Molecular Aspects of Circadian Pharmacology and Relevance for Cancer Chronotherapy
Abstract
:1. Introduction
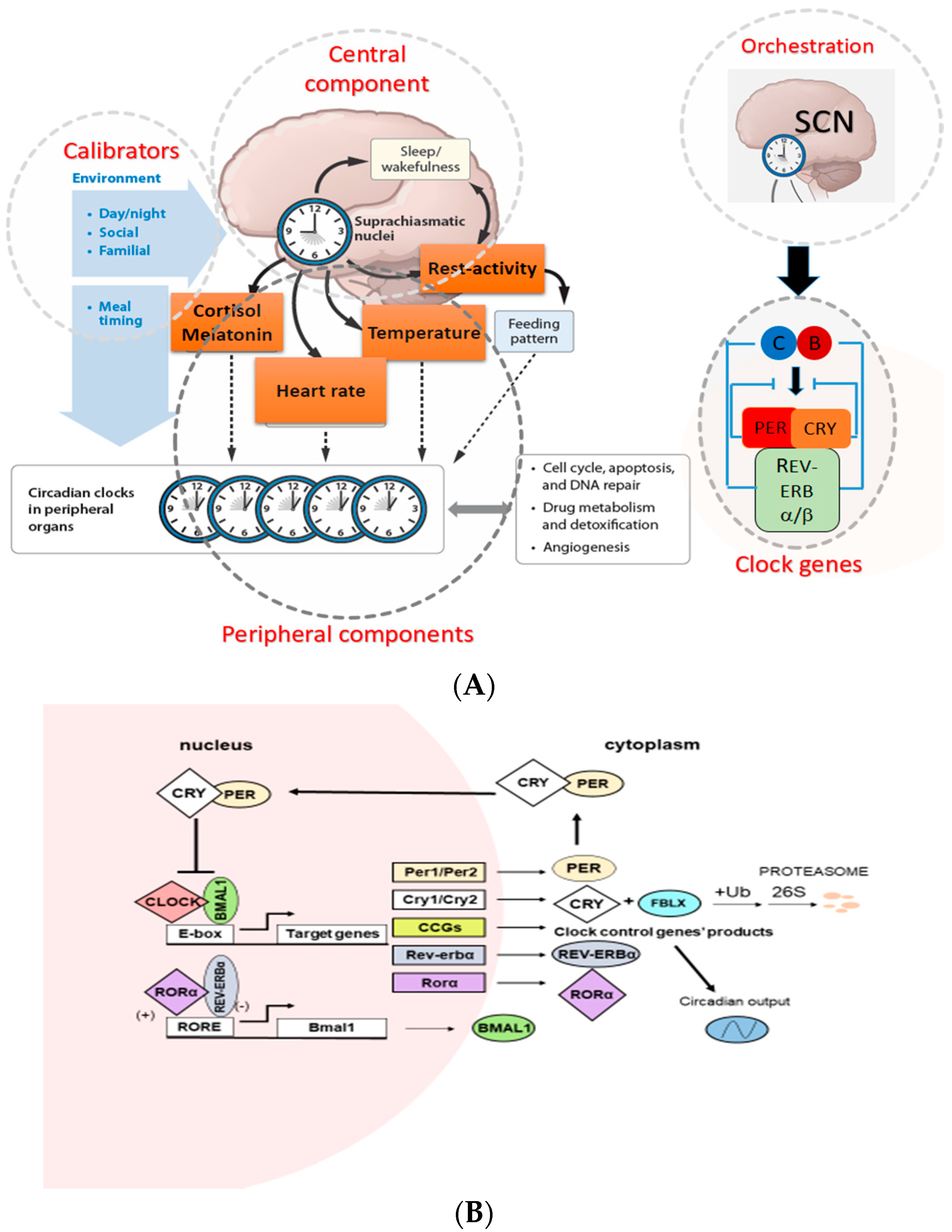
2. The Circadian Timing System (CTS) and Molecular Mechanisms of Circadian Clock
3. Experimental Chronopharmacology of Anticancer Drugs
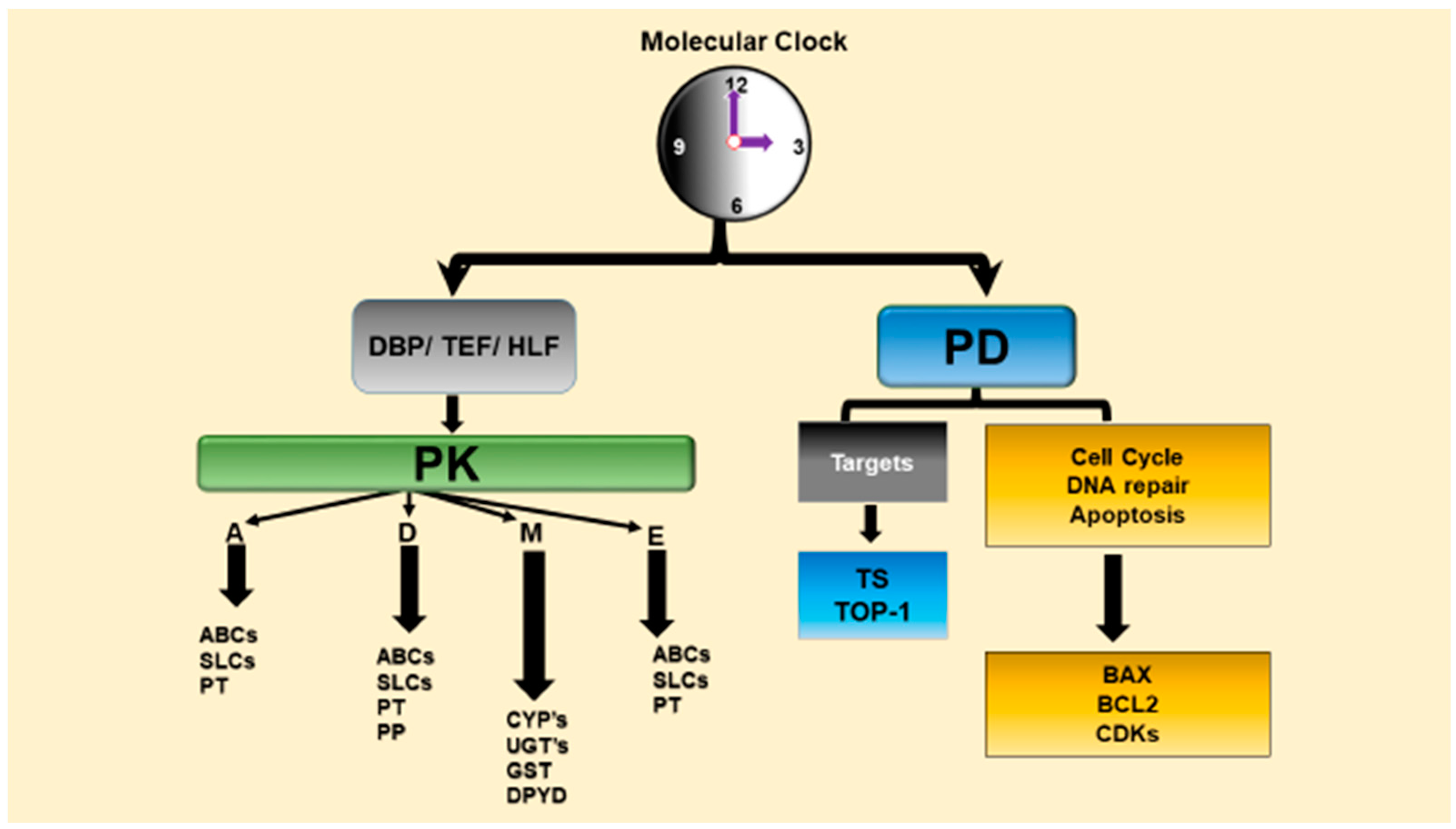
3.1. Interaction of Circadian Clock Network with Drug Metabolism, Detoxification and Transport
3.2. Relevance of Circadian Rhythms for Chronopharmacodynamics of Anticancer Agents
3.3. Relevance of Circadian Rhythms for Chronotoxicity of Anticancer Agents
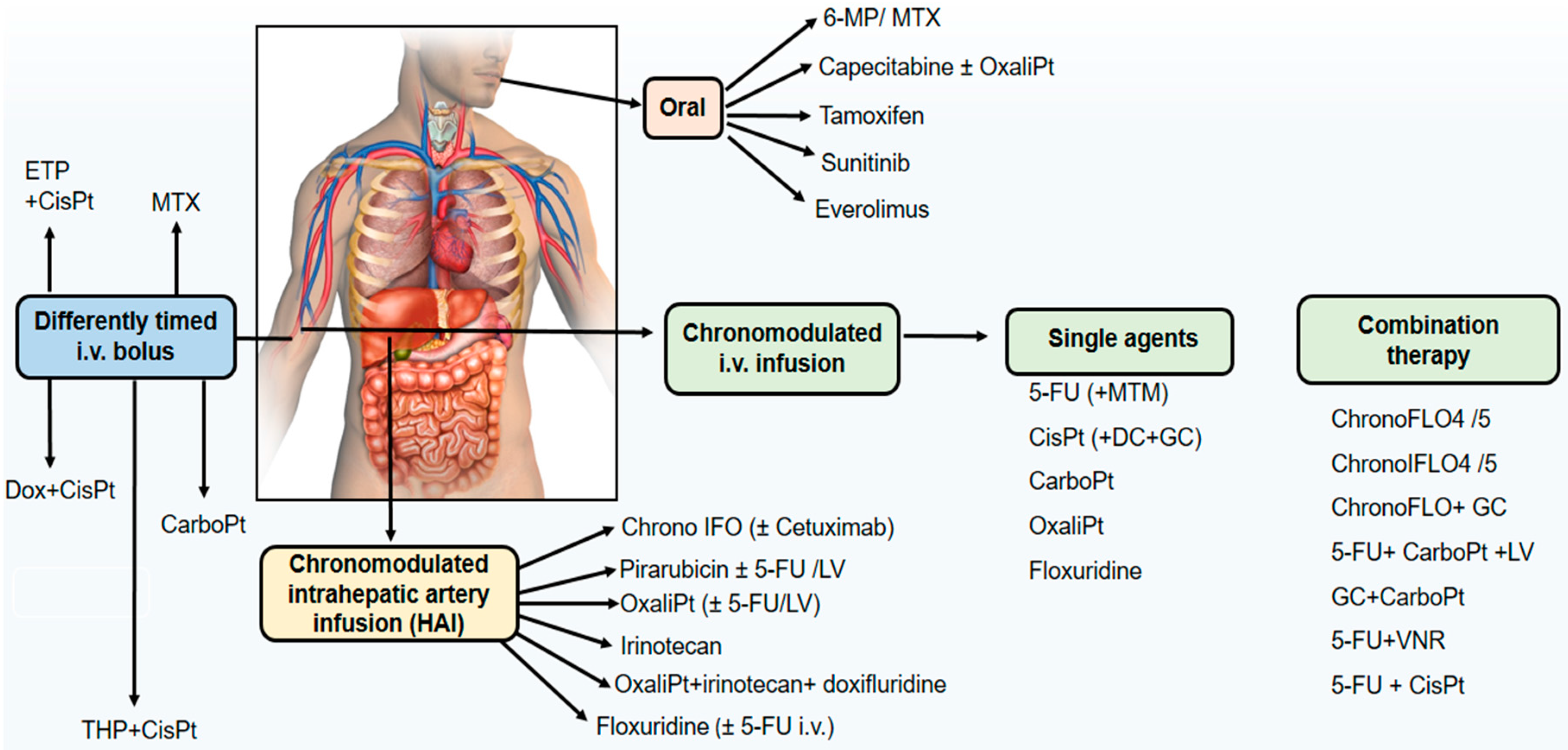
4. Chronotherapeutics in Cancer Chemotherapy
5. Chronopharmaceutics and Innovative Chrono-Drug Delivery Systems in Cancer Treatment
6. Conclusions and Perspectives
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| ABC | ATP-Binding Cassette |
| AGT | O6-alkylguanine transferase |
| Ahr | Aryl hydrocarbon receptor |
| ALAS1 | Aminolevulinic acid synthase |
| ASPS | Advanced sleep phase syndrome |
| ATM | Ataxia telangiectasia mutated |
| BAX | BCL-2-associated X protein |
| BMAL1 | Brain and muscle aryl hydrocarbon receptor nuclear translocator 1 |
| CAR | Constitutive androstan receptor |
| CCGs | Clock controlled genes |
| CDC2 | Cyclin-dependent kinase 2 |
| CES | Carboxylesterase |
| ChronoDDS | Chrono-drug delivery system |
| ChronoPD | Chronopharmacodynamics |
| ChronoPK | Chronopharmacokinetics |
| CKIε | Casein kinase Iε |
| CLOCK | Circadian locomotor output cycles kaput |
| Cmax | Maximum plasma concentration |
| Cry | Cryptochrome |
| CTS | Circadian timing system |
| Cyc | Cyclin |
| CYCLOPS | Cyclic ordering by periodic structure |
| CYP450 | Cytochrome P-450 |
| DBP | Albumin D-site-binding protein |
| DDS | Drug delivery systems |
| DPYD | Dihydropyrimidine dehydrogenase |
| DSPS | Delayed sleep phase syndrome |
| EGFR | Epidermal growth factor receptor |
| ERK/MAPK | Extracellular signal-regulated kinase/mitogen-activated protein kinase |
| FASPS | Familial advanced sleep phase syndrome |
| GSH | Reduced glutathione |
| GST | Glutathione-S-transferases |
| HLF | Hepatic leukemia factor |
| Hsd3b | 3β-hydroxysteroid dehydrogenase |
| IFN-β | Interferon-β |
| ISGF | Interferon-stimulated gene factor |
| MDR1 | Multidrug resistance protein 1 (ABCB1, P-glycoprotein, P-gp in human) |
| Mdr1 | Multidrug resistance protein 1 (abcb1a/b mdr1a/b, P-gp in rodents) |
| MR | Modified Release |
| Mrp | Multidrug resistance-associated protein (Abcc) |
| mTOR | Mammalian target of rapamycin |
| NAT | N-acetyl transferases |
| NFIL3/E4BP4 | Nuclear factor, interleukin 3 regulated |
| NPAS2 | Neuronal PAS-domain protein 2 |
| Oatp | Organic anion transporter polypeptide |
| Oct | Organic cation transporter |
| Octn1 | Oct novel type 1 |
| p21WAF1 | p21 wild-type p53 fragment 1 |
| PARbZip | Proline-acidic amino acid-rich basic leucine zipper |
| PD | Pharmacodynamics |
| PDGF | Platelet-derived growth factor |
| Per | Period |
| P-gp | P-glycoprotein |
| PI3K/AKT | Phosphatidylinositol-3-kinase/protein kinase B |
| PK | Pharmacokinetics |
| POR | P450 oxidoreductase |
| PPAR-α | Peroxisome proliferator activated receptor-alpha |
| PTX | Paclitaxel |
| PTX-NPs | PTX-loaded polymeric nanoparticles |
| PXR | Pregnane X receptor |
| REV-ERB/NR1D | Reverse strand of ERB |
| RORs | Retinoic acid-receptor-related orphan receptor |
| SCN | Suprachiasmatic nuclei |
| SLC | Solute carrier |
| SULT | Sulfotransferases |
| TEF | Thyrotroph embryonic factor |
| Top1 | Topoisomerase 1 |
| TS | Thymidylate synthase |
| UDP | Uridine diphosphate |
| UGT | Glucuronosyl transferase |
| VEGF | Vascular endothelial growth factor |
| WBC | White blood cells |
References
- Smolensky, M.H.; Peppas, N.A. Chronobiology, drug delivery, and chronotherapeutics. Adv. Drug Deliv. Rev. 2007, 59, 828–851. [Google Scholar] [CrossRef] [PubMed]
- Schulz, P.; Steimer, T. Neurobiology of circadian systems. CNS Drugs 2009, 23 (Suppl S2), 3–13. [Google Scholar] [CrossRef] [PubMed]
- Baggs, J.E.; Hogenesch, J.B. Genomics and systems approaches in the mammalian circadian clock. Curr. Opin. Genet. Dev. 2010, 20, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Gachon, F.; Nagoshi, E.; Brown, S.A.; Ripperger, J.; Schibler, U. The mammalian circadian timing system: From gene expression to physiology. Chromosoma 2004, 113, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Green, C.B.; Takahashi, J.S.; Bass, J. The meter of metabolism. Cell 2008, 134, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.; Husse, J.; Oster, H. A time to fast, a time to feast: The crosstalk between metabolism and the circadian clock. Mol. Cells 2009, 28, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.L.; Storm, D.R. Circadian rhythms and memory: Not so simple as cogs and gears. EMBO Rep. 2009, 10, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Franken, P.; Dijk, D.J. Circadian clock genes and sleep homeostasis. Eur. J. Neurosci. 2009, 29, 1820–1829. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, C.P.; Hastings, M.H. Circadian clocks: Genes, sleep, and cognition. Trends Cogn. Sci. 2010, 14, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Toh, K.L.; Jones, C.R.; He, Y.; Eide, E.J.; Hinz, W.A.; Virshup, D.M.; Ptacek, L.J.; Fu, Y.H. An hper2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 2001, 291, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Vanselow, K.; Vanselow, J.T.; Westermark, P.O.; Reischl, S.; Maier, B.; Korte, T.; Herrmann, A.; Herzel, H.; Schlosser, A.; Kramer, A. Differential effects of per2 phosphorylation: Molecular basis for the human familial advanced sleep phase syndrome (fasps). Genes Dev. 2006, 20, 2660–2672. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Toh, K.L.; Jones, C.R.; Shin, J.Y.; Fu, Y.H.; Ptacek, L.J. Modeling of a human circadian mutation yields insights into clock regulation by per2. Cell 2007, 128, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, E.D.; Czeisler, C.A.; Coleman, R.M.; Spielman, A.J.; Zimmerman, J.C.; Dement, W.; Richardson, G.; Pollak, C.P. Delayed sleep phase syndrome. A chronobiological disorder with sleep-onset insomnia. Arch. Gen. Psychiatry 1981, 38, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Uchiyama, M.; Kajimura, N.; Mishima, K.; Inoue, Y.; Kamei, Y.; Kitajima, T.; Shibui, K.; Katoh, M.; Watanabe, T.; et al. A missense variation in human casein kinase i epsilon gene that induces functional alteration and shows an inverse association with circadian rhythm sleep disorders. Neuropsychopharmacology 2004, 29, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Katzenberg, D.; Young, T.; Finn, L.; Lin, L.; King, D.P.; Takahashi, J.S.; Mignot, E. A clock polymorphism associated with human diurnal preference. Sleep 1998, 21, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Lamont, E.W.; Coutu, D.L.; Cermakian, N.; Boivin, D.B. Circadian rhythms and clock genes in psychotic disorders. Isr. J. Psychiatry Relat. Sci. 2010, 47, 27–35. [Google Scholar] [PubMed]
