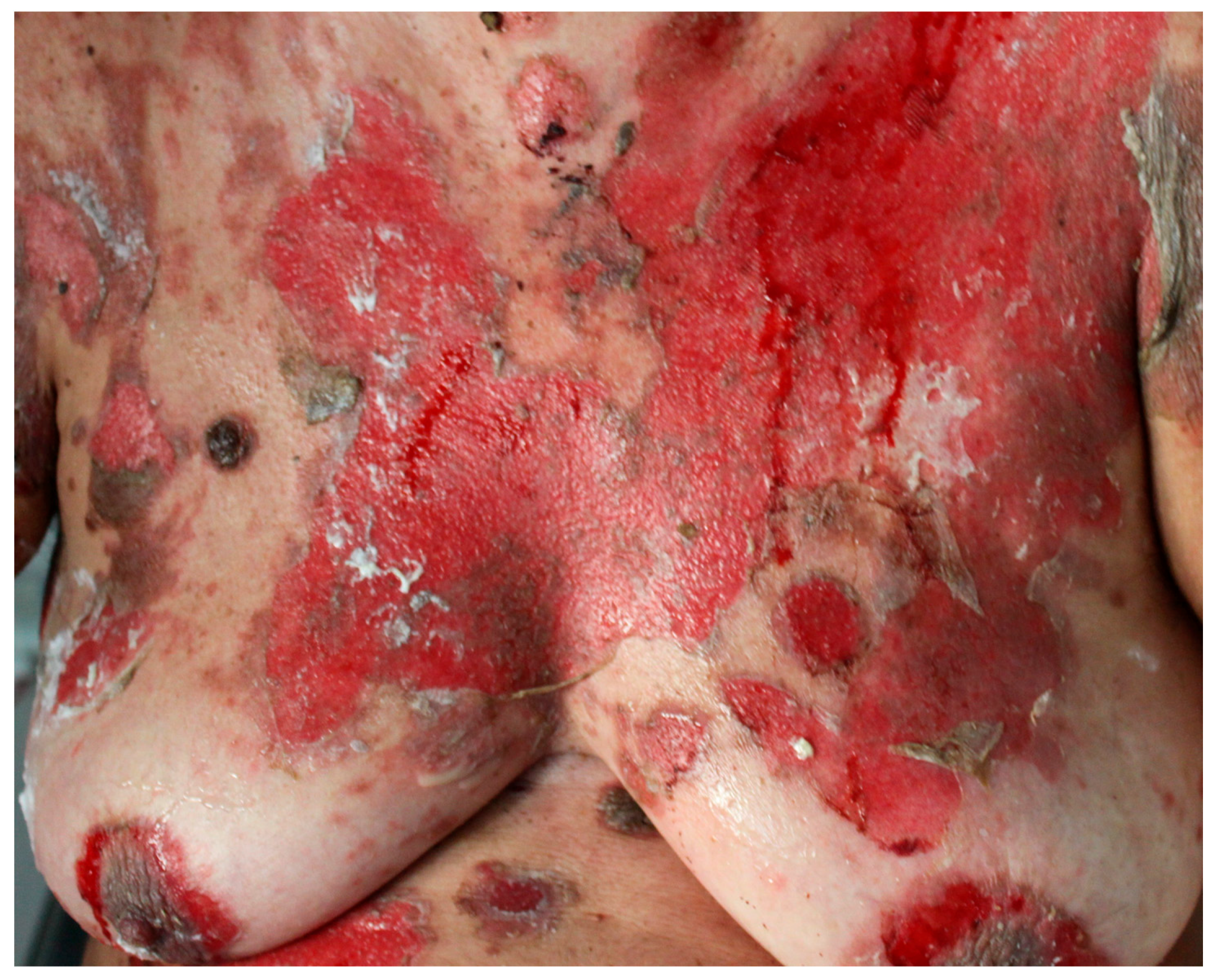
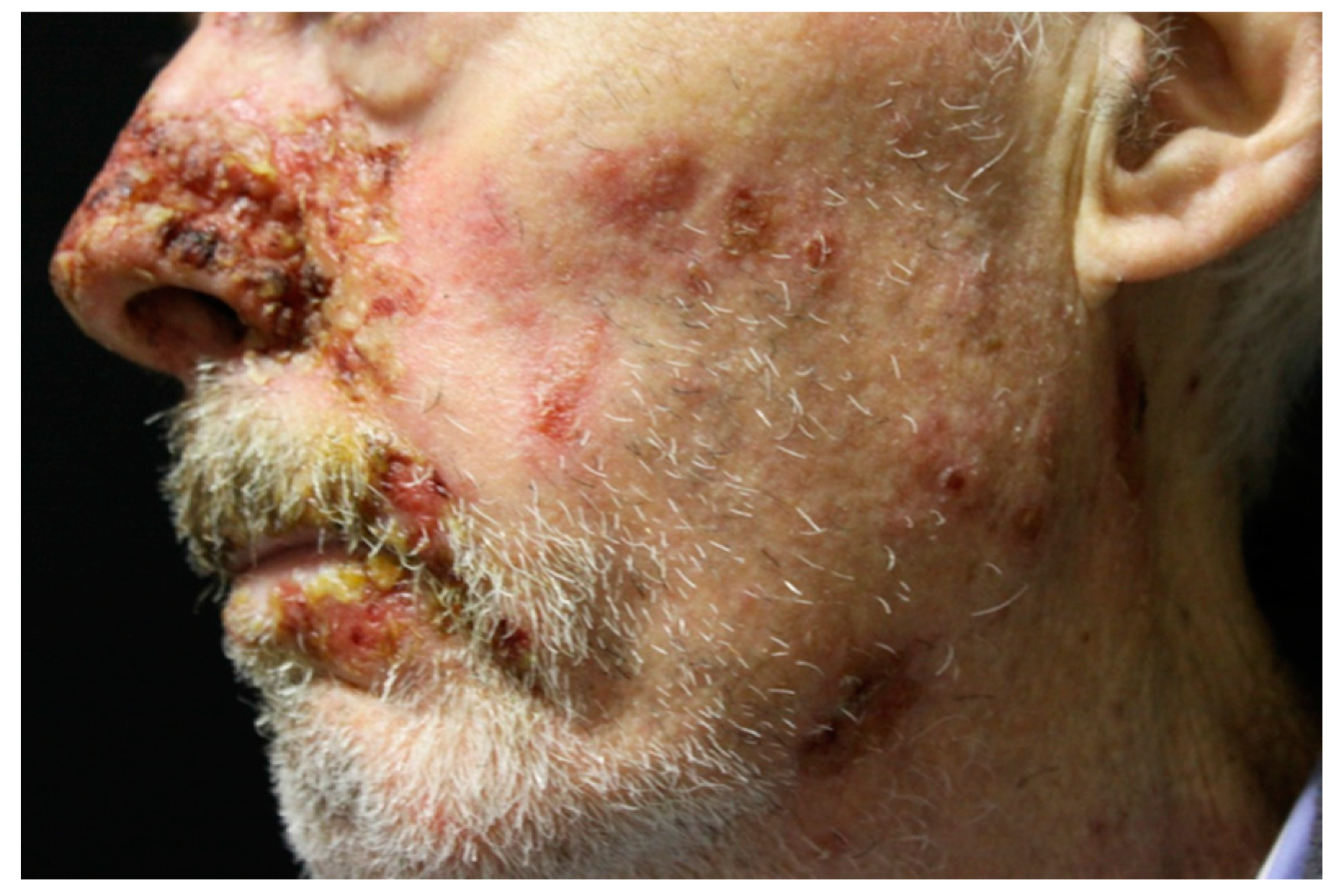
6.3. Skin Lesions
6.4. Pulmonary Manifestations
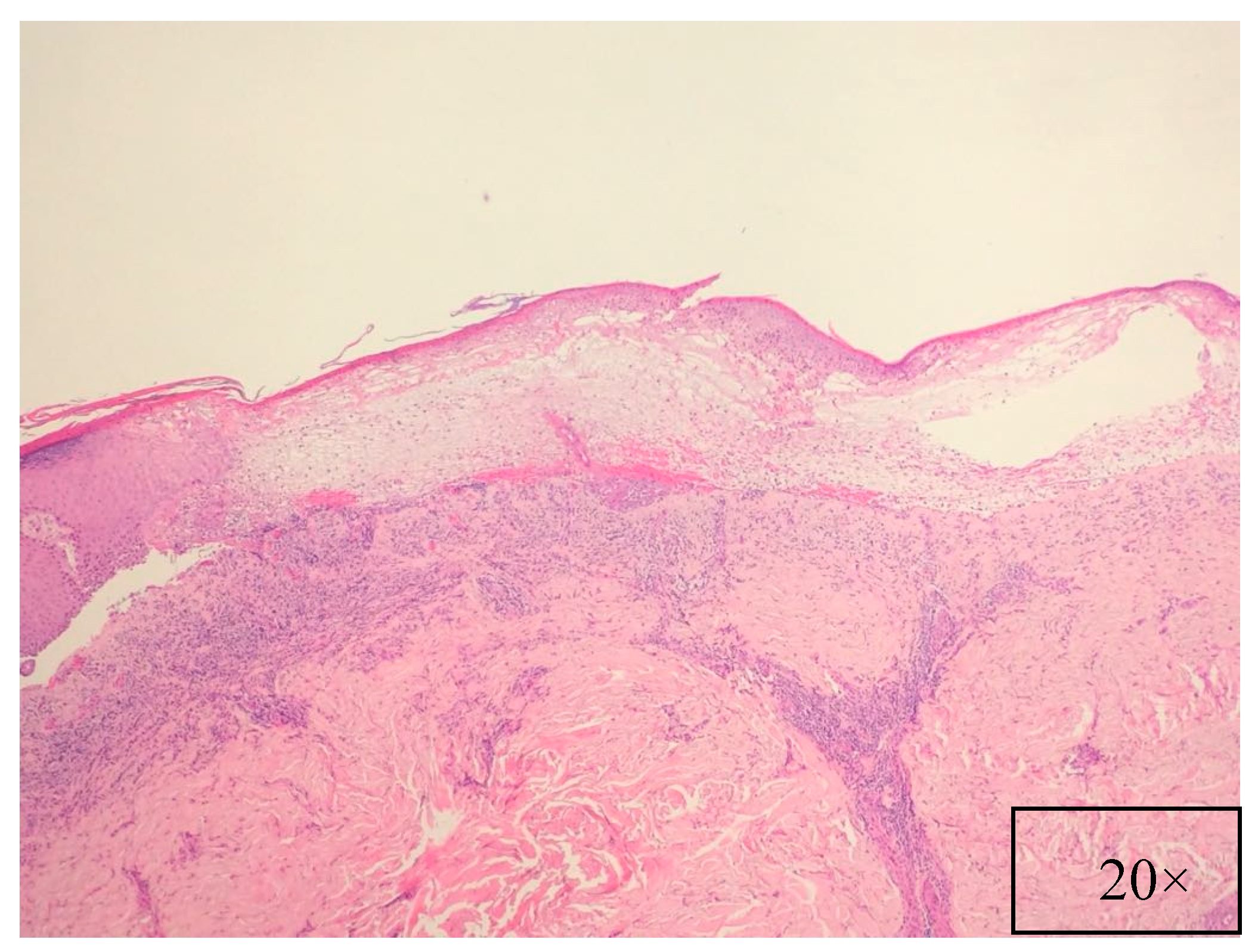
7. Pathology
8. Immunological Studies
9. Diagnosis
10. Differential Diagnosis
11. Treatment Options
12. Prognosis
13. Conclusions
Author Contributions
Conflicts of Interest
References
- Anhalt, G.J.; Kim, S.C.; Stanley, J.R.; Korman, N.J.; Jabs, D.A.; Kory, M.; Izumi, H.; Ratrie, H.; Mutasim, D., 3rd; Ariss-Abdo, L. Paraneoplastic pemphigus. An autoimmune mucocu-taneous disease associated with neoplasia. N. Engl. J. Med. 1990, 323, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Schifter, M.; Fulcher, D.A.; Lin, M.W. Paraneoplastic pemphigus: Two cases of intra-abdominal malignancy presenting solely as treatment refractory oral ulceration. J. Dermatol. 2015, 42, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, V.N.; Srivastava, G. Paraneoplasticpemphigus/paraneoplastic autoimmune multiorgansyn-drome. Int. J. Dermatol. 2009, 48, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.; Sakka, N.; Artsi, O.; Trau, H.; Barzilai, A. Diagnosis and classification of autoimmune blistering diseases. Autoimmun. Rev. 2014, 13, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Ndoye, A.; Bassler, K.D.; Shultz, L.D.; Shields, M.C.; Ruben, B.S.; Webber, R.J.; Pittelkow, M.R.; Lynch, P.J.; Grando, S.A. Classification, clinical manifestations, and im-munopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multi-organ syndrome: A reappraisal of paraneoplastic pemphigus. Arch. Dermatol. 2001, 137, 193–206. [Google Scholar] [PubMed]
- Sinha, A.A. Paraneoplastic pemphigus: Autoimmune-cancer nexus in the skin. Anticancer Agents Med. Chem. 2015, 15, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Anhalt, G.J. Paraneoplastic pemphigus. J. Investig. Dermatol. Symp. Proc. 2004, 9, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, S.; Drenovska, K.; Manuelyan, K. Autoimmune blistering dermatoses as systemic diseases. Clin. Dermatol. 2014, 32, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Czernik, A.; Camilleri, M.; Pittelkow, M.R.; Grando, S.A. Paraneoplastic autoimmune multiorgan syndrome: 20 years after. Int. J. Dermatol. 2011, 50, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Sticherling, M.; Erfurt-Berge, C. Autoimmune blistering diseases of the skin. Autoimmun. Rev. 2012, 11, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Cervini, A.B.; Tosi, V.; Kim, S.H.; Bocian, M.; Chantada, G.; Nousari, C.; Carballo, O.G.; Pierini, A.M. Paraneoplastic pemphigus or paraneoplastic autoimmune multiorgan syndrome. Report of 2 cases in children and a review of the literature. Actas Dermosifiliogr. 2010, 101, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, D.; Anhalt, G.J.; Lazarova, Z.; Aho, S.; Kazerounian, S.; Kouba, D.J.; Mascaro, J.M., Jr.; Nousari, H.C. Paraneoplastic pemphigus in children and adolescents. Br. J. Dermatol. 