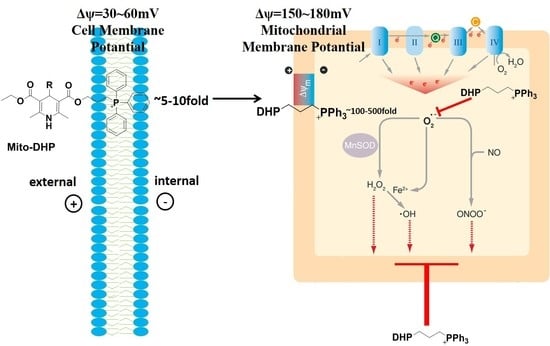
Synthesis and Radioprotective Activity of Mitochondria Targeted Dihydropyridines In Vitro
Abstract
:1. Introduction
2. Results
2.1. Cellular Distributions of Mito-DHPs
2.2. Reactive Oxygen Species (ROS) Detection
2.3. Detection of Histone H2AX (γ-H2AX)
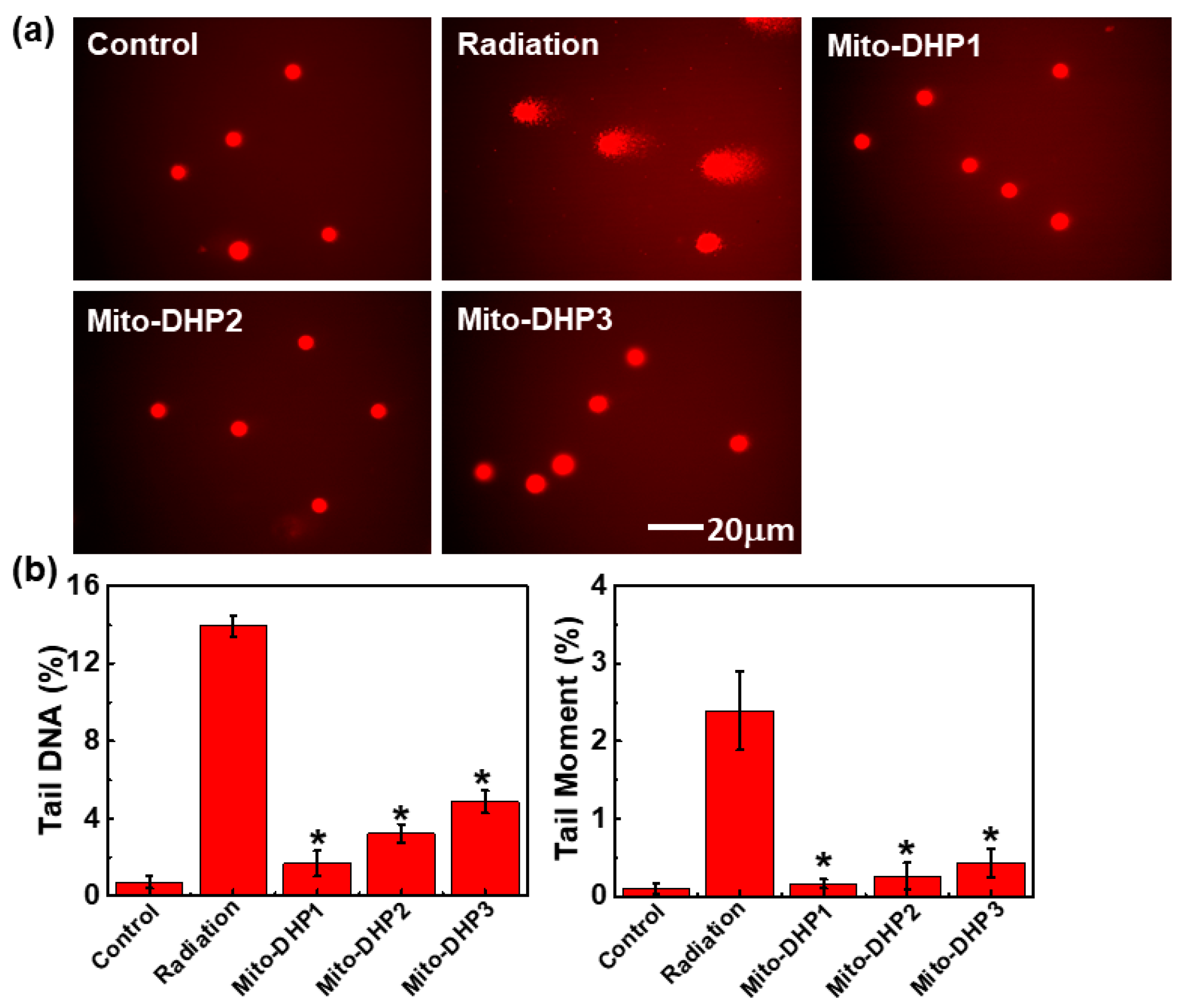
2.4. Comet Assay
3. Discussion
4. Materials and Methods
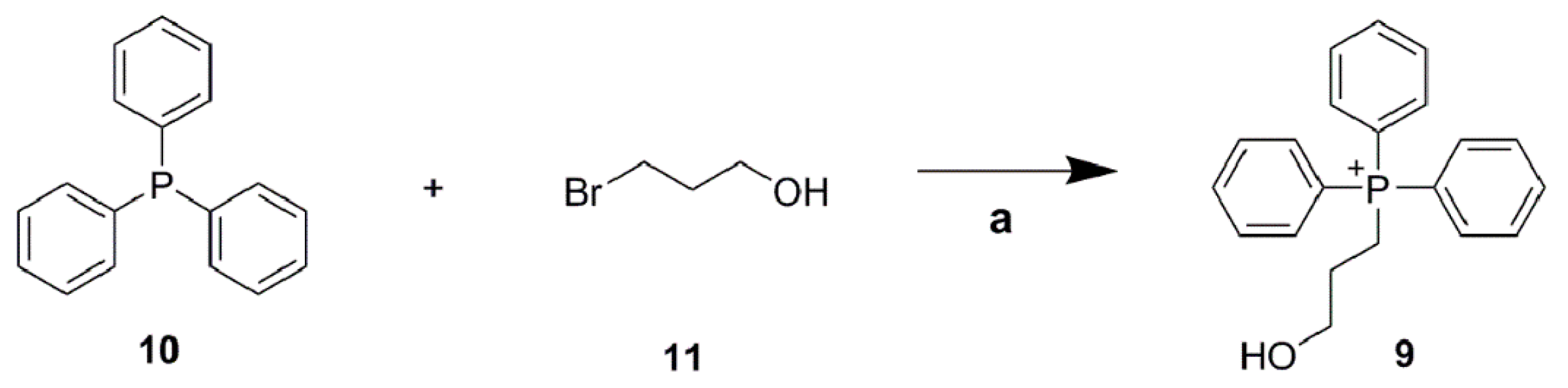
4.1. Chemistry
4.2. Biological Evaluation
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chetty, I.J.; Martel, M.K.; Jaffray, D.A.; Benedict, S.H.; Hahn, S.M.; Berbeco, R.; Deye, J.; Jeraj, R.; Kavanagh, B.; Krishnan, S.; et al. Technology for innovation in radiation oncology. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Kilciksiz, S.; Demirel, C.; Erdal, N.; Gurgul, S.; Tamer, L.; Ayaz, L.; Ors, Y. The effect of N-acetylcysteine on biomarkers for radiation-induced oxidative damage in a rat model. Acta Med. Okayama 2008, 62, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Patt, H.M.; Tyree, E.B.; Straube, R.L.; Smith, D.E. Cysteine protection against X irradiation. Science 1949, 110, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Bacq, Z.M. The amines and particularly cysteamine as protectors against roentgen rays. Acta Radiol. 1954, 41, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kanimozhi, P.; Prasad, N.R. Antioxidant potential of sesamol and its role on radiation-induced DNA damage in whole-body irradiated Swiss albino mice. Environ. Toxicol. Pharmacol. 2009, 28, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Hensley, M.L.; Hagerty, K.L.; Kewalramani, T.; Green, D.M.; Meropol, N.J.; Wasserman, T.H.; Cohen, G.I.; Emami, B.; Gradishar, W.J.; Mitchell, R.B.; et al. American society of clinical oncology 2008 clinical practice guideline update: Use of chemotherapy and radiation therapy protectants. J. Clin. Oncol. 2009, 27, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Lombaert, I.M.; Brunsting, J.F.; Wierenga, P.K.; Kampinga, H.H.; de Haan, G.; Coppes, R.P. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells 2008, 26, 2595–2601. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, R.; Ito, A.; Tomita, M.; Tsukada, T.; Yatagai, F.; Noguchi, M.; Matsumoto, Y.; Kase, Y.; Ando, K.; Okayasu, R.; et al. Contributions of direct and indirect actions in cell killing by high-LET radiations. Radiat. Res. 2009, 171, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Kam, W.W.; Banati, R.B. Effects of ionizing radiation on mitochondria. Free Radic. Biol. Med. 2013, 65, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Hartley, R.C.; Cocheme, H.M.; Murphy, M.P. Mitochondrial pharmacology. Trends Pharmacol. Sci. 2012, 33, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Hartley, R.C.; Murphy, M.P. Mitochondria-targeted small molecule therapeutics and probes. Antioxid. Redox Signal. 2011, 15, 3021–3038. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Raha, S.; Robinson, B.H. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000, 25, 502–508. [Google Scholar] [CrossRef]