- Lamont, E.W.; Legault-Coutu, D.; Cermakian, N.; Boivin, D.B. The role of circadian clock genes in mental disorders. Dialogues Clin. Neurosci. 2007, 9, 333–342. [Google Scholar] [PubMed]
- Mansour, H.A.; Wood, J.; Logue, T.; Chowdari, K.V.; Dayal, M.; Kupfer, D.J.; Monk, T.H.; Devlin, B.; Nimgaonkar, V.L. Association study of eight circadian genes with bipolar i disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav. 2006, 5, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Moons, T.; Claes, S.; Martens, G.J.; Peuskens, J.; Van Loo, K.M.; Van Schijndel, J.E.; De Hert, M.; van Winkel, R. Clock genes and body composition in patients with schizophrenia under treatment with antipsychotic drugs. Schizophr. Res. 2011, 125, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Soria, V.; Martinez-Amoros, E.; Escaramis, G.; Valero, J.; Perez-Egea, R.; Garcia, C.; Gutierrez-Zotes, A.; Puigdemont, D.; Bayes, M.; Crespo, J.M.; et al. Differential association of circadian genes with mood disorders: Cry1 and npas2 are associated with unipolar major depression and clock and vip with bipolar disorder. Neuropsychopharmacology 2010, 35, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Dallaspezia, S.; Colombo, C.; Pirovano, A.; Marino, E.; Smeraldi, E. A length polymorphism in the circadian clock gene per3 influences age at onset of bipolar disorder. Neurosci. Lett. 2008, 445, 184–187. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.J.; Welsh, D.K. Cellular circadian clocks in mood disorders. J. Biol. Rhythms 2012, 27, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Kettner, N.M. The circadian clock in cancer development and therapy. Prog. Mol. Biol. Transl. Sci. 2013, 119, 221–282. [Google Scholar] [PubMed]
- Zee, P.C.; Attarian, H.; Videnovic, A. Circadian rhythm abnormalities. Continuum (Minneap Minn) 2013, 19, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Fuhr, L.; Abreu, M.; Pett, P.; Relogio, A. Circadian systems biology: When time matters. Comput. Struct. Biotechnol. J. 2015, 13, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Mormont, M.C.; Waterhouse, J.; Bleuzen, P.; Giacchetti, S.; Jami, A.; Bogdan, A.; Lellouch, J.; Misset, J.L.; Touitou, Y.; Levi, F. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin. Cancer Res. 2000, 6, 3038–3045. [Google Scholar] [PubMed]
- Ortiz-Tudela, E.; Iurisci, I.; Beau, J.; Karaboue, A.; Moreau, T.; Rol, M.A.; Madrid, J.A.; Levi, F.; Innominato, P.F. The circadian rest-activity rhythm, a potential safety pharmacology endpoint of cancer chemotherapy. Int. J. Cancer 2014, 134, 2717–2725. [Google Scholar] [CrossRef] [PubMed]
- Innominato, P.F.; Roche, V.P.; Palesh, O.G.; Ulusakarya, A.; Spiegel, D.; Levi, F.A. The circadian timing system in clinical oncology. Ann. Med. 2014, 46, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Okyar, A.; Dulong, S.; Innominato, P.F.; Clairambault, J. Circadian timing in cancer treatments. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 377–421. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Okyar, A. Circadian clocks and drug delivery systems: Impact and opportunities in chronotherapeutics. Expert Opin. Drug Deliv. 2011, 8, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Okyar, A.; Piccolo, E.; Ahowesso, C.; Filipski, E.; Hossard, V.; Guettier, C.; La Sorda, R.; Tinari, N.; Iacobelli, S.; Levi, F. Strain- and sex-dependent circadian changes in abcc2 transporter expression: Implications for irinotecan chronotolerance in mouse ileum. PLoS ONE 2011, 6, e20393. [Google Scholar] [CrossRef] [PubMed]
- Dallmann, R.; Okyar, A.; Levi, F. Dosing-time makes the poison: Circadian regulation and pharmacotherapy. Trends Mol. Med. 2016, 22, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Filipski, E.; Berland, E.; Ozturk, N.; Guettier, C.; van der Horst, G.T.; Levi, F.; Okyar, A. Optimization of irinotecan chronotherapy with p-glycoprotein inhibition. Toxicol. Appl. Pharmacol. 2014, 274, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Schibler, U. Circadian rhythms: Mechanisms and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 593–628. [Google Scholar] [CrossRef] [PubMed]
- Gachon, F.; Firsov, D. The role of circadian timing system on drug metabolism and detoxification. Expert Opin. Drug Metab. Toxicol. 2011, 7, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Zarogoulidis, P.; Trakada, G.; Zarogoulidis, K. A chrono-target chemotherapy treatment model for lung cancer treatment. Ther. Deliv. 2013, 4, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Sancar, A.; Lindsey-Boltz, L.A.; Gaddameedhi, S.; Selby, C.P.; Ye, R.; Chiou, Y.Y.; Kemp, M.G.; Hu, J.; Lee, J.H.; Ozturk, N. Circadian clock, cancer, and chemotherapy. Biochemistry 2015, 54, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Ballesta, A.; Innominato, P.F.; Dallmann, R.; Rand, D.A.; Levi, F.A. Systems chronotherapeutics. Pharmacol. Rev. 2017, 69, 161–199. [Google Scholar] [CrossRef] [PubMed]
- Bouchahda, M.; Macarulla, T.; Liedo, G.; Levi, F.; Elez, M.E.; Paule, B.; Karaboue, A.; Artru, P.; Tabernero, J.; Machover, D.; et al. Feasibility of cetuximab given with a simplified schedule every 2 weeks in advanced colorectal cancer: A multicenter, retrospective analysis. Med. Oncol. 2011, 28 (Suppl S1), S253–S258. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Focan, C.; Karaboue, A.; de la Valette, V.; Focan-Henrard, D.; Baron, B.; Kreutz, F.; Giacchetti, S. Implications of circadian clocks for the rhythmic delivery of cancer therapeutics. Adv. Drug Deliv. Rev. 2007, 59, 1015–1035. [Google Scholar] [CrossRef] [PubMed]
- Gekakis, N.; Staknis, D.; Nguyen, H.B.; Davis, F.C.; Wilsbacher, L.D.; King, D.P.; Takahashi, J.S.; Weitz, C.J. Role of the clock protein in the mammalian circadian mechanism. Science 1998, 280, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Hogenesch, J.B.; Gu, Y.Z.; Jain, S.; Bradfield, C.A. The basic-helix-loop-helix-pas orphan mop3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl. Acad. Sci. USA 1998, 95, 5474–5479. [Google Scholar] [CrossRef] [PubMed]
- King, D.P.; Zhao, Y.; Sangoram, A.M.; Wilsbacher, L.D.; Tanaka, M.; Antoch, M.P.; Steeves, T.D.; Vitaterna, M.H.; Kornhauser, J.M.; Lowrey, P.L.; et al. Positional cloning of the mouse circadian clock gene. Cell 1997, 89, 641–653. [Google Scholar] [CrossRef]
- Kume, K.; Zylka, M.J.; Sriram, S.; Shearman, L.P.; Weaver, D.R.; Jin, X.; Maywood, E.S.; Hastings, M.H.; Reppert, S.M. Mcry1 and mcry2 are essential components of the negative limb of the circadian clock feedback loop. Cell 1999, 98, 193–205. [Google Scholar] [CrossRef]
