Two Cycloartenol Synthases for Phytosterol Biosynthesis in Polygala tenuifolia Willd
Abstract
:1. Introduction
2. Results
2.1. De Novo Transcriptome Assembly and Functional Annotation
2.2. Simple Sequence Repeats (SSRs) in the P. tenuifolia Transcriptome
2.3. Identification of Differential Gene Expression
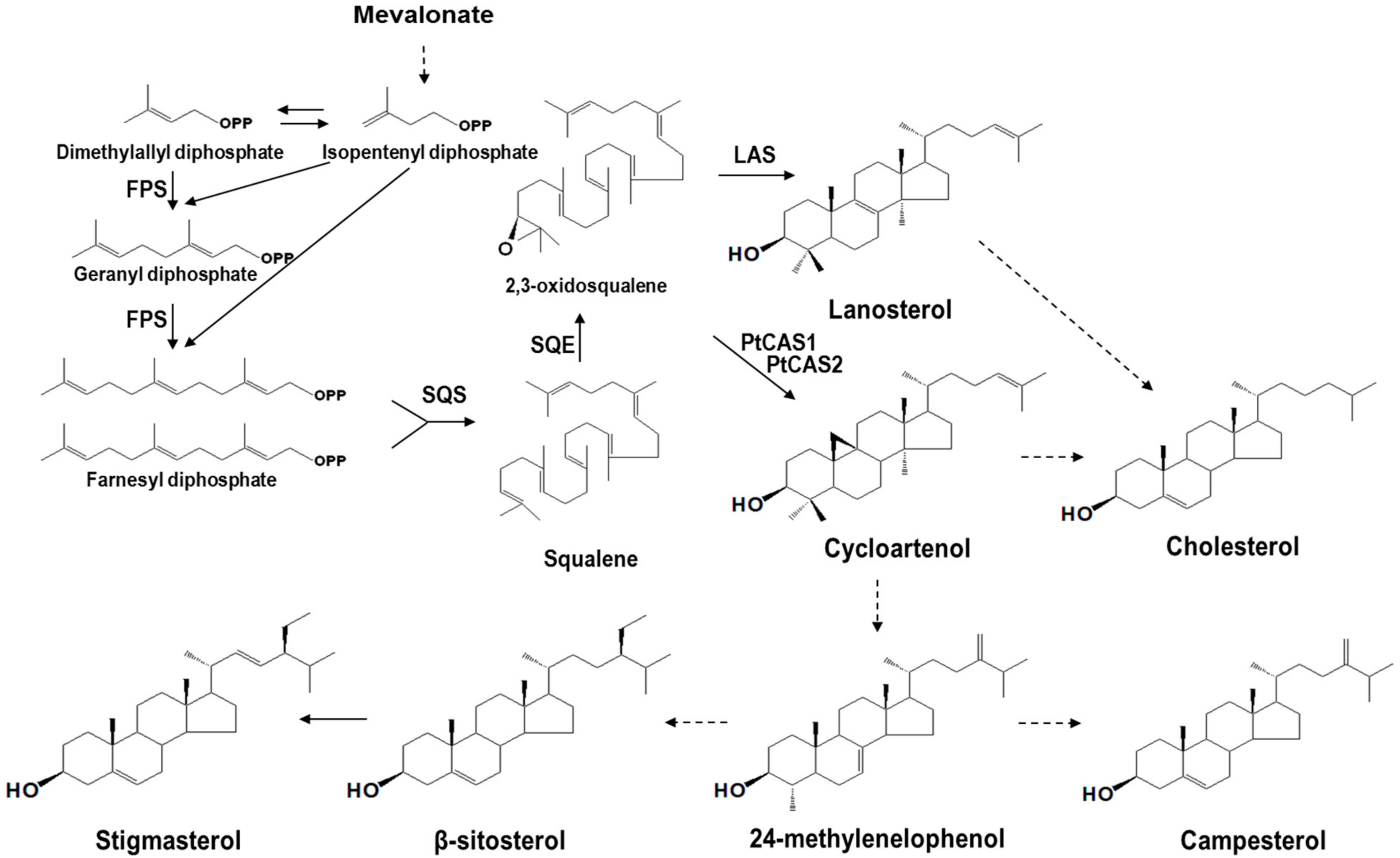
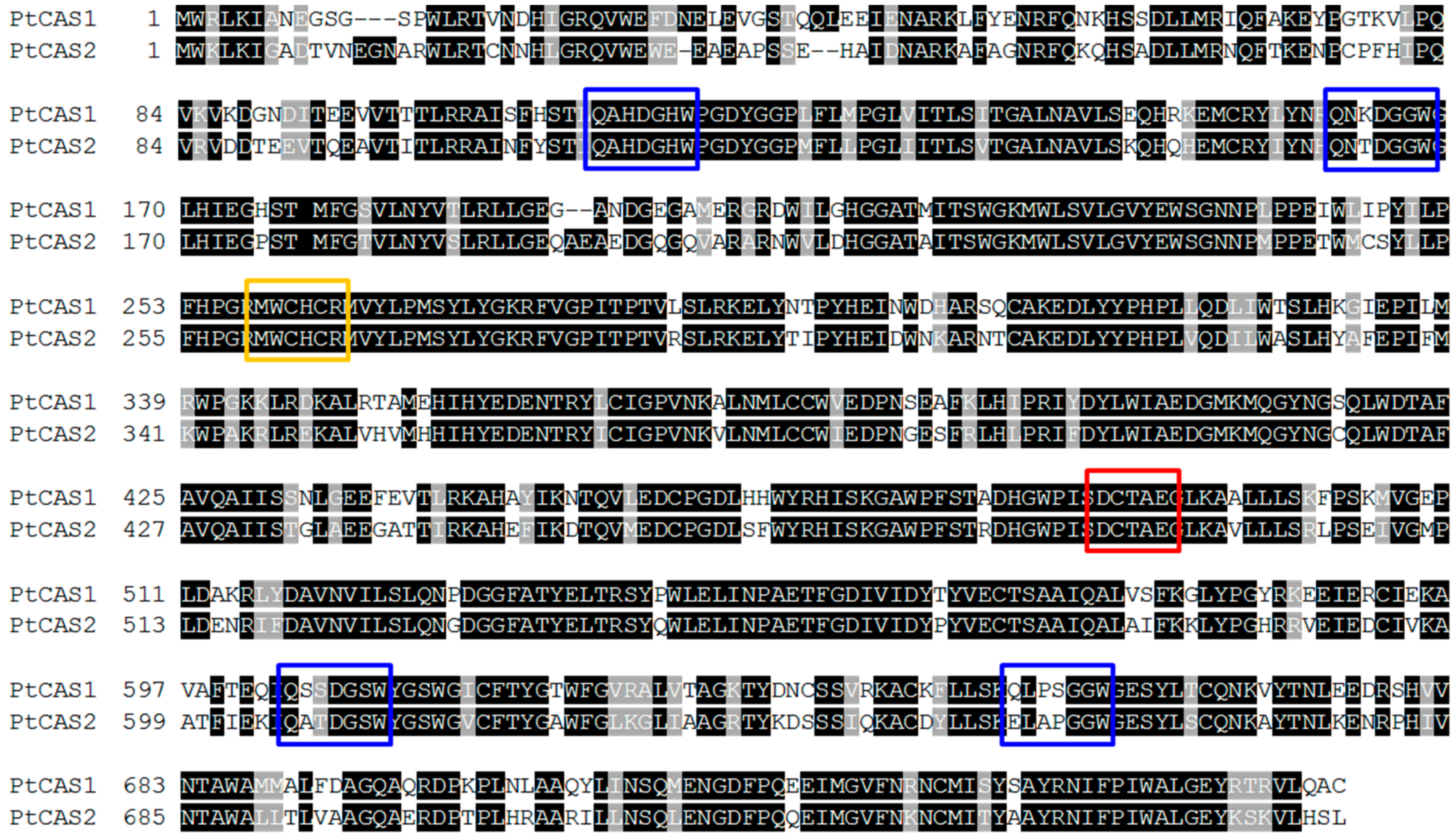
2.4. Functional Characterization of PtCAS1 and PtCAS2 Genes in Yeast Cells
2.5. Expressions of Two CAS Genes in Organs and in Seedlings Treated with MeJA
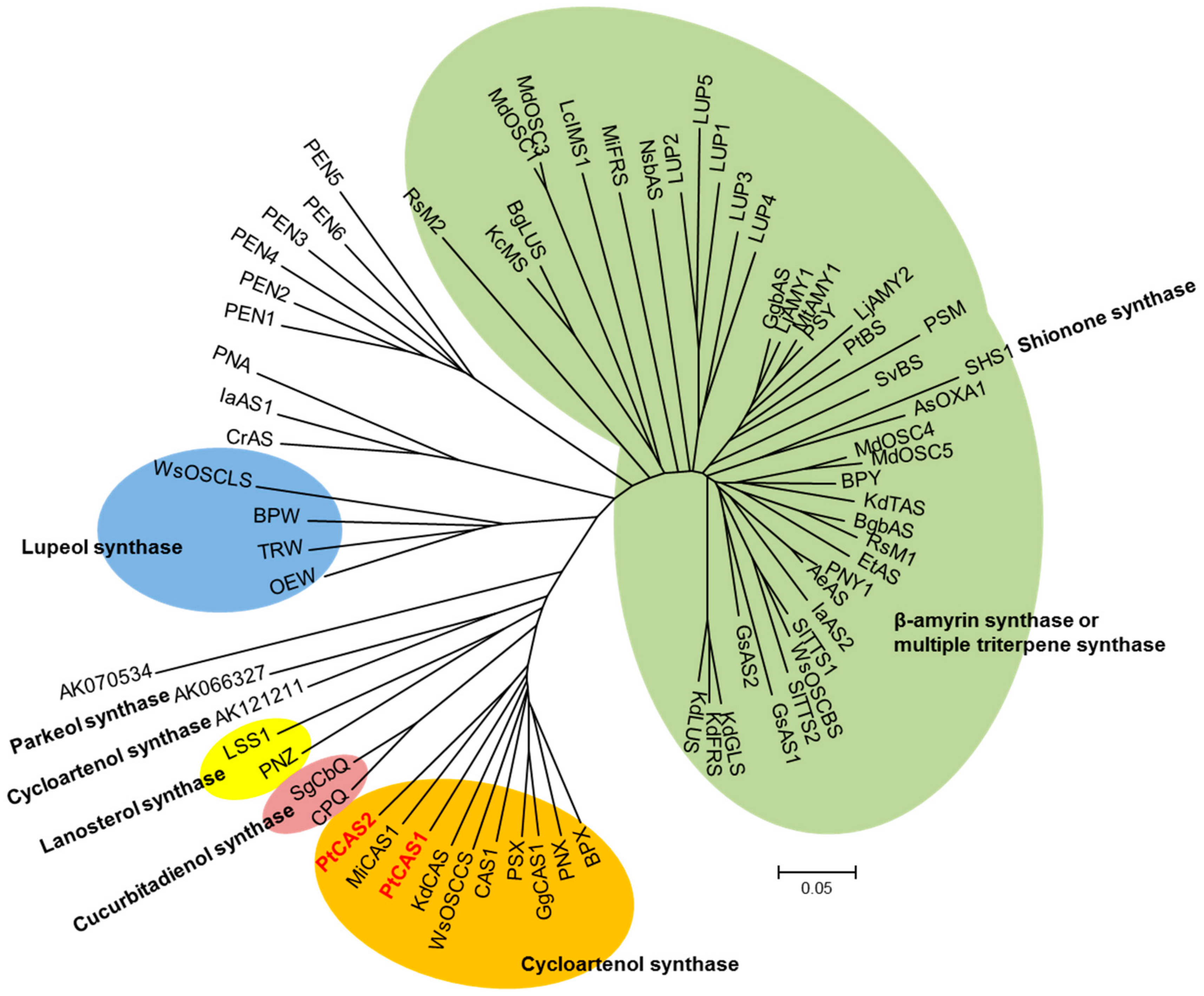
3. Discussion
4. Materials and Methods
4.1. Plant Materials and RNA Extraction
4.2. RNA Library Preparation and Sequencing
4.3. De Novo Assembly and Sequence Annotation
4.4. Phylogenetic Tree Analysis
4.5. Cloning of PtCAS1 and PtCAS2
4.6. Heterologous Expression of PtCAS1 and PtCAS2 Genes in Yeast Mutant
4.7. Identification of Products from Two CASs
4.8. Real-Time RT-PCR Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DEUG | Differential Expression of Unigenes |
| LFC | Log Fold Change |
| FPKM | Fragments Per Kilobase Million |
| OSC | Oxidosqualene Cyclase |
| MeJA | Methyl Jasmonate |
| CAS | Cycloartenol Synthase |
| bAS | β-Amyrin Synthase |
References
- Phillips, D.R.; Rasbery, J.M.; Bartel, B.; Matsuda, S.P.T. Biosynthetic diversity in plant triterpene cyclization. Curr. Opin. Plant Biol. 2006, 9, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Babiychuk, E.; Bouvier-Navé, P.; Compagnon, V.; Suzuki, M.; Muranaka, T.; van Montagu, M.; Kushnir, S.; Schaller, H. Allelic mutant series reveal distinct functions for Arabidopsis cycloartenol synthase 1 in cell viability and plastid biogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Husselstein-Muller, T.; Schaller, H.; Benveniste, P. Molecular