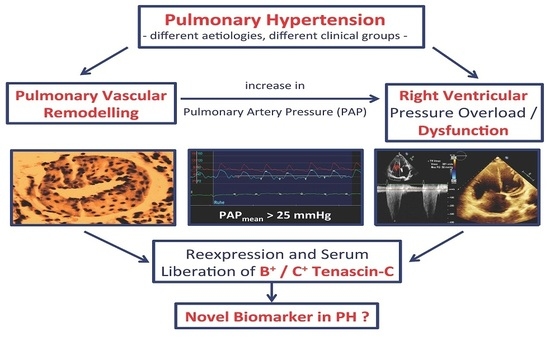
Increased Serum Levels of Fetal Tenascin-C Variants in Patients with Pulmonary Hypertension: Novel Biomarkers Reflecting Vascular Remodeling and Right Ventricular Dysfunction?
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
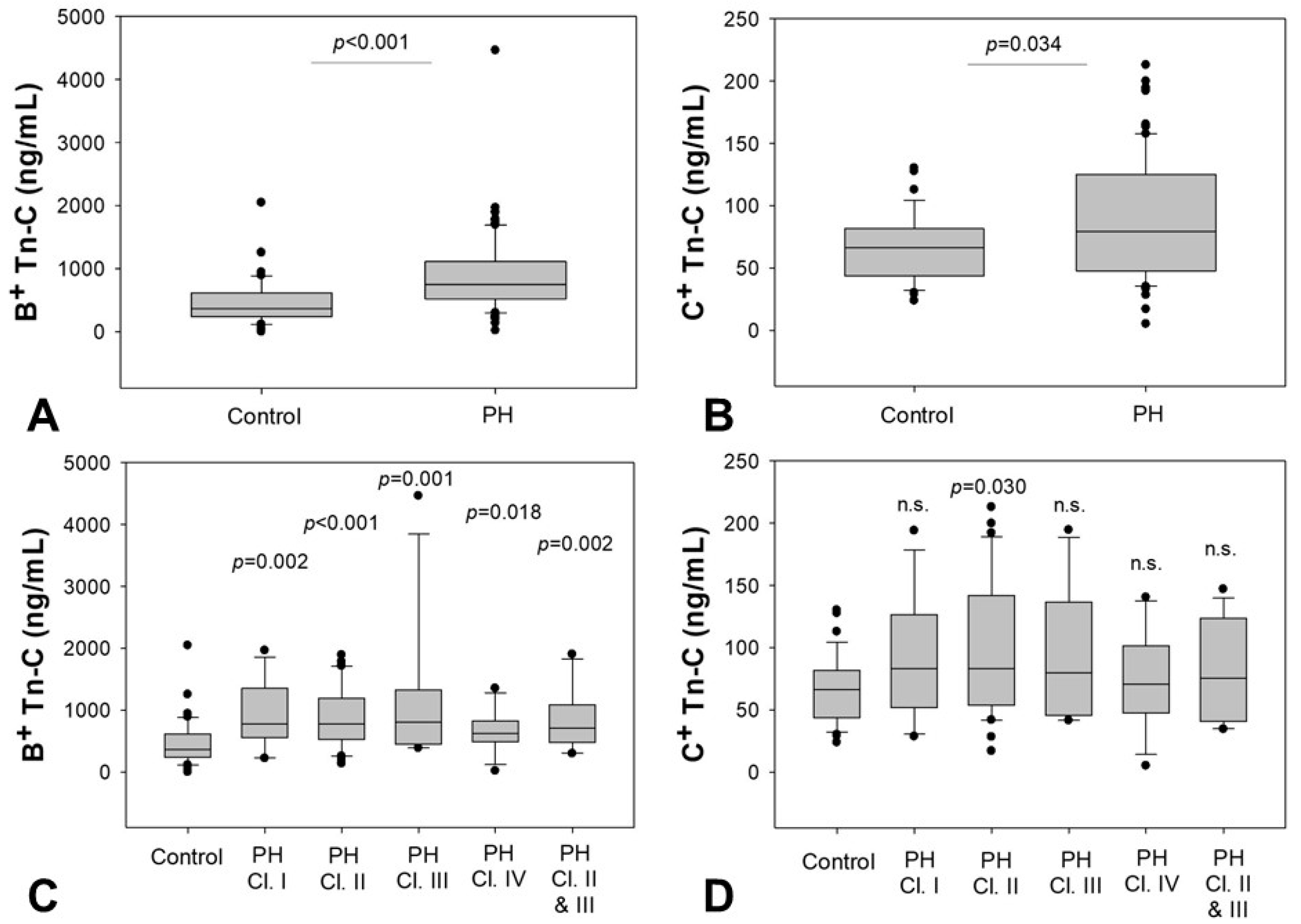
2.2. Serum Levels of B+ and C+ Tn-C
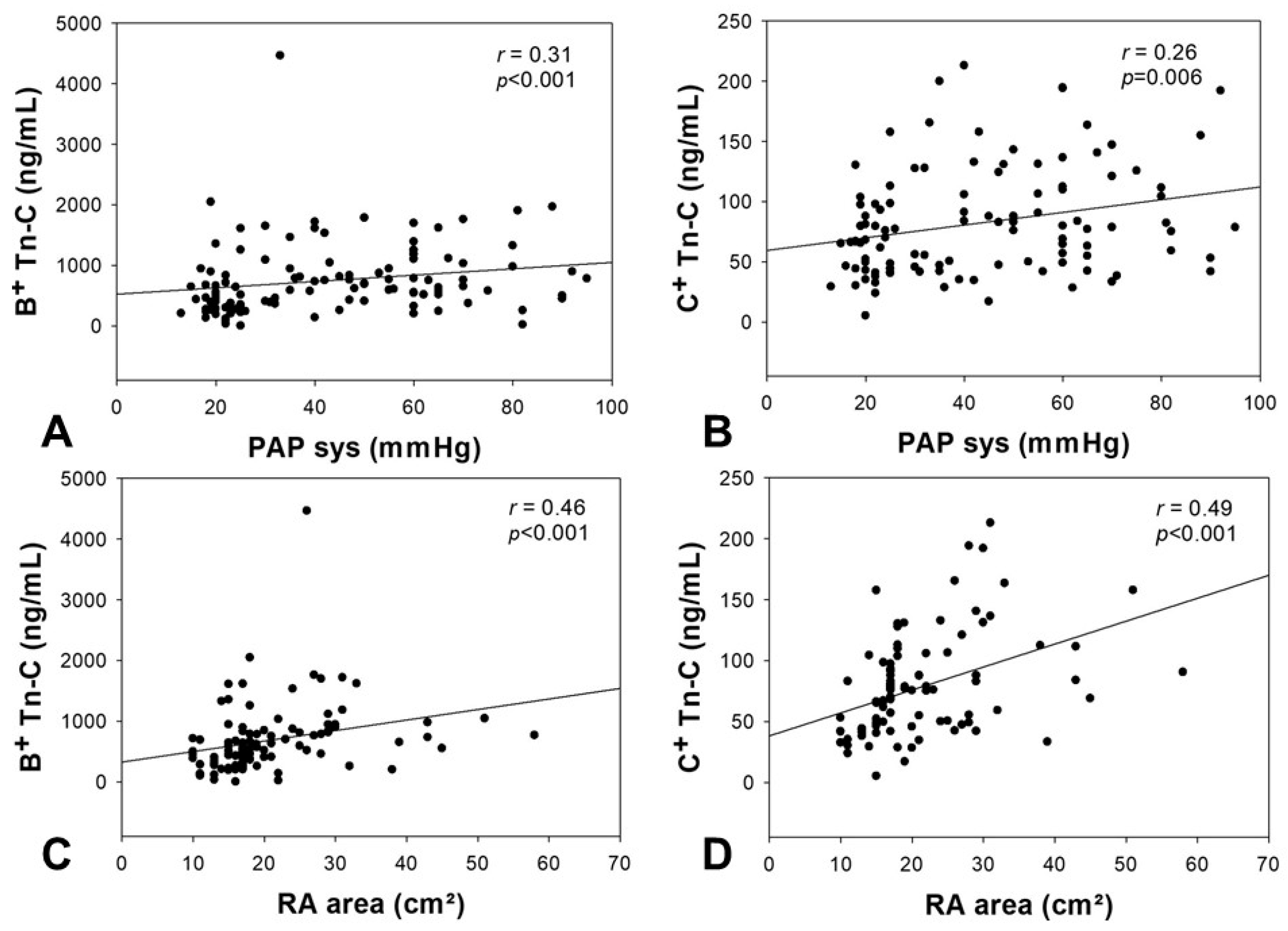
2.3. Correlation of Tn-C Serum Levels with Echocardiographic Parameters
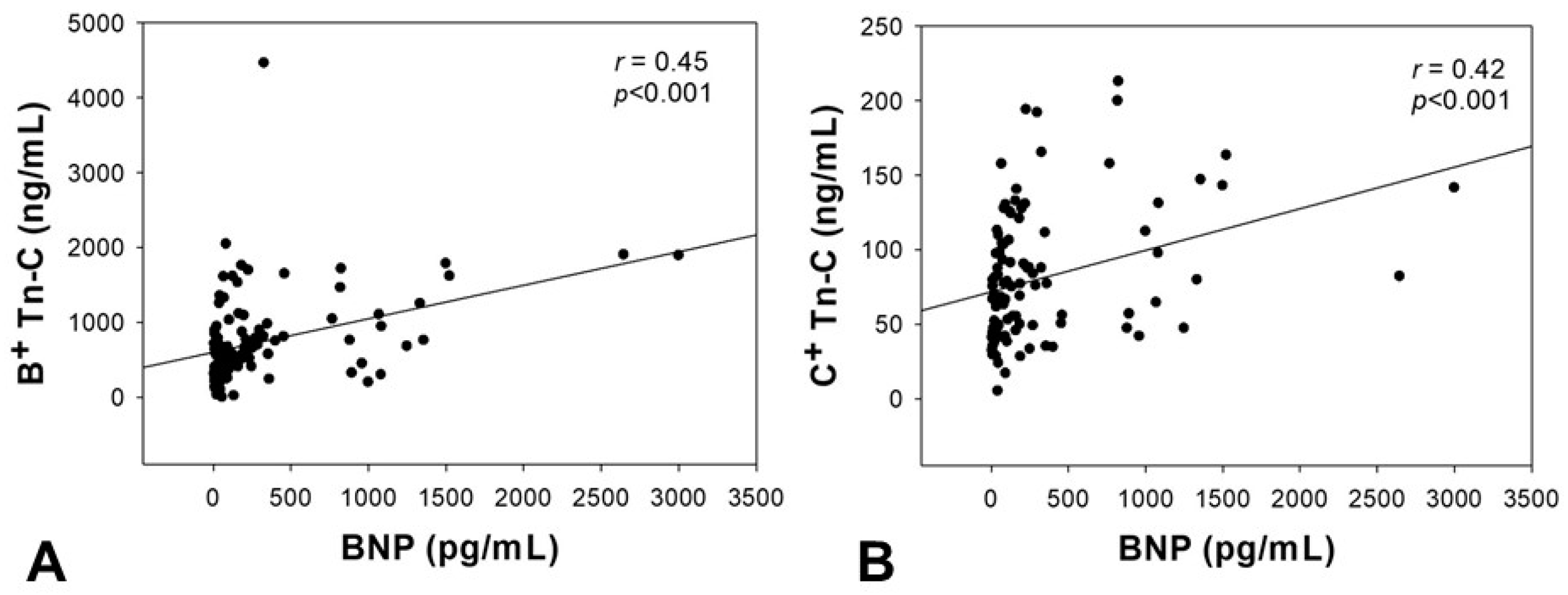
2.4. Correlation of Tn-C Serum Levels with BNP
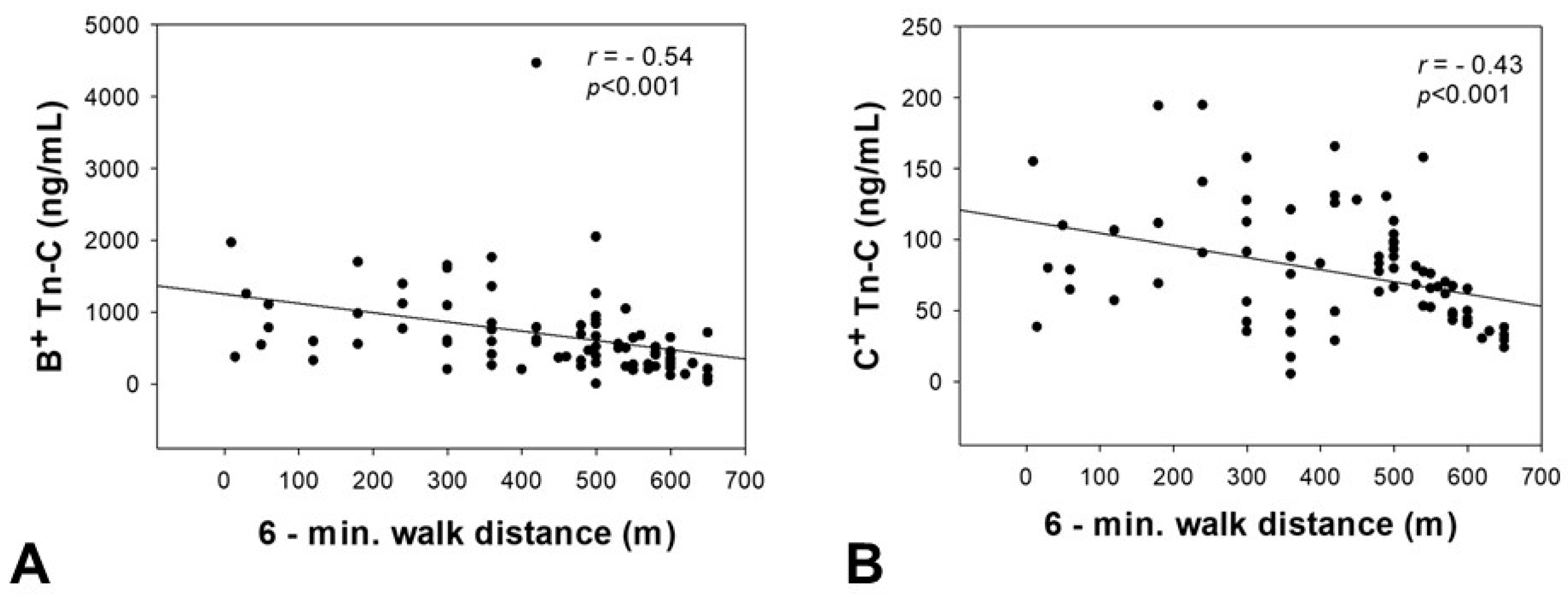
2.5. Correlation of Tn-C Serum Concentrations with the 6-Minute Walk Distance
2.6. Impact of Clinical Parameters on Tn-C Serum Levels
3. Discussion
4. Material and Methods
4.1. Patients
4.2. Quantification of Serum Tn-C Levels and Standard Laboratory Values
4.3. Investigation of Echocardiographic Parameters and 6-Minute Walk Test
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| ASA | Acetyl-salicylic acid |
| BMI | Body mass index |
| BNP | Brain natriuretic peptide |
| CAD | Coronary artery disease |
| CKD | Chronic kidney disease |
| CRP | C-reactive protein |
| ECM | Extracellular matrix |
| IVSDd | Intraventricular septum diameter in diastole |
| LDL | Low-density lipoprotein |
| LV-EF | Left ventricular ejection fraction |
| PH | Pulmonary hypertension |
| PAH | Pulmonary arterial hypertension |
| PAP | Pulmonary arterial pressure |
| RA | Right atrium |
| RV | Right ventricle |
| TAPSE | Tricuspid annular plane systolic excursion |
| TDI | Tissue doppler imaging |
| Tn-C | Tenascin C |
References
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [PubMed]
