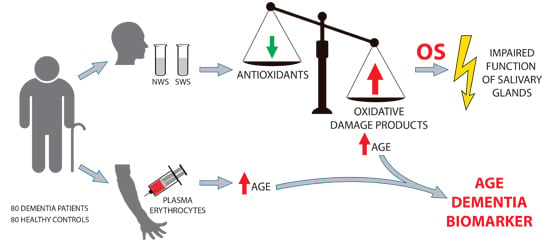
Antioxidant Defence, Oxidative Stress and Oxidative Damage in Saliva, Plasma and Erythrocytes of Dementia Patients. Can Salivary AGE be a Marker of Dementia?
Abstract
:1. Introduction
2. Results
2.1. Clinical Findings
2.2. Dental Examination
2.3. Salivary Flow, Total Protein and pH of Saliva
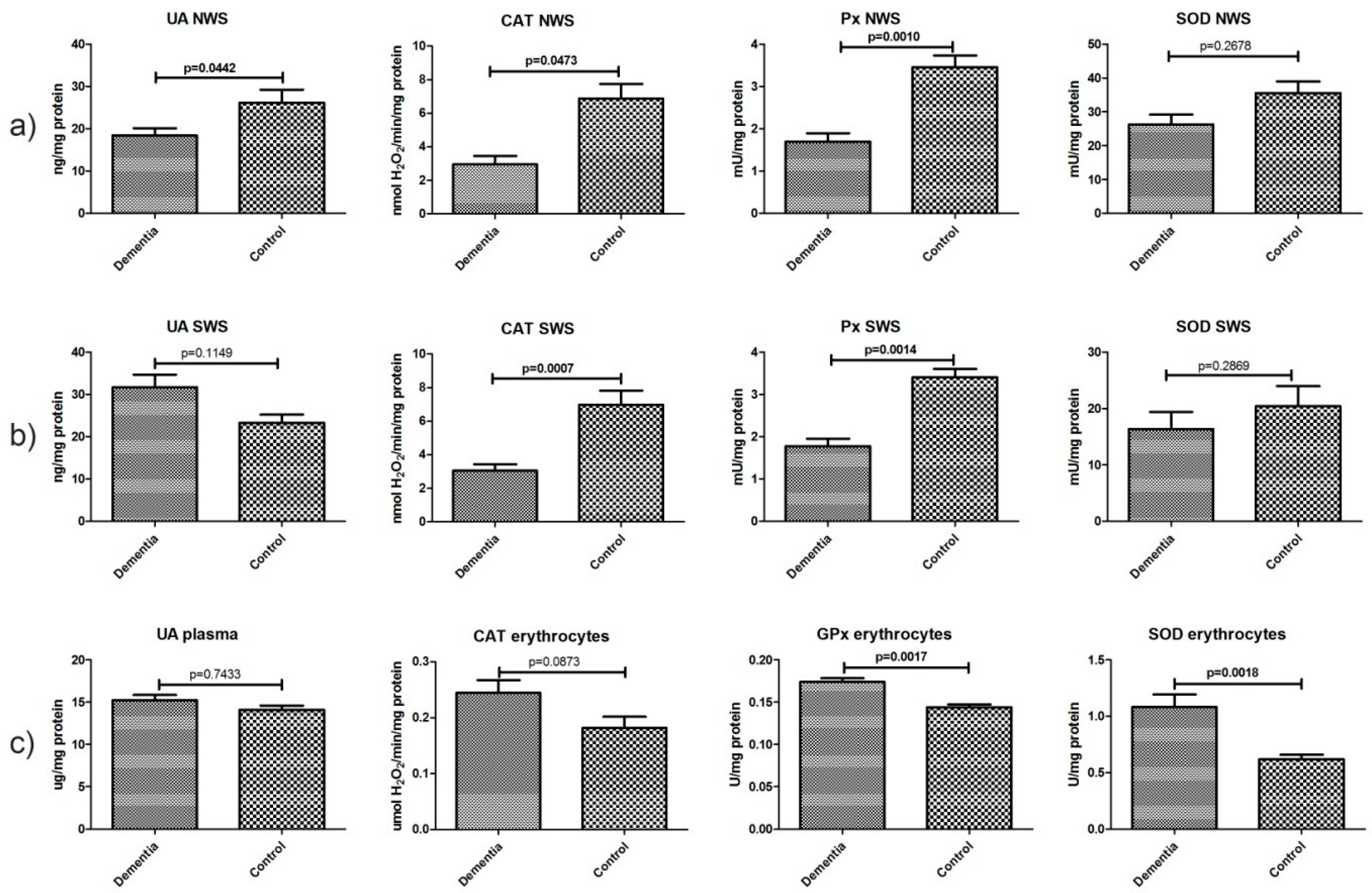
2.4. Non-Enzymatic and Enzymatic Antioxidants
2.5. Total Antioxidant/Oxidant Status
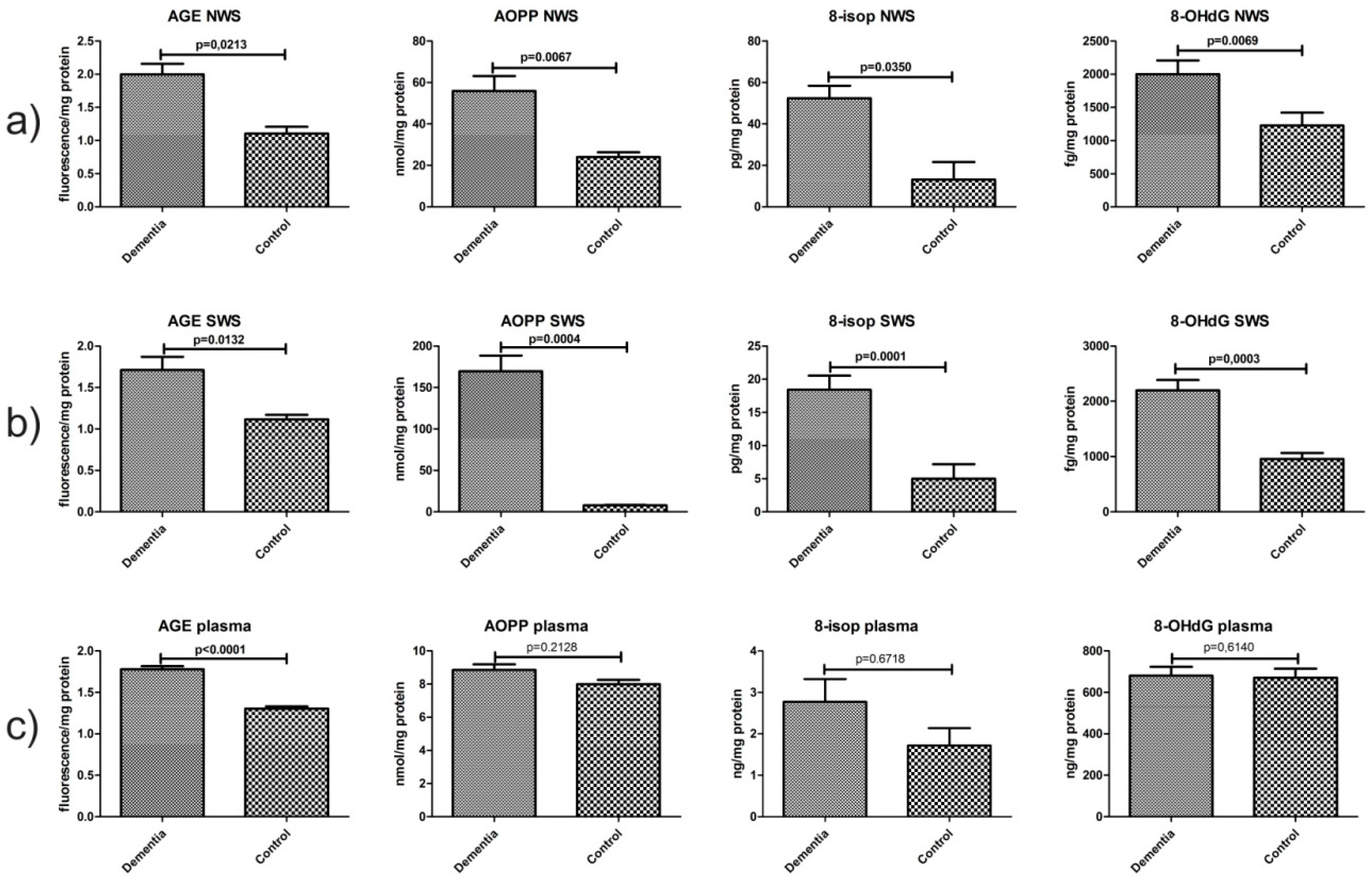
2.6. Oxidative Damage Products
2.7. Correlations
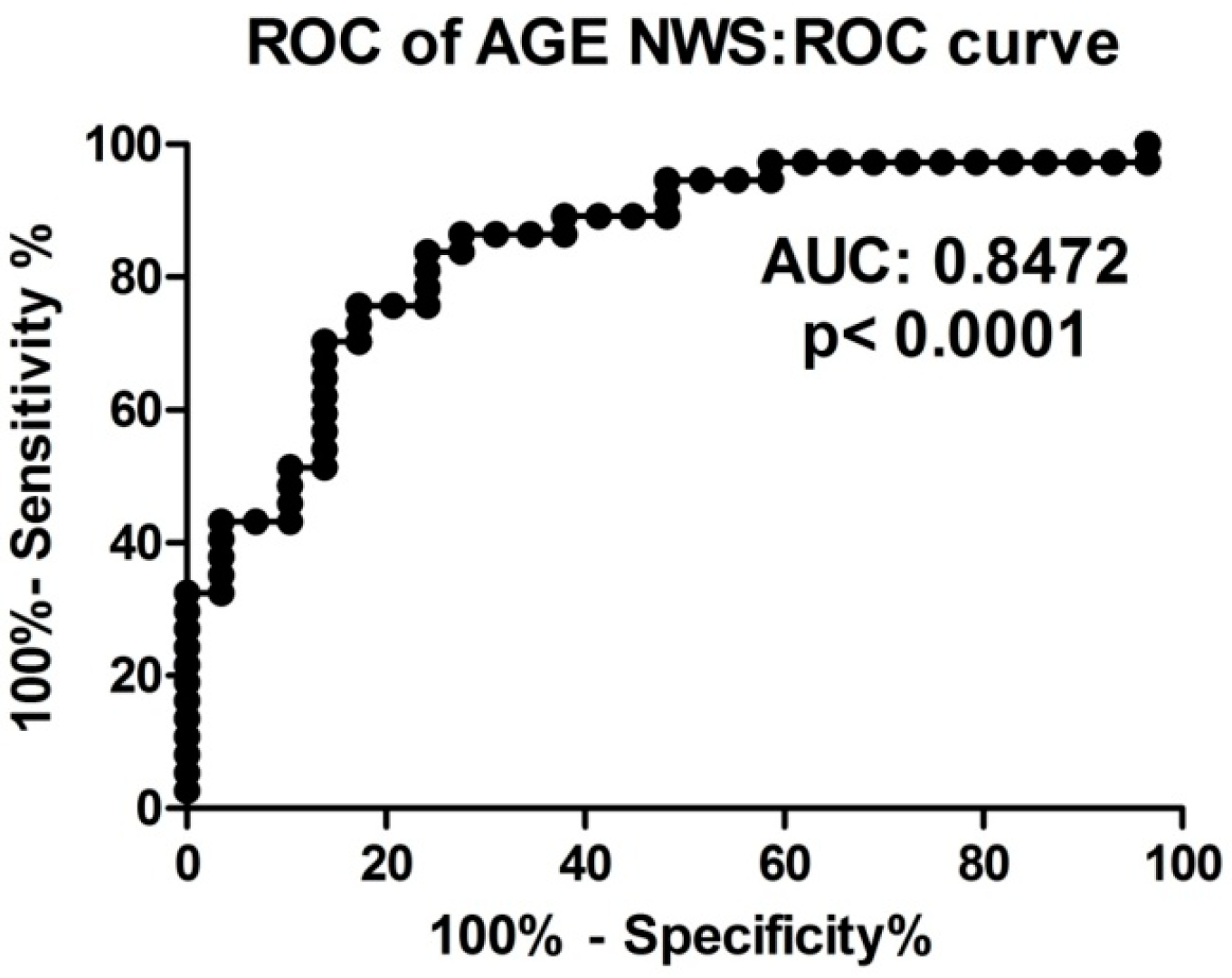
2.8. ROC Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Blood Collection
4.3. Saliva Collection
4.4. Dental Examination
4.5. Biochemical Analysis
4.6. Salivary, Plasma and Erythrocytes Antioxidants
4.7. Salivary and Plasma Oxidative Modification Products
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prince, M.; Comas-Herrera, M.A.; Knapp, M.; Guerchet, M.; Karagiannidou, M.M. World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia: Coverage, Quality and Costs now and in the Future; Alzheimer’s Disease International: London, UK, 2016. [Google Scholar]
- Demirbilek, M.E.; Kilic, N.; Komurcu, H.F.; Akin, K.O. Advanced Oxidation Protein Products in Aged with Dementia. Am. J. Immunol. 2007, 3, 52–55. [Google Scholar] [CrossRef]
- Mousavi, M.; Jonsson, P.; Antti, H.; Adolfsson, R.; Nordin, A.; Bergdahl, J.; Eriksson, K.; Moritz, T.; Nilsson, L.-G.; Nyberg, L. Serum metabolomic biomarkers of dementia. Dement. Geriatr. Cogn. Disord. Extra 2014, 4, 252–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragusa, M.; Bosco, P.; Tamburello, L.; Barbagallo, C.; Condorelli, A.G.; Tornitore, M.; Spada, R.S.; Barbagallo, D.; Scalia, M.; Elia, M.; et al. miRNAs Plasma Profiles in Vascular Dementia: Biomolecular Data and Biomedical Implications. Front. Cell. Neurosci. 