2002, 147, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.E.; Woody, C.; Davis, L.S.; Guill, M.F.; Jerath, R.S. Paraneoplastic autoimmune multiorgan syndrome (para-neoplastic pemphigus) in a child: Case report and review of the literature. Pediatrics 2004, 114, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Geller, S.; Gat, A.; Harel, A.; Mashiah, J.; Zeeli, T.; Eming, R.; Ishii, N.; Hertl, M.; Hashimoto, T.; Sprecher, E. Childhood pemphigus foliaceus with exclusive immunoglobulin G autoantibodies to desmocollins. Pediatr. Dermatol. 2016, 33, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Ohzono, A.; Sogame, R.; Li, X.; Teye, K.; Teye, K.; Tsuchisaka, A.; Numata, S.; Koga, H.; Kawakami, T.; Tsuruta, D.; et al. Clinical and immunological findings in 104 cases of paraneoplastic pemphigus. Br. J. Dermatol. 2015, 173, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Yong, A.A.; Tey, H.L. Paraneoplastic pemphigus. Australas. J. Dermatol. 2013, 54, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Bowen, G.M.; Peters, N.T.; Fivenson, D.P.; Su, L.D.; Nousari, H.C.; Anhalt, G.J.; Cooper, K.D.; Stevens, S.R. Lichenoid dermatitis in paraneoplastic pemphigus: A pathogenic trigger of epitope spreading? Arch. Dermatol. 2000, 136, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Matz, H.; Milner, Y.; Frusic-Zlotkin, M.; Brenner, S. Paraneoplastic pemphigus associated with pancreatic carcinoma. Acta Derm-Venereol. 1997, 77, 289–291. [Google Scholar] [PubMed]
- Wong, K.C.; Ho, K.K. Pemphigus with pemphigoid-like presentation, associated with squa-mous cell carcinoma of the tongue. Australas. J. Dermatol. 2000, 41, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.J.; Kim, S.C.; Kim, H.S.; Bang, D.; Yang, W.I.; Jung, W.H.; Chi, H.S. Paraneoplastic pemphigus associated with follicular dendritic cell sarcoma arising from Castleman’s tumor. J. Am. Acad. Dermatol. 1999, 40, 294–297. [Google Scholar] [CrossRef]
- Van der Wall, R.I.; Pas, H.H.; Anhalt, G.J.; Schulten, E.A.; Jonkman, M.F.; Nieboer, C. PNP as the presenting symptom of lymphoma of the tongue. Oral Oncol. 1998, 34, 567–570. [Google Scholar] [CrossRef]
- Su, Z.; Liu, G.; Liu, J.; Fang, T.; Zeng, Y.; Zhang, H.; Yang, S.; Wang, Y.; Zhang, J.; Wei, J.; et al. Paraneoplastic pemphigus associated with follicular dendritic cell sarcoma: Report of a case and review of literature. Int. J. Clin. Exp. Pathol. 2015, 8, 11983–11994. [Google Scholar] [PubMed]
- Basir, N.; Telisinghe, P.U.; Chong, V.H. Gastric cancer and paraneoplastic pemphigus. Indian J. Surg. 2015, 77, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.R.; Avram, M.M.; Duncan, L.M. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 23-2003. A 79-year-old woman with gastric lymphoma and erosive mucosal and cutaneous lesions. N. Engl. J. Med. 2003, 349, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Schaeppi, H.; Bauer, J.W.; Hametner, R.; Metze, D.; Ortiz-Urda, S.; Salmhofer, W.; Rappersberger, K.; Hintner, H. Localized variant of paraneoplastic pemphigus: Acantho-lysis associated with malignant melanoma. Br. J. Dermatol. 2001, 144, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Anhalt, G.J. Paraneoplastic pemphigus: The role of tumours and drugs. Br. J. Dermatol. 2001, 144, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Bachelez, H.; Dehen, L.; Delmer, A.; Zittoun, R.; Dubertret, L. Lethal paraneoplastic pemphigus following treatment of chronic lymphocytic leukaemia with fludarabine. Ann. Oncol. 1995, 6, 730–731. [Google Scholar] [CrossRef] [PubMed]