- Benza, R.L.; Miller, D.P.; Barst, R.J.; Badesch, D.B.; Frost, A.E.; McGoon, M.D. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012, 142, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.C.; Bye, H.; Brock, M.; Swiss Society of Pulmonary Hypertension. The pathogenesis of pulmonary hypertension—An update. Swiss Med. Wkly. 2015, 145, w14202. [Google Scholar] [CrossRef] [PubMed]
- Imanaka-Yoshida, K.; Hiroe, M.; Nishikawa, T.; Ishiyama, S.; Shimojo, T.; Ohta, Y.; Sakakura, T.; Yoshida, T. Tenascin-C modulates adhesion of cardiomyocytes to extracellular matrix during tissue remodeling after myocardial infarction. Lab. Investig. 2001, 81, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Berndt, A.; Altendorf-Hofmann, A.; Fiedler, N.; Richter, P.; Schumm, J.; Fritzenwanger, M.; Figulla, H.R.; Brehm, B.R. Serum levels of large tenascin-C variants, matrix metalloproteinase-9, and tissue inhibitors of matrix metalloproteinases in concentric versus eccentric left ventricular hypertrophy. Eur. J. Heart Fail. 2009, 11, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Sarli, B.; Topsakal, R.; Kaya, E.G.; Akpek, M.; Lam, Y.Y.; Kaya, M.G. Tenascin-C as predictor of left ventricular remodeling and mortality in patients with dilated cardiomyopathy. J. Investig. Med. 2013, 61, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Brehm, B.R.; Richter, P.; Gruen, K.; Neri, D.; Kosmehl, H.; Hekmat, K.; Renner, A.; Gummert, J.; Figulla, H.R.; et al. Changes in extra cellular matrix remodelling and re-expression of fibronectin and tenascin-C splicing variants in human myocardial tissue of the right atrial auricle: Implications for a targeted therapy of cardiovascular diseases using human SIP format antibodies. J. Mol. Histol. 2010, 41, 39–50. [Google Scholar] [PubMed]