- Shearman, L.P.; Sriram, S.; Weaver, D.R.; Maywood, E.S.; Chaves, I.; Zheng, B.; Kume, K.; Lee, C.C.; van der Horst, G.T.; Hastings, M.H.; et al. Interacting molecular loops in the mammalian circadian clock. Science 2000, 288, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, G.T.; Muijtjens, M.; Kobayashi, K.; Takano, R.; Kanno, S.; Takao, M.; de Wit, J.; Verkerk, A.; Eker, A.P.; van Leenen, D.; et al. Mammalian cry1 and cry2 are essential for maintenance of circadian rhythms. Nature 1999, 398, 627–630. [Google Scholar] [PubMed]
- Vielhaber, E.L.; Duricka, D.; Ullman, K.S.; Virshup, D.M. Nuclear export of mammalian period proteins. J. Biol. Chem. 2001, 276, 45921–45927. [Google Scholar] [CrossRef] [PubMed]
- Ukai-Tadenuma, M.; Kasukawa, T.; Ueda, H.R. Proof-by-synthesis of the transcriptional logic of mammalian circadian clocks. Nat. Cell Biol. 2008, 10, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Kornmann, B.; Schaad, O.; Bujard, H.; Takahashi, J.S.; Schibler, U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007, 5, e34. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Molecular architecture of the circadian clock in mammals. In A time for Metabolism and Hormones; Sassone-Corsi, P., Christen, Y., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Anafi, R.C.; Lee, Y.; Sato, T.K.; Venkataraman, A.; Ramanathan, C.; Kavakli, I.H.; Hughes, M.E.; Baggs, J.E.; Growe, J.; Liu, A.C.; et al. Machine learning helps identify chrono as a circadian clock component. PLoS Biol. 2014, 12, e1001840. [Google Scholar] [CrossRef] [PubMed]
- Kavakli, I.H.; Baris, I.; Tardu, M.; Gul, S.; Oner, H.; Cal, S.; Bulut, S.; Yarparvar, D.; Berkel, C.; Ustaoglu, P.; et al. The photolyase/cryptochrome family of proteins as DNA repair enzymes and transcriptional repressors. Photochem. Photobiol. 2017, 93, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Erkekoglu, P.; Baydar, T. Chronopharmacodynamics of drugs in toxicological aspects: A short review for clinical pharmacists and pharmacy practitioners. J. Res. Pharm. Pract. 2012, 1, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Tahara, Y.; Shibata, S. Chrono-biology, chrono-pharmacology, and chrono-nutrition. J. Pharmacol. Sci. 2014, 124, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Yamaguchi, S.; Mitsui, S.; Emi, A.; Shimoda, F.; Okamura, H. Control mechanism of the circadian clock for timing of cell division in vivo. Science 2003, 302, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Granda, T.G.; Liu, X.H.; Smaaland, R.; Cermakian, N.; Filipski, E.; Sassone-Corsi, P.; Levi, F. Circadian regulation of cell cycle and apoptosis proteins in mouse bone marrow and tumor. FASEB J. 2005, 19, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, F.C.; Rao, A.; Maguire, A. Circadian molecular clocks and cancer. Cancer Lett. 2014, 342, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Paschos, G.K.; Baggs, J.E.; Hogenesch, J.B.; FitzGerald, G.A. The role of clock genes in pharmacology. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 187–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.K.; Yeager, R.L.; Klaassen, C.D. Circadian expression profiles of drug-processing genes and transcription factors in mouse liver. Drug Metab. Dispos. 2009, 37, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Zmrzljak, U.P.; Rozman, D. Circadian regulation of the hepatic endobiotic and xenobitoic detoxification pathways: The time matters. Chem. Res. Toxicol. 2012, 25, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Meyer, U.A. Induction of drug metabolism: The role of nuclear receptors. Pharmacol. Rev. 2003, 55, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, C.Y.; Kong, A.N. Induction of phase i, ii and iii drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005, 28, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Gachon, F. Physiological function of parbzip circadian clock-controlled transcription factors. Ann. Med. 2007, 39, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Dallmann, R.; Brown, S.A.; Gachon, F. Chronopharmacology: New insights and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 339–361. [Google Scholar] [CrossRef] [PubMed]
- Gachon, F.; Olela, F.F.; Schaad, O.; Descombes, P.; Schibler, U. The circadian par-domain basic leucine zipper transcription factors dbp, tef, and hlf modulate basal and inducible xenobiotic detoxification. Cell Metab. 2006, 4, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Higashi, Y.; Matsunaga, N.; Koyanagi, S.; Ohdo, S. Circadian clock-controlled intestinal expression of the multidrug-resistance gene mdr1a in mice. Gastroenterology 2008, 135, 1636–1644.e3. [Google Scholar] [CrossRef] [PubMed]
- Kriebs, A.; Jordan, S.D.; Soto, E.; Henriksson, E.; Sandate, C.R.; Vaughan, M.E.; Chan, A.B.; Duglan, D.; Papp, S.J.; Huber, A.L.; et al. Circadian repressors cry1 and cry2 broadly interact with nuclear receptors and modulate transcriptional activity. Proc. Natl. Acad. Sci. USA 2017, 114, 8776–8781. [Google Scholar] [CrossRef] [PubMed]
- Gorbacheva, V.Y.; Kondratov, R.V.; Zhang, R.; Cherukuri, S.; Gudkov, A.V.; Takahashi, J.S.; Antoch, M.P. Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the clock/bmal1 transactivation complex. Proc. Natl. Acad. Sci. USA 2005, 102, 3407–3412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cho, H.; Yu, R.T.; Atkins, A.R.; Downes, M.; Evans, R.M. Nuclear receptors rock around the clock. EMBO Rep. 2014, 15, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Kang, H.S.; Jetten, A.M.; Xie, W. The emerging role of nuclear receptor roralpha and its crosstalk with lxr in xeno- and endobiotic gene regulation. Exp. Biol. Med. (Maywood) 2008, 233, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Downes, M.; Yu, R.T.; Bookout, A.L.; He, W.; Straume, M.; Mangelsdorf, D.J.; Evans, R.M. Nuclear receptor expression links the circadian clock to metabolism. Cell 2006, 126, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Claudel, T.; Cretenet, G.; Saumet, A.; Gachon, F. Crosstalk between xenobiotics metabolism and circadian clock. FEBS Lett. 2007, 581, 3626–3633. [Google Scholar] [CrossRef] [PubMed]