- Higo, T.; Miyagaki, T.; Nakamura, F.; Shinohara, A.; Asano, H.; Abe, H.; Senda, N.; Yoshizaki, A.; Fukayama, M.; Kurokawa, M. Paraneoplastic pemphigus occurring after bendamustine and rituximab therapy for relapsed follicular lymphoma. Ann. Hematol. 2015, 94, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kossard, S.; Ho, K.K.; Barnetson, R.S.; Ravich, R.B. Paraneoplastic pemphigus triggered by radiotherapy. Australas. J. Dermatol. 1995, 36, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Martel, P.; Loiseau, P.; Joly, P.; Busson, M.; Lepage, V.; Mouquet, H.; Courville, P.; Flageul, B.; Charron, D.; Musette, P.; et al. Paraneoplastic pemphigus is associated with the DRB1*03 allele. J. Autoimmun. 2003, 20, 91–95. [Google Scholar] [CrossRef]
- Liu, Q.; Bu, D.F.; Li, D.; Zhu, X.J. Genotyping of HLA-I and HLA-II alleles in Chinese patients with para-neoplastic pemphigus. Br. J. Dermatol. 2008, 158, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, C.; Ruhrberg, C.; Nie, Z.; Karashima, T.; Mori, O.; Nishikawa, T.; Green, K.J.; Anhalt, G.J.; Di Colandrea, T.; Watt, F.M.; et al. Envoplakin and periplakin are components of the paraneoplastic pemphigus antigen complex. J. Investig. Dermatol. 1998, 111, 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kwon, Y.D.; Lee, I.J.; Chang, S.N.; Lee, T.G. cDNA cloning of the 210-kDa paraneoplastic pemphigus antigen reveals that envoplakin is a component of the antigen complex. J. Investig. Dermatol. 1997, 109, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Oursler, J.R.; Labib, R.S.; Ariss-Abdo, L.; Burke, T.; O’Keefe, E.J.; Anhalt, G.J. Human autoantibodies against desmoplakins in paraneoplastic pemphigus. J. Clin. Investig. 1992, 89, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Borradori, L.; Trueb, R.M.; Jaunin, F.; Limat, A.; Favre, B.; Saurat, J.H. Autoantibodies from a patient with paraneoplastic pemphi-gus bind periplakin, a novel member of the plakin family. J. Investig. Dermatol. 1998, 111, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.; Bracke, S.; van Roy, F.; Pas, H.H.; Bonné, S.; De Schepper, S. Serum plakophilin-3 autoreactivity in paraneoplastic pemphigus. Br. J. Dermatol. 2010, 163, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Brandt, O.; Rafei, D.; Podstawa, E.; Niedermeier, A.; Jonkman, M.F.; Terra, J.B.; Hein, R.; Hertl, M.; Pas, H.H.; Müller, R. Differential IgG recognition of desmoglein 3 by parane-oplastic pemphigus and pemphigus vulgaris sera. J. Investig. Dermatol. 2012, 132, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Amagai, M.; Nishikawa, T.; Nousari, H.C.; Anhalt, G.J.; Hashimoto, T. Antibodies against desmoglein 3 (pemphigus vulgaris antigen) are present in sera from patients with paraneoplastic pemphigus and cause acantholysis in vivo in neonatal mice. Clin. Investig. 1998, 102, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Bahmer, F.; Rose, C.; Zillikens, D.; Schmidt, E. Clinical and immunopathological spectrum of para-neoplastic pemphigus. J. Dtsch. Dermatol. Ges. 2010, 8, 598–606. [Google Scholar] [PubMed]