2016, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Altunoglu, E.; Guntas, G.; Erdenen, F.; Akkaya, E.; Topac, I.; Irmak, H.; Derici, H.; Yavuzer, H.; Gelisgen, R.; Uzun, H. Ischemia-modified albumin and advanced oxidation protein products as potential biomarkers of protein oxidation in Alzheimer’s disease. Geriatr. Gerontol. Int. 2015, 15, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Mao, P. Oxidative Stress and Its Clinical Applications in Dementia. J. Neurodegener. Dis. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Knaś, M.; Zendzian-Piotrowska, M.; Waszkiewicz, N.; Szulimowska, J.; Prokopiuk, S.; Waszkiel, D.; Car, H. Antioxidant profile of salivary glands in high fat diet-induced insulin resistance rats. Oral Dis. 2014, 20, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Mikoluc, B.; Pietrucha, B.; Heropolitanska-Pliszka, E.; Pac, M.; Motkowski, R.; Car, H. Oxidative stress, mitochondrial abnormalities and antioxidant defense in Ataxia-telangiectasia, Bloom syndrome and Nijmegen breakage syndrome. Redox Biol. 2017, 11, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-T.; Chang, W.-N.; Tsai, N.-W.; Huang, C.-C.; Kung, C.-T.; Su, Y.-J.; Lin, W.-C.; Cheng, B.-C.; Su, C.-M.; Chiang, Y.-F.; et al. The Roles of Biomarkers of Oxidative Stress and Antioxidant in Alzheimer’s Disease: A Systematic Review. BioMed Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, K.; Li, L.; Hart, M.; Lindsey, J.R. Accumulation of amyloid-β protein in exocrine glands of transgenic mice overexpressing a carboxyl terminal portion of amyloid protein precursor. Int. J. Exp. Pathol. 2000, 81, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Pareja, F.; Antequera, D.; Vargas, T.; Molina, J.A.; Carro, E. Saliva levels of Abeta1–42 as potential biomarker of Alzheimer’s disease: A pilot study. BMC Neurol. 2010, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Figueira, J.; Jonsson, P.; Nordin Adolfsson, A.; Adolfsson, R.; Nyberg, L.; Öhman, A. NMR analysis of the human saliva metabolome distinguishes dementia patients from matched controls. Mol. BioSyst. 