- Kanno, Y.; Otsuka, S.; Hiromasa, T.; Nakahama, T.; Inouye, Y. Diurnal difference in car mrna expression. Nucl. Recept. 2004, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.F.; Jin, T.; Xu, Y.; Zhang, D.; Wu, Q.; Zhang, Y.K.; Liu, J. Sex differences in the circadian variation of cytochrome p450 genes and corresponding nuclear receptors in mouse liver. Chronobiol. Int. 2013, 30, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, T.; Tomita, M.; Matsunaga, N.; Nakagawa, H.; Koyanagi, S.; Ohdo, S. Molecular basis for rhythmic expression of cyp3a4 in serum-shocked hepg2 cells. Pharmacogenet. Genom. 2007, 17, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Froy, O. Cytochrome p450 and the biological clock in mammals. Curr. Drug Metab. 2009, 10, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Lavery, D.J.; Lopez-Molina, L.; Margueron, R.; Fleury-Olela, F.; Conquet, F.; Schibler, U.; Bonfils, C. Circadian expression of the steroid 15 alpha-hydroxylase (cyp2a4) and coumarin 7-hydroxylase (cyp2a5) genes in mouse liver is regulated by the par leucine zipper transcription factor dbp. Mol. Cell Biol. 1999, 19, 6488–6499. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Cytochrome p450 and chemical toxicology. Chem. Res. Toxicol. 2008, 21, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome p450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Kosir, R.; Zmrzljak, U.P.; Bele, T.; Acimovic, J.; Perse, M.; Majdic, G.; Prehn, C.; Adamski, J.; Rozman, D. Circadian expression of steroidogenic cytochromes p450 in the mouse adrenal gland—Involvement of camp-responsive element modulator in epigenetic regulation of cyp17a1. FEBS J. 2012, 279, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, R.A.; Reddy, A.B.; Maywood, E.S.; Clayton, J.D.; King, V.M.; Smith, A.G.; Gant, T.W.; Hastings, M.H.; Kyriacou, C.P. Circadian cycling of the mouse liver transcriptome, as revealed by cdna microarray, is driven by the suprachiasmatic nucleus. Curr. Biol. 2002, 12, 540–550. [Google Scholar] [CrossRef]
- Tanimura, N.; Kusunose, N.; Matsunaga, N.; Koyanagi, S.; Ohdo, S. Aryl hydrocarbon receptor-mediated cyp1a1 expression is modulated in a clock-dependent circadian manner. Toxicology 2011, 290, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Kakan, X.; Chen, P.; Zhang, J. Clock gene mper2 functions in diurnal variation of acetaminophen induced hepatotoxicity in mice. Exp. Toxicol. Pathol. 2011, 63, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Hamamura, K.; Matsunaga, N.; Ikeda, E.; Kondo, H.; Ikeyama, H.; Tokushige, K.; Itcho, K.; Furuichi, Y.; Yoshida, Y.; Matsuda, M.; et al. Alterations of hepatic metabolism in chronic kidney disease via d-box-binding protein aggravate the renal dysfunction. J. Biol. Chem. 2016, 291, 4913–4927. [Google Scholar] [CrossRef] [PubMed]
- Seng, J.E.; Gandy, J.; Turturro, A.; Lipman, R.; Bronson, R.T.; Parkinson, A.; Johnson, W.; Hart, R.W.; Leakey, J.E. Effects of caloric restriction on expression of testicular cytochrome p450 enzymes associated with the metabolic activation of carcinogens. Arch. Biochem. Biophys. 1996, 335, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Damiola, F. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef] [PubMed]
- Carver, K.A.; Lourim, D.; Tryba, A.K.; Harder, D.R. Rhythmic expression of cytochrome p450 epoxygenases cyp4x1 and cyp2c11 in the rat brain and vasculature. Am. J. Physiol. Cell Physiol. 2014, 307, C989–C998. [Google Scholar] [CrossRef] [PubMed]
- Binkhorst, L.; Kloth, J.S.L.; de Wit, A.S.; de Bruijn, P.; Lam, M.H.; Chaves, I.; Burger, H.; van Alphen, R.J.; Hamberg, P.; van Schaik, R.H.N.; et al. Circadian variation in tamoxifen pharmacokinetics in mice and breast cancer patients. Breast Cancer Res. Treat. 2015, 152, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruckner, J.V.; Ramanathan, R.; Lee, K.M.; Muralidhara, S. Mechanisms of circadian rhythmicity of carbon tetrachloride hepatotoxicity. J. Pharmacol. Exp. Ther. 2002, 300, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Khemawoot, P.; Nishino, K.; Ishizaki, J.; Yokogawa, K.; Miyamoto, K. Circadian rhythm of cytochrome p4502e1 and its effect on disposition kinetics of chlorzoxazone in rats. Eur. J. Pharmacol. 2007, 574, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Nakamura, N.; Yoneda, N.; Qin, T.; Terazono, H.; To, H.; Higuchi, S.; Ohdo, S. Influence of feeding schedule on 24-h rhythm of hepatotoxicity induced by acetaminophen in mice. J. Pharmacol. Exp. Ther. 2004, 311, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Dutertre-Catella, H.; Radionoff, M.; Debray, M.; Benstaali, C.; Rat, P.; Thevenin, M.; Touitou, Y.; Warnet, J.M. Effect of age and photoperiodic conditions on metabolism and oxidative stress related markers at different circadian stages in rat liver and kidney. Life Sci. 2003, 73, 327–335. [Google Scholar] [CrossRef]
- Noshiro, M.; Kawamoto, T.; Furukawa, M.; Fujimoto, K.; Yoshida, Y.; Sasabe, E.; Tsutsumi, S.; Hamada, T.; Honma, S.; Honma, K.; et al. Rhythmic expression of dec1 and dec2 in peripheral tissues: Dec2 is a potent suppressor for hepatic cytochrome p450s opposing dbp. Genes Cells 2004, 9, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, C.M.; Shen, C.S.; Bilir, B.M.; Guibert, E.; Gumucio, J.J. Different hepatocytes express the cholesterol 7 alpha-hydroxylase gene during its circadian modulation in vivo. Hepatology 1995, 21, 1658–1667. [Google Scholar] [PubMed]
- Ishida, H.; Yamashita, C.; Kuruta, Y.; Yoshida, Y.; Noshiro, M. Insulin is a dominant suppressor of sterol 12 alpha-hydroxylase p450 (cyp8b) expression in rat liver: Possible role of insulin in circadian rhythm of cyp8b. J. Biochem. 2000, 127, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Nagatomo, J.; Setoguchi, Y.; Kuroki, N.; Higashi, S.; Setoguchi, T. Circadian rhythms of sterol 12alpha-hydroxylase, cholesterol 7alpha-hydroxylase and dbp involved in rat cholesterol catabolism. Biol. Chem. 2000, 381, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Gielen, J.; Van Cantfort, J.; Robaye, B.; Renson, J. Rat-liver cholesterol 7alpha-hydroxylase. 3. New results about its circadian rhythm. Eur. J. Biochem. 1975, 55, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hirao, J.; Arakawa, S.; Watanabe, K.; Ito, K.; Furukawa, T. Effects of restricted feeding on daily fluctuations of hepatic functions including p450 monooxygenase activities in rats. J. Biol. Chem. 2006, 281, 3165–3171. [Google Scholar] [CrossRef] [PubMed]
- Lavery, D.J.; Schibler, U. Circadian transcription of the cholesterol 7 alpha hydroxylase gene may involve the liver-enriched bzip protein dbp. Genes Dev. 1993, 7, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Noshiro, M.; Nishimoto, M.; Okuda, K. Rat liver cholesterol 7 alpha-hydroxylase. Pretranslational regulation for circadian rhythm. J. Biol. Chem. 1990, 265, 10036–10041. [Google Scholar] [PubMed]
- Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar] [PubMed]
- Furukawa, T.; Manabe, S.; Ohashi, Y.; Sharyo, S.; Kimura, K.; Mori, Y. Daily fluctuation of 7-alkoxycoumarin o-dealkylase activities in the liver of male f344 rats under ad libitum-feeding or fasting condition. Toxicol. Lett. 1999, 108, 11–16. [Google Scholar] [CrossRef]