- Baldinger, A.; Brehm, B.R.; Richter, P.; Bossert, T.; Gruen, K.; Hekmat, K.; Kosmehl, H.; Neri, D.; Figulla, H.R.; Berndt, A.; et al. Comparative analysis of oncofetal fibronectin and tenascin-C expression in right atrial auricular and left ventricular human cardiac tissue from patients with coronary artery disease and aortic valve stenosis. Histochem. Cell Biol. 2011, 135, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Berndt, A.; Neri, D.; Galler, K.; Grun, K.; Porrmann, C.; Reinbothe, F.; Mall, G.; Schlattmann, P.; Renner, A.; et al. Matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1, B+ tenascin-C and ED-A+ fibronectin in dilated cardiomyopathy: Potential impact on disease progression and patients’ prognosis. Int. J. Cardiol. 2013, 168, 5344–5351. [Google Scholar] [CrossRef] [PubMed]
- Wallner, K.; Sharifi, B.G.; Shah, P.K.; Noguchi, S.; DeLeon, H.; Wilcox, J.N. Adventitial remodeling after angioplasty is associated with expression of tenascin mRNA by adventitial myofibroblasts. J. Am. Coll. Cardiol. 2001, 37, 655–661. [Google Scholar] [CrossRef]
- Midwood, K.S.; Hussenet, T.; Langlois, B.; Orend, G. Advances in tenascin-C biology. Cell. Mol. Life Sci. 2011, 68, 3175–3199. [Google Scholar] [CrossRef] [PubMed]
- Borsi, L.; Balza, E.; Gaggero, B.; Allemanni, G.; Zardi, L. The alternative splicing pattern of the tenascin-C pre-mRNA is controlled by the extracellular pH. J. Biol. Chem. 1995, 270, 6243–6245. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.L.; Jones, F.S. Tenascin-C in development and disease: Gene regulation and cell function. Matrix Biol. 2000, 19, 581–596. [Google Scholar] [CrossRef]