2016, 12, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A.; DeCarli, C.; Friedland, R.P.; Baum, B.J. Diminished submandibular salivary flow in dementia of the Alzheimer type. J. Gerontol. 1990, 45, M61–M66. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej, U.; Maciejczyk, M.; Miąsko, A.; Matczuk, J.; Knaś, M.; Żukowski, P.; Żendzian-Piotrowska, M.; Borys, J.; Zalewska, A. Oxidative Modification in the Salivary Glands of High Fat-Diet Induced Insulin Resistant Rats. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Knaś, M.; Maciejczyk, M.; Sawicka, K.; Hady, H.R.; Niczyporuk, M.; Ładny, J.R.; Matczuk, J.; Waszkiel, D.; Żendzian-Piotrowska, M.; Zalewska, A. Impact of morbid obesity and bariatric surgery on antioxidant/oxidant balance of the unstimulated and stimulated human saliva. J. Oral Pathol. Med. 2016, 45, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Al-Maskari, A.Y.; Al-Maskari, M.Y.; Al-Sudairy, S. Oral Manifestations and Complications of Diabetes Mellitus: A review. Sultan Qaboos Univ. Med. J. 2011, 11, 179–186. [Google Scholar] [PubMed]
- Zalewska, A.; Knaś, M.; Maciejczyk, M.; Waszkiewicz, N.; Klimiuk, A.; Choromańska, M.; Matczuk, J.; Waszkiel, D.; Car, H. Antioxidant profile, carbonyl and lipid oxidation markers in the parotid and submandibular glands of rats in different periods of streptozotocin induced diabetes. Arch. Oral Biol. 2015, 60, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, A.; Knaś, M.; Waszkiewicz, N.; Waszkiel, D.; Sierakowski, S.; Zwierz, K. Rheumatoid arthritis patients with xerostomia have reduced production of key salivary constituents. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 483–490. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.M.; Lim, C.; Al-Hamzawi, A.; Alonso, J.; Bruffaerts, R.; Caldas-de-Almeida, J.M.; Florescu, S.; de Girolamo, G.; Hu, C.; de Jonge, P.; et al. Association of Mental Disorders with Subsequent Chronic Physical Conditions. JAMA Psychiatry 2016, 73, 150. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe-Descamps, M.; Labouré, H.; Prot, A.; Septier, C.; Tournier, C.; Feron, G.; Sulmont-Rossé, C. Salivary Flow Decreases in Healthy Elderly People Independently of Dental Status and Drug Intake. J. Texture Stud. 2016, 47, 353–360. [Google Scholar] [CrossRef]
- Nagler, R.M. Salivary glands and the aging process: Mechanistic aspects, health-status and medicinal-efficacy monitoring. Biogerontology 2004, 5, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Kesarwala, A.; Krishna, M.; Mitchell, J. Oxidative stress in oral diseases. Oral Dis. 2016, 22, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Romani, A.; Seripa, D.; Cremonini, E.; Bosi, C.; Magon, S.; Bergamini, C.M.; Valacchi, G.; Pilotto, A.; Zuliani, G. Systemic oxidative stress and conversion to dementia of elderly patients with mild cognitive impairment. BioMed Res. Int. 2014, 2014, 309507. [Google Scholar] [CrossRef] [PubMed]
- Schrag, M.; Mueller, C.; Zabel, M.; Crofton, A.; Kirsch, W.M.; Ghribi, O.; Squitti, R.; Perry, G. Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: A meta-analysis. Neurobiol. Dis. 2013, 59, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Padurariu, M.; Ciobica, A.; Lefter, R.; Serban, I.L.; Stefanescu, C.; Chirita, R. The oxidative stress hypothesis in Alzheimer’s disease. Psychiatr. Danub. 2013, 25, 401–409. [Google Scholar] [PubMed]
- Keller, J.N. Interplay between oxidative damage, protein synthesis, and protein degradation in Alzheimer’s disease. J. Biomed. Biotechnol. 2006, 2006, 12129. [Google Scholar] [CrossRef] [PubMed]