- Hamdan, A.M.; Koyanagi, S.; Wada, E.; Kusunose, N.; Murakami, Y.; Matsunaga, N.; Ohdo, S. Intestinal expression of mouse abcg2/breast cancer resistance protein (bcrp) gene is under control of circadian clock-activating transcription factor-4 pathway. J. Biol. Chem. 2012, 287, 17224–17231. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Ushijima, K.; Ando, H.; Yanagihara, H.; Ishikawa, E.; Tsuruoka, S.; Sugimoto, K.; Fujimura, A. Influence of a time-restricted feeding schedule on the daily rhythm of abcb1a gene expression and its function in rat intestine. J. Pharmacol. Exp. Ther. 2010, 335, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Kotaka, M.; Onishi, Y.; Ohno, T.; Akaike, T.; Ishida, N. Identification of negative transcriptional factor e4bp4-binding site in the mouse circadian-regulated gene mdr2. Neurosci. Res. 2008, 60, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Stearns, A.T.; Balakrishnan, A.; Rhoads, D.B.; Ashley, S.W.; Tavakkolizadeh, A. Diurnal rhythmicity in the transcription of jejunal drug transporters. J. Pharmacol. Sci. 2008, 108, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Koyanagi, S.; Suzuki, N.; Katamune, C.; Matsunaga, N.; Watanabe, N.; Takahashi, M.; Izumi, T.; Ohdo, S. Circadian modulation in the intestinal absorption of p-glycoprotein substrates in monkeys. Mol. Pharmacol. 2015, 88, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Yanagihara, H.; Sugimoto, K.; Hayashi, Y.; Tsuruoka, S.; Takamura, T.; Kaneko, S.; Fujimura, A. Daily rhythms of p-glycoprotein expression in mice. Chronobiol. Int. 2005, 22, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Wada, E.; Koyanagi, S.; Kusunose, N.; Akamine, T.; Masui, H.; Hashimoto, H.; Matsunaga, N.; Ohdo, S. Modulation of peroxisome proliferator-activated receptor-alpha activity by bile acids causes circadian changes in the intestinal expression of octn1/slc22a4 in mice. Mol. Pharmacol. 2015, 87, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Lee, J.H.; Han, D.H.; Cho, S.; Lee, Y.J. Circadian clock is involved in regulation of hepatobiliary transport mediated by multidrug resistance-associated protein 2. J. Pharm. Sci. 2017, 106, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Dulong, S.; Ballesta, A.; Okyar, A.; Levi, F. Identification of circadian determinants of cancer chronotherapy through in vitro chronopharmacology and mathematical modeling. Mol. Cancer Ther. 2015, 14, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Ballesta, A.; Dulong, S.; Abbara, C.; Cohen, B.; Okyar, A.; Clairambault, J.; Levi, F. A combined experimental and mathematical approach for molecular-based optimization of irinotecan circadian delivery. PLoS Comput. Biol. 2011, 7, e1002143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kervezee, L.; Hartman, R.; van den Berg, D.J.; Shimizu, S.; Emoto-Yamamoto, Y.; Meijer, J.H.; de Lange, E.C. Diurnal variation in p-glycoprotein-mediated transport and cerebrospinal fluid turnover in the brain. AAPS J. 2014, 16, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Okyar, A.; Dressler, C.; Hanafy, A.; Baktir, G.; Lemmer, B.; Spahn-Langguth, H. Circadian variations in exsorptive transport: In situ intestinal perfusion data and in vivo relevance. Chronobiol. Int. 2012, 29, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, H.; Meerlo, P.; Elsinga, P.H.; Windhorst, A.D.; Dierckx, R.A.; Colabufo, N.A.; van Waarde, A.; Luurtsema, G. P-glycoprotein function in the rodent brain displays a daily rhythm, a quantitative in vivo pet study. AAPS J. 2016, 18, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Ohdo, S.; Makinosumi, T.; Ishizaki, T.; Yukawa, E.; Higuchi, S.; Nakano, S.; Ogawa, N. Cell cycle-dependent chronotoxicity of irinotecan hydrochloride in mice. J. Pharmacol. Exp. Ther. 1997, 283, 1383–1388. [Google Scholar] [PubMed]
- Porsin, B.; Formento, J.L.; Filipski, E.; Etienne, M.C.; Francoual, M.; Renée, N.; Magné, N.; Lévi, F.; Milano, G. Dihydropyrimidine dehydrogenase circadian rhythm in mouse liver. Eur. J. Cancer 2003, 39, 822–828. [Google Scholar] [CrossRef]
- Tuchman, M.; Roemeling, R.V.; Hrushesky, W.A.; Sothern, R.B.; O’Dea, R.F. Dihydropyrimidine dehydrogenase activity in human blood mononuclear cells. Enzyme 1989, 42, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.E.; Song, R.; Soong, S.J.; Diasio, R.B. Relationship between dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels with evidence for circadian variation of enzyme activity and plasma drug levels in cancer patients receiving 5-fluorouracil by protracted continuous infusion. Cancer Res. 1990, 50, 197–201. [Google Scholar] [PubMed]
- Zeng, Z.L.; Sun, J.; Guo, L.; Li, S.; Wu, M.W.; Qiu, F.; Jiang, W.Q.; Levi, F.; Xian, L.J. Circadian rhythm in dihydropyrimidine dehydrogenase activity and reduced glutathione content in peripheral blood of nasopharyngeal carcinoma patients. Chronobiol. Int. 2005, 22, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Barrat, M.A.; Renee, N.; Mormont, M.C.; Milano, G.; Levi, F. [circadian variations of dihydropyrimidine dehydrogenase (dpd) activity in oral mucosa of healthy volunteers]. Pathol. Biol. (Paris) 2003, 51, 191–193. [Google Scholar] [CrossRef]
- Sawers, L.; Ferguson, M.J.; Ihrig, B.R.; Young, H.C.; Chakravarty, P.; Wolf, C.R.; Smith, G. Glutathione s-transferase p1 (gstp1) directly influences platinum drug chemosensitivity in ovarian tumour cell lines. Br. J. Cancer 2014, 111, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Tew, K.D. The role of glutathione-s-transferase in anti-cancer drug resistance. Oncogene 2003, 22, 7369–7375. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Metzger, G.; Filipski, E.; Boughattas, N.; Lemaigre, G.; Hecquet, B.; Filipski, J.; Levi, F. Pharmacologic modulation of reduced glutathione circadian rhythms with buthionine sulfoximine: Relationship with cisplatin toxicity in mice. Toxicol. Appl. Pharmacol. 1997, 143, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Metzger, G.; Filipski, E.; Lemaigre, G.; Levi, F. Modulation of nonprotein sulphydryl compounds rhythm with buthionine sulphoximine: Relationship with oxaliplatin toxicity in mice. Arch. Toxicol. 1998, 72, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Ohdo, S. Chronotherapeutic strategy: Rhythm monitoring, manipulation and disruption. Adv. Drug Deliv. Rev. 2010, 62, 859–875. [Google Scholar] [CrossRef] [PubMed]
- Aleem, E.; Arceci, R.J. Targeting cell cycle regulators in hematologic malignancies. Front. Cell Dev. Biol. 2015, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Takane, H.; Ohdo, S.; Yamada, T.; Yukawa, E.; Higuchi, S. Chronopharmacology of antitumor effect induced by interferon-beta in tumor-bearing mice. J. Pharmacol. Exp. Ther. 2000, 294, 746–752. [Google Scholar] [PubMed]
- Sancar, A.; Lindsey-Boltz, L.A.; Unsal-Kacmaz, K.; Linn, S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004, 73, 39–85. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.B.; Schumacher, B. P53 in the DNA-damage-repair process. Cold. Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.; Xu, W.; Murphy, D.; James, P.; Sandhu, S. Relevance of DNA damage repair in the management of prostate cancer. Curr. Probl. Cancer 2017, 41, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Pelicano, H.; Liu, J.; Huang, P.; Lee, C. The circadian gene period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 2002, 111, 41–50. [Google Scholar] [CrossRef]