- Velena, A.; Zarkovic, N.; Troselj, K.G.; Bisenieks, E.; Krauze, A.; Poikans, J.; Duburs, G. 1,4-dihydropyridine derivatives: Dihydronicotinamide analogues-model compounds targeting oxidative stress. Oxid. Med. Cell. Longev. 2016, 2016, 1892412. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.B.; Russo, A.; Kuppusamy, P.; Krishna, M.C. Radiation, radicals, and images. Ann. N. Y. Acad. Sci. 2000, 899, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Yamamori, T.; Yasui, H.; Yamazumi, M.; Wada, Y.; Nakamura, Y.; Nakamura, H.; Inanami, O. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic. Biol. Med. 2012, 53, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.K.; van Tuyle, G.; Lin, P.S.; Schmidt-Ullrich, R.; Mikkelsen, R.B. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001, 61, 3894–3901. [Google Scholar] [PubMed]
- Landauer, M.R.; Davis, H.D.; Dominitz, J.A.; Weiss, J.F. Dose and time relationships of the radioprotector WR-2721 on locomotor activity in mice. Pharmacol. Biochem. Behav. 1987, 27, 573–576. [Google Scholar] [CrossRef]
- Wedemeyer, N.; Greve, B.; Uthe, D.; Potter, T.; Denklau, D.; Severin, E.; Hacker-Klom, U.; Kohnlein, W.; Gohde, W. Frequency of CD59 mutations induced in human-hamster hybrid AL cells by low-dose X-irradiation. Mutat. Res. 2001, 473, 73–84. [Google Scholar] [CrossRef]
- Liu, X.; Yu, H.; Zhao, X.; Huang, X.R. Molecular simulations study of novel 1,4-dihydropyridines derivatives with a high selectivity for Cav3.1 calcium channel. Protein Sci. 2015, 24, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Stoyanovsky, D.A.; Belikova, N.A.; Tyurina, Y.Y.; Zhao, Q.; Tungekar, M.A.; Kapralova, V.; Huang, Z.; Mintz, A.H.; Greenberger, J.S.; et al. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat. Res. 2009, 172, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, B.; Montana, V.; Wei, M.D.; Wuskell, J.P.; Loew, L.M. Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys. J. 1988, 53, 785–794. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J. Am. Chem. Soc. 2008, 130, 9638–9639. [Google Scholar] [CrossRef] [PubMed]
- Pilch, D.R.; Sedelnikova, O.A.; Redon, C.; Celeste, A.; Nussenzweig, A.; Bonner, W.M. Characteristics of γ-H2AX foci at DNA double-strand breaks sites. Biochim. Biol. Cell. 2003, 81, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Kinner, A.; Wu, W.; Staudt, C.; Iliakis, G. γ-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008, 36, 5678–5694. [Google Scholar] [CrossRef] [PubMed]