- Knaś, M.; Maciejczyk, M.; Daniszewska, I.; Klimiuk, A.; Matczuk, J.; Kołodziej, U.; Waszkiel, D.; Ładny, J.R.; Żendzian-Piotrowska, M.; Zalewska, A. Oxidative Damage to the Salivary Glands of Rats with Streptozotocin-Induced Diabetes-Temporal Study: Oxidative Stress and Diabetic Salivary Glands. J. Diabetes Res. 2016, 2016, 4583742. [Google Scholar] [CrossRef] [PubMed]
- Ryo, K.; Yamada, H.; Nakagawa, Y.; Tai, Y.; Obara, K.; Inoue, H.; Mishima, K.; Saito, I. Possible involvement of oxidative stress in salivary gland of patients with Sjogren’s syndrome. Pathobiology 2006, 73, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Nagler, R.M.; Salameh, F.; Reznick, A.Z.; Livshits, V.; Nahir, A.M. Salivary gland involvement in rheumatoid arthritis and its relationship to induced oxidative stress. Rheumatology 2003, 42, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C. Salivary flow patterns and the health of hard and soft oral tissues. J. Am. Dent. Assoc. 2008, 139, 18S–24S. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej, U.; Maciejczyk, M.; Niklińska, W.; Waszkiel, D.; Żendzian-Piotrowska, M.; Żukowski, P.; Zalewska, A. Chronic high-protein diet induces oxidative stress and alters the salivary gland function in rats. Arch. Oral Biol. 2017, 84, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Sreebny, L.M.; Zhu, W.X. The use of whole saliva in the differential diagnosis of Sjögren’s syndrome. Adv. Dent. Res. 1996, 10, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.J.; Ship, J.A.; Wu, A.J. Salivary gland function and aging: A model for studying the interaction of aging and systemic disease. Crit. Rev. Oral Biol. Med. 1992, 4, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Cassolato, S.F.; Turnbull, R.S. Xerostomia: Clinical Aspects and Treatment. Gerodontology 2003, 20, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, L.; Kamodyová, N.; Červenka, T.; Celec, P. Salivary markers of oxidative stress in oral diseases. Front. Cell. Infect. Microbiol. 