- Bjarnason, G.A.; Jordan, R.C.; Sothern, R.B. Circadian variation in the expression of cell-cycle proteins in human oral epithelium. Am. J. Pathol. 1999, 154, 613–622. [Google Scholar] [CrossRef]
- Bjarnason, G.A.; Jordan, R.C.; Wood, P.A.; Li, Q.; Lincoln, D.W.; Sothern, R.B.; Hrushesky, W.J.; Ben-David, Y. Circadian expression of clock genes in human oral mucosa and skin: Association with specific cell-cycle phases. Am. J. Pathol. 2001, 158, 1793–1801. [Google Scholar] [CrossRef]
- Wood, P.A.; Du-Quiton, J.; You, S.; Hrushesky, W.J. Circadian clock coordinates cancer cell cycle progression, thymidylate synthase, and 5-fluorouracil therapeutic index. Mol. Cancer Ther. 2006, 5, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, Y.; Hata, K.; Koyanagi, S.; Ohdo, S.; Shimeno, H.; Soeda, S. Circadian regulation of mouse topoisomerase i gene expression by glucocorticoid hormones. Biochem. Pharmacol. 2006, 71, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Flatten, K.; Dai, N.T.; Vroman, B.T.; Loegering, D.; Erlichman, C.; Karnitz, L.M.; Kaufmann, S.H. The role of checkpoint kinase 1 in sensitivity to topoisomerase i poisons. J. Biol. Chem. 2005, 280, 14349–14355. [Google Scholar] [CrossRef] [PubMed]
- Sigmond, J.; Peters, G.J. Pyrimidine and purine analogues, effects on cell cycle regulation and the role of cell cycle inhibitors to enhance their cytotoxicity. Nucleosides Nucleotides Nucleic Acids 2005, 24, 1997–2022. [Google Scholar] [CrossRef] [PubMed]
- Tampellini, M.; Filipski, E.; Liu, X.H.; Lemaigre, G.; Li, X.M.; Vrignaud, P.; Francois, E.; Bissery, M.C.; Levi, F. Docetaxel chronopharmacology in mice. Cancer Res. 1998, 58, 3896–3904. [Google Scholar] [PubMed]
- Granda, T.G.; Filipski, E.; D’Attino, R.M.; Vrignaud, P.; Anjo, A.; Bissery, M.C.; Levi, F. Experimental chronotherapy of mouse mammary adenocarcinoma ma13/c with docetaxel and doxorubicin as single agents and in combination. Cancer Res. 2001, 61, 1996–2001. [Google Scholar] [PubMed]
- Filipski, E.; Lemaigre, G.; Liu, X.H.; Mery-Mignard, D.; Mahjoubi, M.; Levi, F. Circadian rhythm of irinotecan tolerability in mice. Chronobiol. Int. 2004, 21, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Tanaka, K.; Sun, J.; Filipski, E.; Kayitalire, L.; Focan, C.; Levi, F. Preclinical relevance of dosing time for the therapeutic index of gemcitabine-cisplatin. Br. J. Cancer 2005, 92, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F. Circadian rhythms in 5-fluorouracil pharmacology and therapeutic applications. In Fluoropyrimidines in Cancer Therapy; Rustum, Y.M., Ed.; Humana Press: Totowa, NJ, USA, 2003; pp. 107–128. [Google Scholar]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, D.W., 2nd; Hrushesky, W.J.; Wood, P.A. Circadian organization of thymidylate synthase activity in normal tissues: A possible basis for 5-fluorouracil chronotherapeutic advantage. Int. J. Cancer 2000, 88, 479–485. [Google Scholar] [CrossRef]
- Johnston, P.G.; Drake, J.C.; Trepel, J.; Allegra, C.J. Immunological quantitation of thymidylate synthase using the monoclonal antibody ts 106 in 5-fluorouracil-sensitive and -resistant human cancer cell lines. Cancer Res. 1992, 52, 4306–4312. [Google Scholar] [PubMed]
- Johnston, P.G.; Lenz, H.J.; Leichman, C.G.; Danenberg, K.D.; Allegra, C.J.; Danenberg, P.V.; Leichman, L. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 1995, 55, 1407–1412. [Google Scholar] [PubMed]
- McGowan, C.H.; Russell, P. Cell cycle regulation of human wee1. EMBO J. 1995, 14, 2166–2175. [Google Scholar] [PubMed]
- Koyanagi, S.; Kuramoto, Y.; Nakagawa, H.; Aramaki, H.; Ohdo, S.; Soeda, S.; Shimeno, H. A molecular mechanism regulating circadian expression of vascular endothelial growth factor in tumor cells. Cancer Res. 2003, 63, 7277–7283. [Google Scholar] [PubMed]
- Nakagawa, H.; Takiguchi, T.; Nakamura, M.; Furuyama, A.; Koyanagi, S.; Aramaki, H.; Higuchi, S.; Ohdo, S. Basis for dosing time-dependent change in the anti-tumor effect of imatinib in mice. Biochem. Pharmacol. 2006, 72, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Buchdunger, E.; Cioffi, C.L.; Law, N.; Stover, D.; Ohno-Jones, S.; Druker, B.J.; Lydon, N.B. Abl protein-tyrosine kinase inhibitor sti571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J. Pharmacol. Exp. Ther. 2000, 295, 139–145. [Google Scholar] [PubMed]
- Druker, B.J.; Talpaz, M.; Resta, D.J.; Peng, B.; Buchdunger, E.; Ford, J.M.; Lydon, N.B.; Kantarjian, H.; Capdeville, R.; Ohno-Jones, S.; et al. Efficacy and safety of a specific inhibitor of the bcr-abl tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001, 344, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Kim, S.J.; Karashima, T.; Shepherd, D.L.; Fan, D.; Tsan, R.; Killion, J.J.; Logothetis, C.; Mathew, P.; Fidler, I.J. Effects of blocking platelet-derived growth factor-receptor signaling in a mouse model of experimental prostate cancer bone metastases. J. Natl. Cancer Inst. 2003, 95, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Emi, M.; Arihiro, K.; Tanabe, K.; Uchida, Y.; Toge, T. Chemosensitization by sti571 targeting the platelet-derived growth factor/platelet-derived growth factor receptor-signaling pathway in the tumor progression and angiogenesis of gastric carcinoma. Cancer 2005, 103, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Anderson, F.E.; Jung, Y.J.; Dziema, H.; Obrietan, K. Circadian regulation of mammalian target of rapamycin signaling in the mouse suprachiasmatic nucleus. Neuroscience 2011, 181, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, H.; Matsunaga, N.; Fujioka, T.; Okazaki, F.; Akagawa, Y.; Tsurudome, Y.; Ono, M.; Kuwano, M.; Koyanagi, S.; Ohdo, S. Circadian regulation of mtor by the ubiquitin pathway in renal cell carcinoma. Cancer Res. 2014, 74, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; An, F.; Xu, X.; Zhao, L.; Liu, L.; Liu, N.; Wang, P.; Liu, J.; Wang, L.; Li, M. Chronopharmacodynamics and mechanisms of antitumor effect induced by erlotinib in xenograft-bearing nude mice. Biochem. Biophys. Res. Commun. 2015, 460, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Soreide, K. Egfr and downstream genetic alterations in kras/braf and pi3k/akt pathways in colorectal cancer: Implications for targeted therapy. Discov. Med. 2012, 14, 207–214. [Google Scholar] [PubMed]
- Steins, M.; Thomas, M.; Geissler, M. Erlotinib. Recent Res. Cancer Res. 2014, 201, 109–123. [Google Scholar]
- Goffin, J.R.; Zbuk, K. Epidermal growth factor receptor: Pathway, therapies, and pipeline. Clin. Ther. 2013, 35, 1282–1303. [Google Scholar] [CrossRef] [PubMed]