- Franz, M.; Jung, C.; Lauten, A.; Figulla, H.R.; Berndt, A. Tenascin-C in cardiovascular remodeling: Potential impact for diagnosis, prognosis estimation and targeted therapy. Cell Adhes. Migr. 2015, 9, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Hessel, M.H.; Bleeker, G.B.; Bax, J.J.; Henneman, M.M.; den Adel, B.; Klok, M.; Schalij, M.J.; Atsma, D.E.; van der Laarse, A. Reverse ventricular remodelling after cardiac resynchronization therapy is associated with a reduction in serum tenascin-C and plasma matrix metalloproteinase-9 levels. Eur. J. Heart Fail. 2007, 9, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Berndt, A.; Grun, K.; Kuethe, F.; Fritzenwanger, M.; Figulla, H.R.; Jung, C. Serum levels of tenascin-C variants in congestive heart failure patients: Comparative analysis of ischemic, dilated, and hypertensive cardiomyopathy. Clin. Lab. 2014, 60, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, M. Elastase and the pathobiology of unexplained pulmonary hypertension. Chest 1998, 114 (Suppl. 3), 213S–224S. [Google Scholar] [CrossRef] [PubMed]
- Schermuly, R.T.; Yilmaz, H.; Ghofrani, H.A.; Woyda, K.; Pullamsetti, S.; Schulz, A.; Gessler, T.; Dumitrascu, R.; Weissmann, N.; Grimminger, F.; et al. Inhaled iloprost reverses vascular remodeling in chronic experimental pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2005, 172, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Correia-Pinto, J.; Henriques-Coelho, T.; Roncon-Albuquerque, R., Jr.; Lourenco, A.P.; Melo-Rocha, G.; Vasques-Novoa, F.; Gillebert, T.C.; Leite-Moreira, A.F. Time course and mechanisms of left ventricular systolic and diastolic dysfunction in monocrotaline-induced pulmonary hypertension. Basic Res. Cardiol. 