cloning and expression in yeast of 2,3-oxidosqualene-triterpenoid cyclases from Arabidopsis thaliana. Plant Mol. Biol. 2001, 45, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shibuya, M.; Yokota, S.; Ebizuka, Y. Oxidosqualene cyclases from cell suspension cultures of Betula platyphylla var. japonica: Molecular evolution of oxidosqualene cyclases in higher plants. Biol. Pharm. Bull. 2003, 26, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, X.; He, J.; Xia, M.; Xu, L.; Yang, S. Structure analysis of triterpenesaponins in Polygala tenuifolia by electrospray ionization ion trap multiple-stage mass spectrometry. J. Mass Spectrometry 2007, 42, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.C., Jr.; de Andrade, S.F.; Cechinel Filho, V. A pharmacognostic approach to the Polygala genus: Phytochemical and pharmacological aspects. Chem. Biodivers. 2012, 9, 181–209. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Santino, A.; Taurino, M.; de Domenico, S.; Bonsegna, S.; Poltronieri, P.; Pastor, V.; Flors, V. Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep. 2013, 32, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Xu, X.; Zhang, F.; Wang, Y.; Guo, S.; Qin, X.; Du, G. Analysis of Polygala tenuifolia transcriptome and description of secondary metabolite biosynthetic pathways by Illumina sequencing. Int. J. Genom. 2015, 2015, 782635. [Google Scholar]
- Jensen, L.J.; Julien, P.; Kuhn, M.; von Mering, C.; Muller, J.; Doerks, T.; Bork, P. eggNOG: Automated construction and annotation of orthologous groups of genes. Nucleic Acids Res. 2008, 36, D250–D254. [Google Scholar] [CrossRef] [PubMed]