- Lauriola, M.; Enuka, Y.; Zeisel, A.; D’Uva, G.; Roth, L.; Sharon-Sevilla, M.; Lindzen, M.; Sharma, K.; Nevo, N.; Feldman, M.; et al. Diurnal suppression of egfr signalling by glucocorticoids and implications for tumour progression and treatment. Nat. Commun. 2014, 5, 5073. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.C.; Chalmers, A.J.; El-Khamisy, S.F. Topoisomerase i inhibition in colorectal cancer: Biomarkers and therapeutic targets. Br. J. Cancer 2012, 106, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.L.; Luo, H.Y.; Yang, J.; Wu, W.J.; Chen, D.L.; Huang, P.; Xu, R.H. Overexpression of the circadian clock gene bmal1 increases sensitivity to oxaliplatin in colorectal cancer. Clin. Cancer Res. 2014, 20, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Dy, G.K.; Adjei, A.A. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J. Clin. 2013, 63, 249–279. [Google Scholar] [CrossRef] [PubMed]
- To, H.; Ohdo, S.; Shin, M.; Uchimaru, H.; Yukawa, E.; Higuchi, S.; Fujimura, A.; Kobayashi, E. Dosing time dependency of doxorubicin-induced cardiotoxicity and bone marrow toxicity in rats. J. Pharm. Pharmacol. 2003, 55, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Filipski, E.; Amat, S.; Lemaigre, G.; Vincenti, M.; Breillout, F.; Levi, F.A. Relationship between circadian rhythm of vinorelbine toxicity and efficacy in p388-bearing mice. J. Pharmacol. Exp. Ther. 1999, 289, 231–235. [Google Scholar] [PubMed]
- Li, X.M.; Levi, F. Circadian physiology is a toxicity target of the anticancer drug gemcitabine in mice. J. Biol. Rhythms 2007, 22, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Kanekal, S.; Crepin, D.; Guettier, C.; Carriere, J.; Elliott, G.; Levi, F. Circadian pharmacology of l-alanosine (sdx-102) in mice. Mol. Cancer Ther. 2006, 5, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Boughattas, N.A.; Levi, F.; Fournier, C.; Lemaigre, G.; Roulon, A.; Hecquet, B.; Mathe, G.; Reinberg, A. Circadian rhythm in toxicities and tissue uptake of 1,2-diamminocyclohexane(trans-1)oxalatoplatinum(ii) in mice. Cancer Res. 1989, 49, 3362–3368. [Google Scholar] [PubMed]
- Iurisci, I.; Filipski, E.; Reinhardt, J.; Bach, S.; Gianella-Borradori, A.; Iacobelli, S.; Meijer, L.; Levi, F. Improved tumor control through circadian clock induction by seliciclib, a cyclin-dependent kinase inhibitor. Cancer Res. 2006, 66, 10720–10728. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Mechkouri, M.; Roulon, A.; Bailleul, F.; Horvath, C.; Reinberg, A.; Mathe, G. Circadian rhythm in tolerance of mice for etoposide. Cancer Treat. Rep. 1985, 69, 1443–1445. [Google Scholar] [PubMed]
- Luan, J.J.; Zhang, Y.S.; Liu, X.Y.; Wang, Y.Q.; Zuo, J.; Song, J.G.; Zhang, W.; Wang, W.S. Dosing-time contributes to chronotoxicity of clofarabine in mice via means other than pharmacokinetics. Kaohsiung J. Med. Sci. 2016, 32, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Aizawa, K.; Sakai, S.; Jingami, S.; Fukunaga, E.; Yoshida, M.; Hamada, A.; Saito, H. The relationship between treatment time of gemcitabine and development of hematologic toxicity in cancer patients. Biol. Pharm. Bull. 2011, 34, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- Giacchetti, S.; Cur´e, H.; Adenis, A.; Tubiana, N.; Vernillet, L.; Chedouba-Messali, L.; Chevalier, V.; Germa, C.; Chollet, P.; Levi, F. Randomized multicenter trial of irinotecan (cpt) chronomodulated (chrono) versus standard (std) infusion in patients (pts) with metastatic colorectal cancer (mcc). Eur. J. Cancer 2001, 37, S309. [Google Scholar] [CrossRef]
- Kloth, J.S.; Binkhorst, L.; de Wit, A.S.; de Bruijn, P.; Hamberg, P.; Lam, M.H.; Burger, H.; Chaves, I.; Wiemer, E.A.; van der Horst, G.T.; et al. Relationship between sunitinib pharmacokinetics and administration time: Preclinical and clinical evidence. Clin. Pharmacokinet. 2015, 54, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C.Y.; Ji, S.G.; Xu, X.; Wang, P.P.; Zhang, B.; Zhao, L.Y.; Liu, L.; Lin, P.P.; Liu, L.K.; et al. Chronopharmacokinetics of erlotinib and circadian rhythms of related metabolic enzymes in lewis tumor-bearing mice. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Zidani, R.; Misset, J.L. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International organization for cancer chronotherapy. Lancet 1997, 350, 681–686. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, J.; Yang, K.; Ma, Y.; Yang, F. Retrospective analysis of chronomodulated chemotherapy versus conventional chemotherapy with paclitaxel, carboplatin, and 5-fluorouracil in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Onco Targets Ther. 2013, 6, 1507–1514. [Google Scholar] [PubMed]
- Bajetta, E.; Pietrantonio, F.; Buzzoni, R.; Ferrario, E.; Valvo, F.; Mariani, L.; Dotti, K.F.; Biondani, P.; Formisano, B.; Gevorgyan, A.; et al. Chronomodulated capecitabine and adjuvant radiation in intermediate-risk to high-risk rectal cancer: A phase ii study. Am. J. Clin. Oncol. 2014, 37, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Yabushita, Y.; Nakagawa, K.; Endo, I. [circadian chronotherapy for metastatic liver tumor]. Nihon Rinsho 2013, 71, 2158–2164. [Google Scholar] [PubMed]
- Patil, S.S.; Shahiwala, A. Patented pulsatile drug delivery technologies for chronotherapy. Expert Opin. Ther. Pat. 2014, 24, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Karaboue, A.; Etienne-Grimaldi, M.C.; Paintaud, G.; Focan, C.; Innominato, P.; Bouchahda, M.; Milano, G.; Chatelut, E. Pharmacokinetics of irinotecan, oxaliplatin and 5-fluorouracil during hepatic artery chronomodulated infusion: A translational european optiliv study. Clin. Pharmacokinet. 2017, 56, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.A.; Boige, V.; Hebbar, M.; Smith, D.; Lepere, C.; Focan, C.; Karaboue, A.; Guimbaud, R.; Carvalho, C.; Tumolo, S.; et al. Conversion to resection of liver metastases from colorectal cancer with hepatic artery infusion of combined chemotherapy and systemic cetuximab in multicenter trial optiliv. Ann. Oncol. 2016, 27, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Youan, B.B. Chronopharmaceutical drug delivery systems: Hurdles, hype or hope? Adv. Drug Deliv. Rev. 2010, 62, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Fu, S.; Peng, Q.; Han, Y.; Xie, J.; Zan, N.; Chen, Y.; Fan, J. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: In vitro and in vivo evaluation. Int. J. Pharm. 2017, 516, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, R.; Ji, M.; Zou, S.L.; Zhu, L.N. Cisplatin-based chronotherapy for advanced non-small cell lung cancer patients: A randomized controlled study and its pharmacokinetics analysis. Cancer Chemother. Pharmacol. 2015, 76, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Giacchetti, S.; Dugue, P.A.; Innominato, P.F.; Bjarnason, G.A.; Focan, C.; Garufi, C.; Tumolo, S.; Coudert, B.; Iacobelli, S.; Smaaland, R.; et al. Sex moderates circadian chemotherapy effects on survival of patients with metastatic colorectal cancer: A meta-analysis. Ann. Oncol. 2012, 23, 3110–3116. [Google Scholar] [CrossRef] [PubMed]