2009, 104, 535–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schumann, C.; Lepper, P.M.; Frank, H.; Schneiderbauer, R.; Wibmer, T.; Kropf, C.; Stoiber, K.M.; Rudiger, S.; Kruska, L.; Krahn, T.; et al. Circulating biomarkers of tissue remodelling in pulmonary hypertension. Biomarkers 2010, 15, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Fassler, R.; Sasaki, T.; Timpl, R.; Chu, M.L.; Werner, S. Differential regulation of fibulin, tenascin-C, and nidogen expression during wound healing of normal and glucocorticoid-treated mice. Exp. Cell Res. 1996, 222, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Gratchev, A.; Kzhyshkowska, J.; Utikal, J.; Goerdt, S. Interleukin-4 and dexamethasone counterregulate extracellular matrix remodelling and phagocytosis in type-2 macrophages. Scand. J. Immunol. 2005, 61, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ekblom, M.; Fassler, R.; Tomasini-Johansson, B.; Nilsson, K.; Ekblom, P. Downregulation of tenascin expression by glucocorticoids in bone marrow stromal cells and in fibroblasts. J. Cell Biol. 1993, 123, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Celik, A.; Kocyigit, I.; Calapkorur, B.; Korkmaz, H.; Doganay, E.; Elcik, D.; Ozdogru, I. Tenascin-C may be a predictor of acute pulmonary thromboembolism. J. Atheroscler. Thromb. 2011, 18, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Hessel, M.; Steendijk, P.; den Adel, B.; Schutte, C.; van der Laarse, A. Pressure overload-induced right ventricular failure is associated with re-expression of myocardial tenascin-C and elevated plasma tenascin-C levels. Cell. Physiol. Biochem. 2009, 24, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Task Force, M.; Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar]