2015, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Hegab, Z.; Gibbons, S.; Neyses, L.; Mamas, M.A. Role of advanced glycation end products in cardiovascular disease. World J. Cardiol. 2012, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- Borys, J.; Maciejczyk, M.; Krȩtowski, A.J.; Antonowicz, B.; Ratajczak-Wrona, W.; Jabłońska, E.; Załęski, P.; Waszkiel, D.; Ładny, J.R.; Żukowski, P.; et al. The Redox Balance in Erythrocytes, Plasma, and Periosteum of Patients with Titanium Fixation of the Jaw. Front. Physiol. 2017, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Oral health Surveys: Basic Methods, 4th ed.; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Lobene, R.R.; Mankodi, S.M.; Ciancio, S.G.; Lamm, R.A.; Charles, C.H.; Ross, N.M. Correlations among Gingival Indices: A methodology study. J. Periodontol. 1989, 60, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Mansson-Rahemtulla, B.; Baldone, D.C.; Pruitt, K.M.; Rahemtulla, F. Specific assays for peroxidases in human saliva. Arch. Oral Biol. 1986, 31, 661–668. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M. The Bicinchoninic Acid (BCA) Assay for Protein Quantitation. In Basic Protein and Peptide Protocols; Humana Press: Totowa, NJ, USA, 1994; Volume 32, pp. 5–8. [Google Scholar]
- Kalousová, M.; Skrha, J.; Zima, T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol. Res. 2002, 51, 597–604. [Google Scholar] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Alzheimer’s Dementia (AD) | Vascular Dementia (VaD) | Mixed Dementia (MxD) | All Patients with Dementia | Control Group | |
|---|---|---|---|---|---|---|
| Sex | Male n | 10 | 8 | 7 | 25 | 25 |
| Female n | 14 | 22 | 19 | 55 | 55 | |
| Age mean (SEM) | 77.87 (1.427) | 80.63 (1.107) | 81.81 (1.325) | 80.12 (0.7455) | 80.12 (0.7455) | |
| Education in years mean (SEM) | 7.636 (0.8337) | 7.48 (0.5806) | 5.6 (0.7158) | 7.081 (0.4223) | 7.52 (0.315) | |
| MMSE score mean (SEM) | 13.14 (0.5516) * | 13.31 (0.5089) * | 14 (0.6211) * | 13.45 (0.3201) * | 27.42 (0.3465) * | |