- Numata, S.; Teye, K.; Tsuruta, D.; Sogame, R.; Ishii, N.; Koga, H.; Natsuaki, Y.; Tsuchisaka, A.; Hamada, T.; Karashima, T.; et al. Anti-alpha-2-macroglobulinlike-1 autoantibodies are detected frequently and may be pathogenic in paraneoplastic pemphigus. J. Investig. Dermatol. 2013, 133, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Schepens, I.; Jaunin, F.; Begre, N.; Läderach, U.; Marcus, K.; Hashimoto, T.; Favre, B.; Borradori, L. The protease inhibitor alpha-2-macroglobulin-like-1 is the p170 antigen recognized by paraneoplastic pemphigus autoantibodies in human. PLoS ONE 2010, 18, e12250. [Google Scholar]
- Tsuchisaka, A. Epiplakin Is a Paraneoplastic Pemphigus Autoantigen and Related to Bronchiolitis Obliterans in Japanese Patients. J. Investig. Dermatol. 2016, 136, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Taintor, A.R.; Leiferman, K.M.; Hashimoto, T.; Ishii, N.; Zone, J.J.; Hull, C.M. A novel case of IgA paraneoplastic pemphigus asso-ciated with chronic lymphocytic leukemia. J. Am. Acad. Dermatol. 2007, 56, S73–S76. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, M.; Nakano, T.; Taniguchi, T.; Katsuoka, K.; Tadera, N.; Miyazaki, K.; Teye, K.; Koga, H.; Hashimoto, T. IgA paraneoplastic pemphigus in angioimmunoblastic T-cell lymphoma with antibodies to desmocollin 1, type vii collagen and laminin 332. Acta Derm. Venereol. 2013, 93, 235–236. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, S.H.; Woodley, D.T.; Smoller, B.R.; Anhalt, G.J. Paraneoplastic pemphigus with autoantibody deposition in bronchial epithelium after autologous bone marrow transplantation. JAMA 1992, 267, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Preisz, K.; Horvath, A.; Sardy, M.; Somlai, B.; Hársing, J.; Amagai, M.; Hashimoto, T.; Nagata, Y.; Fekete, S.; Kárpáti, S. Exacerbation of paraneoplastic pemphigus by cyclophosphamide treatment: Detection of novel autoantigens and bronchial autoantibodies. Br. J. Dermatol. 2004, 150, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Mentink, L.F.; de Jong, M.C.; Kloosterhuis, G.J.; Zuiderveen, J.; Jonkman, M.F.; Pas, H.H. Coexistence of IgA antibodies to desmogleins 1 and 3 in pemphigus vulgaris, pemphigus foliaceus and paraneoplastic pemphigus. Br. J. Dermatol. 2007, 156, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Billet, S.E.; Grando, S.A.; Pittelkow, M.R. Paraneoplastic autoimmune multiorgan syndrome: Re-view of the literature and support for a cytotoxic role in pathogenesis. Autoimmunity 2006, 39, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Brinck, U.; Letschert, M.; Blaschke, V.; Dames, K.; Braess, J.; Wörmann, B.; Rünger, T.M.; Neumann, C. Graft-versus-host disease like immunophenotype and apoptotic keratinocyte death in paraneoplastic pemphigus. Br. J. Dermatol. 1999, 141, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Cummins, D.L.; Mimouni, D.; Tzu, J.; Owens, N.; Anhalt, G.J.; Meyerle, J.H. Lichenoid paraneoplastic pemphigus in the absence of detectable antibodies. J. Am. Acad. Dermatol. 2007, 56, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Wade, M.S.; Black, M.M. Paraneoplastic pemphigus: A brief update. Australas. J. Dermatol. 2005, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, B. Paraneoplastic pemphigus. J. Dermatol. 2007, 34, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Bialy-Golan, A.; Brenner, S.; Anhalt, G.J. Paraneoplastic pemphigus: Oral involvement as the sole manifestation. Acta Derm. Venereol. 1996, 76, 253–254. [Google Scholar] [PubMed]