- Ling, Y.; Johnson, M.K.; Kiely, D.G.; Condliffe, R.; Elliot, C.A.; Gibbs, J.S.; Howard, L.S.; Pepke-Zaba, J.; Sheares, K.K.; Corris, P.A.; et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: Results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am. J. Respir. Crit. Care Med. 2012, 186, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Bootz, F.; Neri, D. Immunocytokines: A novel class of products for the treatment of chronic inflammation and autoimmune conditions. Drug Discov. Today 2016, 21, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Casi, G.; Neri, D. Antibody-drug conjugates: Basic concepts, examples and future perspectives. J. Control Release 2012, 161, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Rybak, J.N.; Trachsel, E.; Scheuermann, J.; Neri, D. Ligand-based vascular targeting of disease. ChemMedChem 2007, 2, 22–40. [Google Scholar] [CrossRef] [PubMed]
| Clinical Parameter | Control Persons (n = 40) | PH Patients (n = 80) | p-Value |
|---|---|---|---|
| Age (years) | 66 ± 7 | 70 ± 13 | n.s. |
| BMI (kg/m2) | 27.9 ± 4.7 | 28.6 ± 6.0 | n.s. |
| Gender, male (%) | 33 | 39 | n.s. |
| Systolic BP (mmHg) | 146 ± 33 | 146 ± 33 | n.s. |
| Diastolic BP (mmHg) | 81 ± 17 | 78 ± 13 | n.s. |
| Functional class | 1.5 ± 0.6 | 2.6 ± 0.8 | <0.001 |
| Laboratory: | |||
| BNP (pg/mL) | 56 ± 70 | 445 ± 584 | <0.001 |
| CRP (mg/L) | 2.7 ± 2.6 | 11.7 ± 17.5 | 0.002 |
| Creatinine (μmol/l) | 76 ± 16 | 111 ± 50 | <0.001 |
| LDL (mmol/l) | 3.6 ± 1.0 | 2.7 ± 1.0 | <0.001 |
| Haemoglobin (mmol/l) | 8.7 ± 0.8 | 7.9 ± 1.3 | <0.001 |
| Leukocytes (Gpt/l) | 7.1 ± 1.4 | 7.5 ± 2.2 | n.s. |
| Co-Morbidities: | |||
| Hypertension (%) | 95 | 83 | 0.03 |
| Hyperlipidemia (%) | 88 | 56 | <0.001 |
| Obesity (%) (BMI>30 kg/m2) | 42 | 38 | n.s. |
| CAD (%) | 0 | 26 | <0.001 |
| CKD (%) (GFR < 50 mL/min) | 8 | 50 | <0.001 |