| Hypertension n | 8 | 11 | 18 | 37 | 34 | |
| Coronary heart disease n | 4 | 5 | 6 | 15 | 14 | |
| Atherosclerosis n | 3 | 3 | 2 | 8 | 9 | |
| Osteoporosis n | 1 | 1 | 0 | 2 | 3 | |
| Polypharmacy | No drugs n | 4 | 1 | 8 | 13 | 12 |
| Non-polypharmacy (1–5 drugs) n | 14 | 18 | 13 | 45 | 43 | |
| Polypharmacy (>5 drugs) n | 6 | 11 | 5 | 22 | 20 | |
| Characteristic | Alzheimer’s Dementia (AD) n = 24 | Vascular Dementia (VaD) n = 30 | Mixed Dementia (MxD) n = 26 | All Patients with Dementia n = 80 | Control Group n = 80 |
|---|---|---|---|---|---|
| DMFT mean (SEM) | 28.00 | 30.09 | 29.96 | 29.50 | 29.32 |
| (1.62) | (0.6885) | (0.8902) | (0.5907) | (0.5295) | |
| PBI mean (SEM) | 1.554 | 1.973 | 1.520 | 1.763 | 1.087 |
| (0.341) | (0.239) | (0.2687) | (0.1619) | (0.06926) | |
| GI mean (SEM) | 1.750 | 2.175 | 2.063 | 2.026 | 1.582 |
| (0.3493) | (0.1377) | (0.1475) | (0.1191) | (0.06122) | |
| CR mean (SEM) | 0.1875 | 0.5957 | 0.1304 | 0.3248 | 0.21 |
| (0.1008) | (0.3765) | (0.09544) | (0.1827) | (0.128) |
| Parameter | Dementia n = 80 | Control Group n = 80 |
|---|---|---|
| pH NWS | 7.038 | 7.649 |
| (0.09722) | (0.05961) * | |
| pH SWS | 6.672 | 7.328 |
| (0.1285) | (0.1007) * |
| Parameter | AUC | Cutt-Off | Sensitivity | Specificity | ||||
|---|---|---|---|---|---|---|---|---|
| NWS | SWS | NWS | SWS | NWS | SWS | NWS | SWS | |
| Non-enzymatic and Enzymatic Antioxidants | ||||||||
| UA | 0.6039 (0.07348) | 0.6243 (0.07156) | >18.12 | <25.45 | 57.58 | 58.06 | 57.58 | 58.06 |
| CAT | 0.7495 (0.05887) | 0.7758 (0.05452) | >3.233 | >3.487 | 64.86 | 70.27 | 63.33 | 67.65 |
| Px | 0.8342 (0.04984) | 0.8728 (0.04459) | >2.555 | >2.574 | 72.97 | 83.78 | 73.33 | 82.35 |
| SOD | 0.6343 (0.06919) | 0.5686 (0.06920) | >25.00 | >10.71 | 60 | 55.56 | 56.67 | 55.88 |
| Total Antioxidant/Oxidant Status | ||||||||
| TAC | 0.737 (0.06168) | 0.8358 (0.05134) | >0.1586 | >1.514 | 66.67 | 80.56 | 66.67 | 79.41 |