- Lee, S.E.; Kim, S.C. Paraneoplastic pemphigus. Dermatol. Sin. 2010, 28, 1–14. [Google Scholar] [CrossRef]
- Healy, W.J.; Peters, S.; Nana-Sinkam, S.P. A middle-aged man presenting with unexplained mucosal erosions and progressive dyspnoea. BMJ Case Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, V.K.; Sharma, V.; Chauhan, P.S.; Mehta, K.S.; Sharma, A.L.; Abhinav, C.; Khatri, G.; Prabha, N.; Sharma, S.; Negi, M. Paraneoplastic pemphigus: A paraneoplastic autoimmune multiorgan syndrome or autoimmune multiorganopathy? Case Rep. Dermatol. Med. 2012, 2012, 207126. [Google Scholar] [CrossRef] [PubMed]
- Mar, W.A.; Glaesser, R.; Struble, K.; Stephens-Groff, S.; Bangert, J.; Hansen, R.C. Paraneoplastic pemphigus with bronchiolitis obliterans in a child. Pediatr. Dermatol. 2003, 20, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Chorzelski, T.; Hashimoto, T.; Maciejewska, B.; Amagai, M.; Anhalt, G.J.; Jablonska, S. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J. Am. Acad. Dermatol. 1999, 41, 393–400. [Google Scholar] [CrossRef]
- Meyers, S.J.; Varley, G.A.; Meisler, D.M.; Camisa, C.; Wander, A.H. Conjunctival involvement in paraneoplastic pemphigus. Am. J. Ophthalmol. 1992, 114, 621–624. [Google Scholar] [CrossRef]
- Ng, P.P.; Rencic, A.; Nousari, H.C. Paraneoplastic pemphigus: A refractory autoimmune mucocutaneous disease. J. Cutan. Med. Surg. 2002, 6, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Yokokura, H.; Demitsu, T.; Kakurai, M.; Umemoto, N.; Azuma, R.; Yamada, T.; Suzuki, M.; Jimbu, Y.; Yoneda, K.; Ishii, N.; et al. Paraneoplastic pemphigus mimicking erosive mucosal lichen planus associated with primary hepatocellular carcinoma. J. Dermatol. 2006, 33, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Broussard, K.C.; Leung, T.G.; Moradi, A.; Thorne, J.E.; Fine, J.D. Autoimmune bullous diseases with skin and eye involvement: Cicatricial pemphigoid, pemphigus vulgaris, and pemphigus paraneoplastica. Clin. Dermatol. 2016, 34, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Mutasim, D.F.; Pelc, N.J.; Anhalt, G.J. Paraneoplastic pemphigus. Dermatol. Clin. 1993, 11, 473–481. [Google Scholar] [PubMed]
- Tankel, M.; Tannenbaum, S.; Parekh, S. Paraneoplastic pemphigus presenting as an unusual bullous eruption. J. Am. Acad. Dermatol. 1993, 29, 825–828. [Google Scholar] [CrossRef]
- Sapadin, A.N.; Anhalt, G.J. Paraneoplastic pemphigus with a pemphigus vegetans-like plaque as the only cutaneous manifestation. J. Am. Acad. Dermatol. 1998, 39, 867–871. [Google Scholar] [CrossRef]