- Sowmithra, K.; Shetty, N.J.; Jha, S.K.; Chaubey, R.C. Evaluation of genotoxicity of the acute γ radiation on earthworm Eisenia fetida using single cell gel electrophoresis technique (Comet Assay). Mutat. Res. 2015, 794, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jenrow, K.A.; Brown, S.L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. 2014, 32, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ueno, M.; Nakanishi, I.; Anzai, K. Density of hydroxyl radicals generated in an aqueous solution by irradiating carbon-ion beam. Chem. Pharm. Bull. 2015, 63, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Saenko, Y.; Cieslar-Pobuda, A.; Skonieczna, M.; Rzeszowska-Wolny, J. Changes of reactive oxygen and nitrogen species and mitochondrial functioning in human K562 and Hl60 cells exposed to ionizing radiation. Radiat. Res. 2013, 180, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Sriram, A.; Fernandez-Ayala, D.; Gubina, N.; Lohmus, M.; Nelson, G.; Logan, A.; Cooper, H.M.; Navas, P.; Enriquez, J.A.; et al. Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell. Metab. 2016, 23, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Desagher, S.; Martinou, J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef]
- Ande, S.R.; Chen, J.; Maddika, S. The ubiquitin pathway: An emerging drug target in cancer therapy. Eur. J. Pharmacol. 2009, 625, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Gogvadze, V.; Robertson, J.D.; Zhivotovsky, B.; Orrenius, S. Cytochrome c release occurs via Ca2+-dependent and Ca2+-independent mechanisms that are regulated by Bax. J. Biol. Chem. 2001, 276, 19066–19071. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.F.; Da Ros, T.; Blaikie, F.H.; Prime, T.A.; Porteous, C.M.; Severina, I.I.; Skulachev, V.P.; Kjaergaard, H.G.; Smith, R.A.; Murphy, M.P. Accumulation of lipophilic dications by mitochondria and cells. Biochem. J. 2006, 400, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Liu, Q.; Feng, K.; Chen, B.; Tung, C.H.; Wu, L.Z. Facile photoreduction of graphene oxide by an NAD(P)H model: Hantzsch 1,4-dihydropyridine. Langmuir 2012, 28, 8224–8229. [Google Scholar] [CrossRef] [PubMed]
- Ogle, J.; Stradins, J.; Baumane, L. Formation and decay of free cation-radicals in the course of electro-oxidation of 1,2- and 1,4-dihydropyridines (Hantzsch esters). Electrochim. Acta 1994, 39, 73–79. [Google Scholar] [CrossRef]
- Schon, E.A.; DiMauro, S.; Hirano, M. Human mitochondrial DNA: Roles of inherited and somatic mutations. Nat. Rev. Genet. 2012, 13, 878–890. [Google Scholar] [CrossRef] [PubMed]
- Patti, M.E.; Corvera, S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr. Rev. 2010, 31, 364–395. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Piaceri, I.; Rinnoci, V.; Bagnoli, S.; Failli, Y.; Sorbi, S. Mitochondria and Alzheimer’s disease. J. Neurol. Sci. 2012, 322, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.G. Recent advances in mitochondrial research. Circ. Res. 2013, 113, e107–e110. [Google Scholar] [CrossRef] [PubMed]