| TOS | 0.9213 (0.03193) | 0.7712 (0.05522) | <1.837 | <1.554 | 80.56 | 63.89 | 80 | 64.71 |
| OSI | 0.9080 (0.03687) | 0.9044 (0.03488) | <1.064 | <0.1259 | 80.56 | 77.78 | 79.31 | 76.47 |
| Oxidative Damage Products | ||||||||
| AGE | 0.8472 (0.04834) | 0.7035 (0.06587) | <1.451 | <1.258 | 75.68 | 64.86 | 75.86 | 64.71 |
| AOPP | 0.8135 (0.05092) | 1 (0.0) | <31.24 | <43.49 | 70.27 | 100 | 70 | 100 |
| 8-isop | 0.9286 (0.06883) | 0.9231 (0.05504) | <18.67 | <8.236 | 92.86 | 84.62 | 94.12 | 87.5 |
| 8-OHdG | 0.7559 (0.09378) | 0.9899 (0.01631) | <1361 | <1567 | 70 | 88.89 | 70.59 | 90.91 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choromańska, M.; Klimiuk, A.; Kostecka-Sochoń, P.; Wilczyńska, K.; Kwiatkowski, M.; Okuniewska, N.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M. Antioxidant Defence, Oxidative Stress and Oxidative Damage in Saliva, Plasma and Erythrocytes of Dementia Patients. Can Salivary AGE be a Marker of Dementia? Int. J. Mol. Sci. 2017, 18, 2205. https://doi.org/10.3390/ijms18102205
Choromańska M, Klimiuk A, Kostecka-Sochoń P, Wilczyńska K, Kwiatkowski M, Okuniewska N, Waszkiewicz N, Zalewska A, Maciejczyk M. Antioxidant Defence, Oxidative Stress and Oxidative Damage in Saliva, Plasma and Erythrocytes of Dementia Patients. Can Salivary AGE be a Marker of Dementia? International Journal of Molecular Sciences. 2017; 18(10):2205. https://doi.org/10.3390/ijms18102205
Chicago/Turabian StyleChoromańska, Magdalena, Anna Klimiuk, Paula Kostecka-Sochoń, Karolina Wilczyńska, Mikołaj Kwiatkowski, Natalia Okuniewska, Napoleon Waszkiewicz, Anna Zalewska, and Mateusz Maciejczyk. 2017. "Antioxidant Defence, Oxidative Stress and Oxidative Damage in Saliva, Plasma and Erythrocytes of Dementia Patients. Can Salivary AGE be a Marker of Dementia?" International Journal of Molecular Sciences 18, no. 10: 2205. https://doi.org/10.3390/ijms18102205