- Marathe, K.; Lu, J.; Morel, K.D. Bullous diseases: Kids are not just little people. Clin. Dermatol. 2015, 33, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, F.; Pittelkow, M.R.; Ryu, J.H. Constrictive bronchiolitis associated with paraneoplastic autoimmune multi-organ syndrome. Respirology 2009, 14, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Maeyama, Y.; Karashima, T.; Nakama, T.; Kusuhara, M.; Yasumoto, S.; Hashimoto, T. Immunoserological analyses of 55 patients with pemphigus at the Dermatological Department of Kurume University Hospital: An 11-year retrospective study (1996–2006). Int. J. Dermatol. 2008, 47, 1321–1322. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Schlumberger, W.; Stöcker, W.; Recke, A.; Schmidt, E.; Hashimoto, T.; Zhu, X.J.; Zillikens, D.; Komorowski, L. Development of ELISA for the specific determination of autoantibodies against envoplakin and periplakin in paraneoplastic pemphigus. Clin. Chim. Acta 2009, 410, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Culican, S.; Silvestrini, R.A.; Vu, J.; Schifter, M.; Fulcher, D.A.; Lin, M.W. Comparative study of five serological assays for the diagnosis of paraneoplastic pemphigus. Pathology 2015, 47, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Joly, P.; Richard, C.; Gilbert, D.; Courville, P.; Chosidow, O.; Roujeau, J.C.; Beylot-Barry, M.; D’incan, M.; Martel, P.; Lauret, P.; et al. Sensitivity and specificity of clinical, histologic, and immuno-logic features in the diagnosis of paraneoplastic pemphigus. J. Am. Acad. Dermatol. 2000, 43, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Helou, J.; Allbritton, J.; Anhalt, G. Accuracy of indirect immunofluorescence in the diagnosis of para-neoplastic pemphigus. J. Am. Acad. Dermatol. 1995, 32, 441–447. [Google Scholar] [CrossRef]
- Cozzani, E.; Dal Bello, M.G.; Mastrogiacomo, A.; Drosera, M.; Parodi, A. Antidesmoplakin antibodies in pemphigus vulgaris. Br. J. Dermatol. 2006, 154, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Kazerounian, S.; Mahoney, M.G.; Uitto, J.; Aho, S. Envoplakin and periplakin, the paraneoplastic pemphigus antigens, are also recognized by pemphigus foliaceus autoantibodies. J. Investig. Dermatol. 2000, 115, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Amagai, M.; Watanabe, K.; Chorzelski, T.P.; Bhogal, B.S.; Black, M.M.; Stevens, H.P.; Boorsma, D.M.; Korman, N.J.; Gamou, S. Characterization of paraneoplastic pemphigus autoanti-gens by immunoblot analysis. J. Investig. Dermatol. 1995, 104, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Camisa, C.; Helm, T.N. Paraneoplastic pemphigus is a distinct neoplasia-induced autoimmune disease. Arch. Dermatol. 1993, 129, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Iannella, G.; Greco, A.; Granata, G.; Manno, A.; Pasquariello, B.; Angeletti, D.; Didona, D.; Magliulo, G. Granulomatosis with polyangiitis and facial palsy: Literature review and insight in the autoimmune pathogenesis. Autoimmun. Rev. 2016, 15, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Iannella, G.; Greco, A.; Didona, D.; Didona, B.; Granata, G.; Manno, A.; Pasquariello, B.; Magliulo, G. Vitiligo: Pathogenesis, clinical variants and treatment approaches. Autoimmun. Rev. 2016, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Hashimoto, T.; Kim, S.C. No mucosal involvement in a patient with paraneoplastic pemphigus associated with thymoma and myasthenia gravis. Br. J. Dermatol. 