- Monden, Y.; Tahara, M. Genetic linkage analysis using DNA markers in sweetpotato. Breed. Sci. 2017, 67, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.A.; Rafii, M.Y.; Mahmud, T.M.; Hanafi, M.M.; Miah, G. Molecular markers: A potential resource for ginger genetic diversity studies. Mol. Biol. Rep. 2016, 43, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Rasbery, J.M.; Shan, H.; LeClair, R.J.; Norman, M.; Matsuda, SPT.; Bartel, B. Arabidopsis thaliana squalene epoxidase 1 is essential for root and seed development. J. Biol. Chem. 2007, 282, 17002–17013. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.L.; Lee, D.Y.; Um, Y.; Lee, J.H.; Park, C.G.; Jetter, R.; Kim, O.T. Isolation and characterization of an oxidosqualene cyclase gene encoding a β-amyrin synthase involved in Polygala tenuifolia Willd. saponin biosynthesis. Plant Cell Rep. 2014, 33, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Kushiro, T.; Shibuya, M.; Ebizuka, Y. β-amyrin synthase—Cloning of oxidosqualene cyclase that catalyzes the formation of the most popular triterpene among higher plants. Eur. J. Biochem. 1998, 256, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Huang, P.Y.; Inoue, K. Up-regulation of soyasaponin biosynthesis by methyl jasmonate in cultured cells of Glycyrrhiza glabra. Plant Cell Physiol. 2003, 44, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Achnine, L.; Xu, R.; Matsuda, SPT.; Dixon, R.A. A genomic approach to the early stages of triterpene saponin biosynthesis in Medicago truncatula. Plant J. 2002, 32, 1033–1048. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Bansal, S.; Mishra, B.; Sangwan, R.S.; Asha Jadaun, J.S.; Sangwan, N.S. RNAi and homologous over-expression based functional approaches reveal triterpenoid synthase gene-cycloartenol synthase is involved in downstream withanolide biosynthesis in Withania somnifera. PLoS ONE 2016, 11, e0149691. [Google Scholar] [CrossRef] [PubMed]