- Demiral, A.N.; Yerebakan, O.; Simsir, V.; Alpsoy, E. Amifostine-induced toxic epidermal necrolysis during radiotherapy: A case report. Jpn. J. Clin. Oncol. 2002, 32, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kuwahara, Y.; Li, L.; Baba, T.; Shin, R.W.; Ohkubo, Y.; Ono, K.; Fukumoto, M. Analysis of common deletion (CD) and a novel deletion of mitochondrial DNA induced by ionizing radiation. Int. J. Radiat. Biol. 2007, 83, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.V.; Carrithers, S.L.; Parkinson, S.J.; Skurk, C.; Nuss, C.; Pooler, P.M.; Owen, C.S.; Lefer, A.M.; Waldman, S.A. Hypotensive mechanisms of amifostine. J. Clin. Pharmacol. 1996, 36, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Winn, L.M. The effects of 1,4-benzoquinone on c-Myb and topoisomerase II in K-562 cells. Mutat. Res. 2008, 645, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Zhang, Y.; Guliaev, A.B.; Shen, H.; Hang, B.; Singer, B.; Wang, Z. The p-benzoquinone DNA adducts derived from benzene are highly mutagenic. DNA Repair 2005, 4, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Whysner, J.; Reddy, M.V.; Ross, P.M.; Mohan, M.; Lax, E.A. Genotoxicity of benzene and its metabolites. Mutat. Res. 2004, 566, 99–130. [Google Scholar] [CrossRef]
- Forni, L.G.; Willson, R.L. Thiyl and phenoxyl free radicals and NADH Direct observation of one-electron oxidation. Biochem. J. 1986, 240, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Velena, A.; Zilbers, J.; Duburs, G. Derivatives of 1,4-dihydropyridines as modulators of ascorbate-induced lipid peroxidation and high-amplitude swelling of mitochondria, caused by ascorbate, sodium linoleate and sodium pyrophosphate. Cell Biochem. Funct. 1999, 17, 237–252. [Google Scholar] [CrossRef]
- Zhang, J. Synthesis and antioxidant activity of a series of novel 3-chalcone-substituted 1,4-dihydropyridine derivatives. Pharm. Chem. J. 2012, 18, 120–124. [Google Scholar] [CrossRef]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, J.; Li, Y.; Wang, F.; Yang, F.; Xu, W. Synthesis and Radioprotective Activity of Mitochondria Targeted Dihydropyridines In Vitro. Int. J. Mol. Sci. 2017, 18, 2233. https://doi.org/10.3390/ijms18112233
Zhang Y, Wang J, Li Y, Wang F, Yang F, Xu W. Synthesis and Radioprotective Activity of Mitochondria Targeted Dihydropyridines In Vitro. International Journal of Molecular Sciences. 2017; 18(11):2233. https://doi.org/10.3390/ijms18112233
Chicago/Turabian StyleZhang, Yurui, Junying Wang, Yuanyuan Li, Feng Wang, Fujun Yang, and Wenqing Xu. 2017. "Synthesis and Radioprotective Activity of Mitochondria Targeted Dihydropyridines In Vitro" International Journal of Molecular Sciences 18, no. 11: 2233. https://doi.org/10.3390/ijms18112233