2008, 159, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Frew, J.W.; Murrell, D.F. Current management strategies in paraneoplastic pemphigus (paraneoplastic autoimmune multiorgan syndrome). Dermatol. Clin. 2011, 29, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Gergely, L.; Váróczy, L.; Vadász, G.; Remenyik, E.; Illés, A. Successful treatment of B cell chronic lymphocytic leukemia-associated severe paraneoplastic pemphigus with cyclosporin A. Acta Haematol. 2003, 109, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Martínez De Pablo, M.I.; Iranzo, P.; Llambrich, A.; Baradad, M.; Herrero, C. Paraneoplastic pemphigus associated with non-Hodgkin B-cell lymphoma and good response to prednisone. Acta Derm. Venereol. 2005, 85, 233–235. [Google Scholar] [PubMed]
- Vezzoli, P.; Berti, E.; Marzano, A.V. Rationale and efficacy for the use of rituximab in paraneoplastic pemphigus. Expert Rev. Clin. Immunol. 2008, 4, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.V.; Marks, J.G.; Billingsley, E.M. Use of mycophenolate mofetil in the treatment of paraneoplastic pemphigus. Br. J. Dermatol. 2000, 142, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, M.S.; Schifter, M.; Sullivan, J.; Stapleton, K. Paraneoplastic pemphigus in two patients with B-cell non-Hodgkin’s lymphoma: Significant responses to cyclophosphamide and prednisolone. Am. J. Hematol. 2000, 63, 105–106. [Google Scholar] [CrossRef]
- Tan-Lim, R.; Bystryn, J.C. Effect of plasmapheresis therapy on circulating levels of pemphigus antibodies. J. Am. Acad. Dermatol. 1990, 22, 35–40. [Google Scholar] [CrossRef]
- Izaki, S.; Yoshizawa, Y.; Kitamura, K.; Kato, H.; Hashimoto, H.; Korman, N.J.; Hamamatsu, Y.; Ohashi, N.; Ogasa, S. Paraneoplastic pemphigus: Potential therapeutic effect of plasmapheresis. Br. J. Dermatol. 1996, 134, 987–989. [Google Scholar] [CrossRef] [PubMed]
- Granata, G.; Greco, A.; Iannella, G.; Granata, M.; Manno, A.; Savastano, E.; Magliulo, G. Posterior reversible encephalopathy syndrome—Insight into pathogenesis, clinical variants and treatment approaches. Autoimmun. Rev. 2015, 14, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Hertl, M.; Zillikens, D.; Borradori, L.; Bruckner-Tuderman, L.; Burckhard, H.; Eming, R.; Engert, A.; Goebeler, M.; Hofmann, S.; Hunzelmann, N.; et al. Recommendations for the use of rituximab (anti-CD20 antibody) in the treatment of autoimmune bullous skin diseases. J. Dtsch. Dermatol. Ges. 2008, 6, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Burris, H.A.; Morrissey, L.H.; Litchy, S.; Scullin, D.C., Jr.; Bearden, J.D., 3rd; Richards, P.; Greco, F.A. Rituximab monoclonal antibody as initial systemic therapy for patients with lowgrade non-Hodgkins lymphoma. Blood 2000, 95, 3052–3056. [Google Scholar] [PubMed]
- Hohwy, T.; Bang, K.; Steiniche, T.; Peterslund, N.A.; d’Amore, F. Alemtuzumab-induced remission of both severe para-neoplastic pemphigus and leukaemic bone marrow infiltration in a case of treatment-resistant B-cell chronic lymphocytic leukaemia. Eur. J. Haematol. 2004, 73, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, X.; Li, R.; Tu, P.; Wang, R.; Zhang, L.; Li, T.; Chen, X.; Wang, A.; Yang, S.; et al. Paraneoplastic pemphigus associated with Castleman tumor: A commonly reported subtype of paraneoplastic pemphigus in China. Arch. Dermatol. 2005, 141, 1285–1293. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Criterion |