- Kribii, R.; Arró, M.; Del Arco, A.; González, V.; Balcells, L.; Delourme, D.; Ferrer, A.; Karst, F.; Boronat, A. Cloning and characterization of the Arabidopsis thaliana SQS1 gene encoding squalene synthase—Involvement of the C-terminal region of the enzyme in the channeling of squalene through the sterol pathway. Eur. J. Biochem. 1997, 249, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.D.; Han, J.Y.; Huh, G.H.; Choi, Y.E. Expression and functional characterization of three squalene synthase genes associated with saponin biosynthesis in Panax ginseng. Plant Cell Physiol. 2011, 52, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.T.; Kim, M.Y.; Huh, S.M.; Bai, D.G.; Ahn, J.C.; Hwang, B. Cloning of a cDNA probably encoding oxidosqualene cyclase associated with asiaticoside biosynthesis from Centella asiatica (L.) Urban. Plant Cell Rep. 2005, 24, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Cho, J.H.; Park, S.; Han, J.Y.; Back, K.; Choi, Y.E. Gene regulation patterns in triterpene biosynthetic pathway driven by overexpression of squalene synthase and methyl jasmonate elicitation in Bupleurum falcatum. Planta 2011, 233, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Dhar, N.; Rana, S.; Razdan, S.; Bhat, W.W.; Hussain, A.; Dhar, R.S.; Vaishnavi, S.; Hamid, A.; Vishwakarma, R.; Lattoo, S.K. Cloning and functional characterization of three branch point oxidosqualene cyclases from Withania somnifera (L.) dunal. J. Biol. Chem. 2014, 289, 17249–17267. [Google Scholar] [CrossRef] [PubMed]
- Kawano, N.; Ichinose, K.; Ebizuka, Y. Molecular cloning and functional expression of cDNAs encoding oxidosqualene cyclases from Costus speciosus. Biol. Pharm. Bull. 2003, 25, 477–482. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Nishiyama, T.; Shimizu, K.; Kadota, K. TCC: An R package for comparing tag count data with robust normalization strategies. BMC Bioinform. 