| Diabetes (%) | 20 | 50 | 0.002 |
| Smoking (%) | 29 | 52 | 0.027 |
| Medication: | |||
| ASA (%) | 25 | 18 | n.s. |
| Beta blocker (%) | 63 | 60 | n.s. |
| ACE Inhibitor/Sartans (%) | 85 | 78 | n.s. |
| Statins (%) | 38 | 59 | 0.026 |
| Prednisolon (%) | 0 | 11 | 0.027 |
| ICS (%) | 0 | 21 | 0.002 |
| Characteristics | Control (n = 40) | PH Class I (n = 13) | PH Class II (n = 30) | PH Class III (n = 11) | PH Class IV (n = 12) | PH Class II & III (n = 14) | p-Value between Different PH-Classes |
|---|---|---|---|---|---|---|---|
| Age (years) | 66.0 ± 6.8 | 64.5 ± 11.5 | 75.1 ± 8.1 | 58.5 ± 22.3 | 69.8 ± 11.5 | 74.9 ± 7.9 | <0.001 |
| BMI (kg/m2) | 28.0 ± 4.7 | 29.0 ± 8.4 | 27.9 ± 3.9 | 25.9 ± 7.5 | 30.6 ± 5.2 | 29.9 ± 6.5 | n.s. |
| Functional class | 1.5 ± 0.6 | 2.6 ± 0.8 | 2.6 ± 0.7 | 2.4 ± 1.1 | 2.3 ± 0.7 | 2.8 ± 0.7 | n.s. |
| Laboratory: | |||||||
| BNP (pg/mL) | 56 ± 70 | 113 ± 82 | 635 ± 678 | 469 ± 469 | 111 ± 87 | 627 ± 731 | 0.021 |
| CRP (mg/L) | 2.7 ± 2.6 | 7.8 ± 10.7 | 13.8 ± 21.0 | 10.5 ± 12.9 | 5.2 ± 5.8 | 16.1 ± 22.0 | n.s. |
| Creatinine (μmol/L) | 76 ± 16 | 88 ± 44 | 127 ± 59 | 93 ± 24 | 99 ± 40 | 121 ± 47 | n.s. |
| LDL (mmol/L) | 3.6 ± 1.0 | 2.5 ± 1.0 | 2.5 ± 1.0 | 3.0 ± 1.0 | 3.0 ± 0.9 | 2.7 ± 1.2 | n.s. |
| Haemoglobin (mmol/L) | 8.7 ± 0.8 | 7.7 ± 1.5 | 7.5 ± 1.2 | 8.5 ± 1.0 | 8.9 ± 1.4 | 7.6 ± 1.6 | 0.012 |
| Leukocytes (Gpt/L) | 7.1 ± 1.4 | 6.7 ± 2.4 | 7.6 ± 1.7 | 8.3 ± 1.5 | 7.4 ± 1.8 | 7.7 ± 3.4 | n.s. |
| Parameter | Control (n = 40) | PH Class I (n = 13) | PH Class II (n = 30) | PH Class III (n = 11) | PH Class IV (n = 12) | PH Class II & III (n = 14) |
|---|---|---|---|---|---|---|
| LV-EF (%) | 68 ± 7 | 63 ± 7 | 56 ± 13 | 63 ± 9 | 60 ± 6 | 52 ± 12 |
| IVSDd (mm) | 12 ± 2 | 12 ± 3 | 13 ± 3 | 13 ± 2 | 11 ± 2 | 12 ± 2 |