|---|---|
| Clinical features | Painful erosions involving mucosae with or without a multiform skin eruption producing blisters and erosions, occurring in association with an occult or evident neoplasm |
| Histopathology | Suprabasal intraepithelial acantholysis, vacuolar interface changes, necrosis of individual keratinocytes, and/or lichenoid inflammation |
| Direct immunofluorescence | Combined presence of IgG and complement (C3) granular-linear deposition within the epidermal intercellular spaces and along the basement-membrane zone |
| Indirect immunofluorescence | Presence of circulating antibodies that target the intercellular zone of stratified squamous or transitional epithelia |
| Immunoprecipitation | Typical complex of proteins, including desmoplakin I (250 kD), bullous pemphigoid antigen (230 kD), envoplakin (210 kD), desmoplakin II (210 kD), periplakin (190 kD) and α-2-macroglobulin-like-1 (170 kD) |
| CLINIC | Bullous Lesions on Skin and Mucous Membranes | |
|---|---|---|
| PATHOLOGY | Acantholysis (intraepidermal bulla) | Sub-epidermal cleavage |
| DIF | Combined presence of IgG and complement (C3) granular-linear deposition within the epidermal intercellular spaces and along the basement-membrane zone | |
| IIF | Presence of circulating antibodies that target the intercellular zone of stratified squamous or transitional epithelia | |
| LABORATORY | AAB directed to several proteins, including desmoplakin I (250 kD), bullous pemphigoid antigen (230 kD), envoplakin (210 kD), desmoplakin II (210 kD), periplakin (190 kD) and α-2-macroglobulin-like-1 (170 kD) IgG anti-DSG 1 and 3 IgG anti-DSG 1 IgA anti-DSC | |
| DIAGNOSIS | PNP PV PF IgAP | Exclusion of PNP |
| Differential Diagnosis |
|---|
| Pemphigus vulgaris Bullous pemphigoid Major aphthous stomatitis Oral lichen planus Lichen planus Drug eruption Erythema multiforme GVHD Stevens–Johnson syndrome Toxic epidermal necrolysis |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolino, G.; Didona, D.; Magliulo, G.; Iannella, G.; Didona, B.; Mercuri, S.R.; Moliterni, E.; Donati, M.; Ciofalo, A.; Granata, G.; et al. Paraneoplastic Pemphigus: Insight into the Autoimmune Pathogenesis, Clinical Features and Therapy. Int. J. Mol. Sci. 2017, 18, 2532. https://doi.org/10.3390/ijms18122532
Paolino G, Didona D, Magliulo G, Iannella G, Didona B, Mercuri SR, Moliterni E, Donati M, Ciofalo A, Granata G, et al. Paraneoplastic Pemphigus: Insight into the Autoimmune Pathogenesis, Clinical Features and Therapy. International Journal of Molecular Sciences. 2017; 18(12):2532. https://doi.org/10.3390/ijms18122532
Chicago/Turabian StylePaolino, Giovanni, Dario Didona, Giuseppe Magliulo, Giannicola Iannella, Biagio Didona, Santo Raffaele Mercuri, Elisa Moliterni, Michele Donati, Andrea Ciofalo, Guido Granata, and et al. 2017. "Paraneoplastic Pemphigus: Insight into the Autoimmune Pathogenesis, Clinical Features and Therapy" International Journal of Molecular Sciences 18, no. 12: 2532. https://doi.org/10.3390/ijms18122532