2013, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yeats, T.; Han, H.; Jetter, R. Cloning and characterization of oxidosqualene cyclases from Kalanchoe daigremontiana: Enzymes catalyzing up to 10 rearrangement steps yielding friedelin and other triterpenoids. J. Biol. Chem. 2010, 285, 29703–29712. [Google Scholar] [CrossRef] [PubMed]



| Dataset Name | Control | MeJA (24 h) |
|---|---|---|
| No. raw reads | 71,835,350 | 76,653,282 |
| No. clean reads | 66,602,004 | 71,347,274 |
| No. of unigenes | 129,035 | |
| Average length of unigenes | 1065 bp | |
| Unigenes expressed in each sample (No.) | 121,987 | 102,869 |
| Different Types of SSRs | Number | Proportion (%) | Type No. | Total Length (bp) | Average Length (bp) |
|---|---|---|---|---|---|
| Dinucleotide | 574 | 29.4 | 12 | 102,317 | 15 |
| Trinucleotide | 1208 | 61.9 | 60 | 216,304 | 13 |
| Tetranucleotide | 112 | 5.7 | 72 | 20,302 | 17 |
| Pentanucleotide | 25 | 1.3 | 21 | 4439 | 21 |
| Hexanucleotide | 31 | 1.6 | 31 | 5559 | 25 |
| Enzyme | GeneID | Description | Identity (%) | e-Value | LFC | FPKM | |
|---|---|---|---|---|---|---|---|
| CON | MeJA | ||||||
| SQS | TBIU012384 | squalene synthase (Polygala tenuifolia) | 99 | 0 | 3.31 | 6.8 | 47.19 |
| SQE | TBIU001989 | squalene epoxidase (Astragalus membranaceus) | 83 | 2.0 × 10−66 | 0.765 | 2.84 | 3.37 |
| TBIU001990 | squalene monooxygenase (Theobroma cacao) | 88 | 2.0 × 10−83 | 13.8 | 0 | 17.77 | |
| TBIU020398 | PREDICTED: squalene epoxidase 3-like (Tarenaya hassleriana) | 86 | 4.0 × 10−41 | −2.08 | 1.6 | 0.26 | |
| TBIU062165 | Squalene monooxygenase, putative (Ricinus communis) | 88 | 3.0 × 10−14 | 1.82 | 1.63 | 4.03 | |
| TBIU068277 | PREDICTED: squalene monooxygenase-like (Eucalyptus grandis) | 88 | 3.0 × 10−71 | −1.02 | 8.19 | 2.83 | |