| RVd basal (mm) | 35 ± 2 | 41 ± 9 | 46 ± 7 | 47 ± 10 | 44 ± 7 | 49 ± 9 |
| TAPSE (mm) | 25 ± 2 | 22 ± 5 | 15 ± 3 | 19 ± 7 | 18 ± 3 | 16 ± 4 |
| TDI-S’ (RV) (cm/s) | 14 ± 2 | 12 ± 1 | 8 ± 1 | 9 ± 0 | 11 ± 1 | 9 ± 2 |
| RA area (cm2) | 15.4 ± 2.3 | 20.8 ± 8.3 | 27.9 ± 9.6 | 19.7 ± 8.8 | 22.2 ± 5.2 | 31.0 ± 13.7 |
| PAP sys (mmHg) | 21 ± 4 | 59 ± 22 | 52 ± 16 | 57 ± 22 | 50 ± 20 | 56 ± 16 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohm, I.; Grün, K.; Müller, L.M.; Kretzschmar, D.; Fritzenwanger, M.; Yilmaz, A.; Lauten, A.; Jung, C.; Schulze, P.C.; Berndt, A.; et al. Increased Serum Levels of Fetal Tenascin-C Variants in Patients with Pulmonary Hypertension: Novel Biomarkers Reflecting Vascular Remodeling and Right Ventricular Dysfunction? Int. J. Mol. Sci. 2017, 18, 2371. https://doi.org/10.3390/ijms18112371
Rohm I, Grün K, Müller LM, Kretzschmar D, Fritzenwanger M, Yilmaz A, Lauten A, Jung C, Schulze PC, Berndt A, et al. Increased Serum Levels of Fetal Tenascin-C Variants in Patients with Pulmonary Hypertension: Novel Biomarkers Reflecting Vascular Remodeling and Right Ventricular Dysfunction? International Journal of Molecular Sciences. 2017; 18(11):2371. https://doi.org/10.3390/ijms18112371
Chicago/Turabian StyleRohm, Ilonka, Katja Grün, Linda Marleen Müller, Daniel Kretzschmar, Michael Fritzenwanger, Atilla Yilmaz, Alexander Lauten, Christian Jung, P. Christian Schulze, Alexander Berndt, and et al. 2017. "Increased Serum Levels of Fetal Tenascin-C Variants in Patients with Pulmonary Hypertension: Novel Biomarkers Reflecting Vascular Remodeling and Right Ventricular Dysfunction?" International Journal of Molecular Sciences 18, no. 11: 2371. https://doi.org/10.3390/ijms18112371