| OSC | TBIU017624 | β-amyrin synthase (Polygala tenuifolia) | 100 | 0 | 4.39 | 7.69 | 112.6 |
| TBIU030037 | cycloartenol synthase1 (Polygala tenuifolia) | 99 | 3.0 × 10−102 | 1.03 | 11.05 | 15.74 | |
| TBIU030038 | cycloartenol synthase (Glycine max) | 91 | 5.0 × 10−12 | 0.473 | 2.15 | 2.09 | |
| TBIU030039 | cycloartenol synthase (Betula platyphylla) | 81 | 1.0 × 10−71 | 0.013 | 6.32 | 4.46 | |
| TBIU030040 | PREDICTED : cycloartenol synthase (Ziziphus jujube) | 80 | 0 | 0.699 | 2.45 | 2.78 | |
| TBIU030041 | cycloartenol synthase1 (Polygala tenuifolia) | 87 | 0 | 0.932 | 9.48 | 12.63 | |
| TBIU030042 | PREDICTED : cycloartenol synthase (Ziziphus jujube) | 81 | 0 | 0.043 | 18.15 | 13.06 | |
| TBIU030043 | cycloartenol synthase1 (Polygala tenuifolia) | 87 | 0 | 0.368 | 1.75 | 1.58 | |
| TBIU030044 | cycloartenol synthase1 (Polygala tenuifolia) | 99 | 5.0 × 10−51 | 1.12 | 9.07 | 13.84 | |
| TBIU106220 | β-amyrin synthase (Polygala tenuifolia) | 100 | 1.0 × 10−14 | 2.44 | 3.47 | 13.21 | |
| TBIU119757 | β-amyrin synthase (Polygala tenuifolia) | 99 | 9.0 × 10−56 | 2.81 | 3.24 | 15.97 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, M.L.; Lee, W.M.; Kim, O.T. Two Cycloartenol Synthases for Phytosterol Biosynthesis in Polygala tenuifolia Willd. Int. J. Mol. Sci. 2017, 18, 2426. https://doi.org/10.3390/ijms18112426
Jin ML, Lee WM, Kim OT. Two Cycloartenol Synthases for Phytosterol Biosynthesis in Polygala tenuifolia Willd. International Journal of Molecular Sciences. 2017; 18(11):2426. https://doi.org/10.3390/ijms18112426
Chicago/Turabian StyleJin, Mei Lan, Woo Moon Lee, and Ok Tae Kim. 2017. "Two Cycloartenol Synthases for Phytosterol Biosynthesis in Polygala tenuifolia Willd" International Journal of Molecular Sciences 18, no. 11: 2426. https://doi.org/10.3390/ijms18112426