- Akgun, Z.; Saglam, S.; Yucel, S.; Gural, Z.; Balik, E.; Cipe, G.; Yildiz, S.; Kilickap, S.; Okyar, A.; Kaytan-Saglam, E. Neoadjuvant chronomodulated capecitabine with radiotherapy in rectal cancer: A phase ii brunch regimen study. Cancer Chemother. Pharmacol. 2014, 74, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Pilanci, K.N.; Saglam, S.; Okyar, A.; Yucel, S.; Pala-Kara, Z.; Ordu, C.; Namal, E.; Ciftci, R.; Iner-Koksal, U.; Kaytan-Saglam, E. Chronomodulated oxaliplatin plus capecitabine (xelox) as a first line chemotherapy in metastatic colorectal cancer: A phase ii brunch regimen study. Cancer Chemother. Pharmacol. 2016, 78, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Giacchetti, S.; Li, X.M.; Ozturk, N.; Cuvier, C.; Machowiak, J.; Arrondeau, J.; Chang-Marchand, Y.; Espie, M.; Okyar, A.; Levi, F. Consistent dosing-time dependent tolerability of everolimus (ev) in a pilot study in women with metastatic breast cancers (mbc) and in a mouse chronopharmacology investigation. Cancer Res. 2017, 77, 2. [Google Scholar] [CrossRef]
- Xiong, H.; Chiu, Y.L.; Ricker, J.L.; LoRusso, P. Results of a phase 1, randomized study evaluating the effects of food and diurnal variation on the pharmacokinetics of linifanib. Cancer Chemother. Pharmacol. 2014, 74, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Zhang, L.; Rowbottom, L.; McDonald, R.; Bjarnason, G.A.; Tsao, M.; Barnes, E.; Danjoux, C.; Popovic, M.; Lam, H.; et al. Effects of circadian rhythms and treatment times on the response of radiotherapy for painful bone metastases. Ann. Palliat. Med. 2017, 6, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Roche, V.P.; Mohamad-Djafari, A.; Innominato, P.F.; Karaboue, A.; Gorbach, A.; Levi, F.A. Thoracic surface temperature rhythms as circadian biomarkers for cancer chronotherapy. Chronobiol. Int. 2014, 31, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Tudela, E.; Innominato, P.F.; Rol, M.A.; Levi, F.; Madrid, J.A. Relevance of internal time and circadian robustness for cancer patients. BMC Cancer 2016, 16, 285. [Google Scholar] [CrossRef] [PubMed]
- Anafi, R.C.; Francey, L.J.; Hogenesch, J.B.; Kim, J. Cyclops reveals human transcriptional rhythms in health and disease. Proc. Natl. Acad. Sci. USA 2017, 114, 5312–5317. [Google Scholar] [CrossRef] [PubMed]
| Enzyme | Parameter | Method | Tissue/Cell Line | Species | Strain | Sex | Age | Peak | Trough | Cosinor | ANOVA | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyp11a1 | mRNA | RT-PCR | Adrenal gland | Mouse | WT | - | - | ZT0 | ZT12 | - | - | [81] |
| Cyp11b1 | mRNA | RT-PCR | Adrenal gland | Mouse | Crem KO | - | - | ZT0 | ZT12 | - | - | [81] |
| Cyp17 | mRNA | Microarray | Liver | Mouse | CD-1 | Male | Mature | ZT14-18 | ZT2 | - | - | [82] |
| Cyp17a1 | mRNA | RT-PCR | Adrenal gland | Mouse | Crem KO | - | - | ZT12 | ZT0 | - | - | [81] |
| Cyp1a1 | mRNA | RT-PCR | Liver | Mouse | Kunming | Male | 8 W | ZT6 | ZT18-22 | p < 0.05 | - | [75] |
| Cyp1a1 | mRNA | RT-PCR | Liver | Mouse | Kunming | Female | 8 W | ZT6 | ZT22 | p < 0.05 | - | [75] |
| Cyp1a1 | mRNA | RT-PCR | Lung | Mouse | WT | - | - | ZT14 | ZT6 | - | p < 0.05 | [83] |
| Cyp1a1 | Protein | Western blot | Lung | Mouse | WT | - | - | ZT18 | ZT6 | - | - | [83] |
| Cyp1a1 | Activity | P450-Glo kit | Lung | Mouse | WT | - | - | ZT18 | ZT2 | - | p < 0.05 | [83] |
| Cyp1a2 | mRNA | RT-PCR | Liver | Mouse | C57BL/6 | Male | 8 W | ZT13 | ZT1 | - | - | [84] |
| Cyp1a2 | mRNA | RT-PCR | Liver | Mouse | Kunming | Male | 8 W | ZT6 | ZT18-22 | p < 0.05 | - | [75] |
| Cyp1a2 | mRNA | RT-PCR | Liver | Mouse | Kunming | Female | 8 W | ZT6 | ZT22 | p < 0.05 | - | [75] |
| Cyp21a1 | mRNA | RT-PCR | Adrenal gland | Mouse | WT | - | - | ZT12 | ZT0 | - | - | [81] |
| Cyp26a1 | mRNA | RT-PCR | Liver | Mouse | ICR | Male | 6 W | ZT6-10 | ZT2 | - | - | [85] |
| Cyp27a1 | mRNA | RT-PCR | Liver | Mouse | Kunming | Male | 8 W | ZT10 | ZT22 | p < 0.05 | - | [75] |
| Cyp27a1 | mRNA | RT-PCR | Liver | Mouse | Kunming | Female | 8 W | ZT10 | ZT22 | p < 0.05 | - | [75] |
| Cyp2a1 | Activity | THA | Testis | Rat | Fischer 344 | Male | 18 W | ZT8 | ZT20 | - | - | [86] |
| Cyp2a4 | mRNA | Microarray | Liver | Mouse | CD-1 | Male | Mature | ZT14-18 | ZT2 | - | - | [82] |
| Cyp2a4 | mRNA | RPA | Liver | Mouse | 129/Ola | Male | 10–16 W | ZT11-15 | ZT23 | - | - | [78] |
| Cyp2a4 | mRNA | RT-PCR | Liver | Mouse | Kunming | Male | 8 W | ZT10 | ZT22 | p < 0.05 | - | [75] |
| Cyp2a5 | mRNA | RPA | Liver | Mouse-NTF | - | - | 10–16 W | ZT13 | ZT0 | - | - | [87] |
| Cyp2a5 | mRNA | RPA | Liver | Mouse-DTF | - | - | 10–16 W | ZT0 | ZT12 | - | - | [87] |
| Cyp2a5 | mRNA | ADDER | Liver | Mouse-NTF | - | - | 10–16 W | ZT16 | ZT4 | - | - | [87] |
| Cyp2a5 | mRNA | ADDER | Liver | Mouse-DTF | - | - | 10–16 W | ZT4 | ZT16 | - | - | [87] |
| Cyp2a5 | mRNA | RBA | Liver | Mouse | 129/Ola | Male | 10–16 W | ZT11-15 | ZT23-3 | - | - | [78] |
| Cyp2b10 | mRNA | Northern blot (PTB induced) | Liver | Mouse | - | - | - | ZT16 | ZT4 | - | - | [66] |
| Cyp2b10 | mRNA | Northern blot (PTB induced) | Intestine | Mouse | - | - | - | ZT16 | ZT4 | - | - | [66] |
| Cyp2b10 | mRNA | RT-PCR | Liver | Mouse | Kunming | Male | 8 W | ZT14-18 | ZT22-2 | p < 0.05 | - | [75] |
| Cyp2b10 | mRNA | RT-PCR | Liver | Mouse | Kunming | Female | 8 W | ZT18 | ZT6 | p < 0.05 | - | [75] |
| Cyp2b10 | mRNA | Branched DNA assay | Liver | Mouse | C57BL/6 | Male | 9 W | ZT21 | ZT9 | - | - | [60] |
| Cyp2c11 | mRNA | RT-PCR | Hippocampus | Rat | Wistar | Male | - | ZT2.58 | - | - | p = 0.0017 | [88] |
| Cyp2c11 | mRNA | RT-PCR | Middle cerebral artery | Rat | Wistar | Male | - | ZT5.11 | - | - | p = 0.025 | [88] |
| Cyp2c11 | mRNA | RT-PCR | Iinferior vena cava | Rat | Wistar | Male | - | ZT4.20 | - | - | p = 0.011 | [88] |
| Cyp2c50 | pre-mRNA | RT-PCR | Liver | Mouse | - | - | - | ZT20 | ZT4 | - | - | [66] |
| Cyp2d10 | mRNA | RT-PCR | Liver | Mouse | FVB | Female | 8–12 W | ZT20 | ZT8 | p < 0.0413 | - | [89] |
| Cyp2d22 | mRNA | RT-PCR | Liver | Mouse | FVB | Female | 8–12 W | ZT20 | ZT8 | p < 0.0413 | - | [89] |
| Cyp2e1 | mRNA | Microarray | Liver | Mouse | CD-1 | Male | Mature | ZT14-18 | ZT2 | - | - | [82] |
| Cyp2e1 | Activity | p-Nitrophenol hydroxylation | Liver | Rat | Sprague Dawley | Male | - | ZT12 | ZT0 | - | - | [90] |
| Cyp2e1 | mRNA | RT-PCR | Liver | Rat | Wistar | Male | 8 W | ZT12 | ZT21 | - | p < 0.05 | [91] |
| Cyp2e1 | Protein | Western blot | Liver | Rat | Wistar | Male | 8 W | ZT18 | ZT3 | - | p < 0.01 | [91] |
| Cyp2e1 | Activity | Hydroxylation/HPLC | Liver | Rat | Wistar | Male | 8 W | ZT21 | ZT3 | - | p < 0.05 | [91] |
| Cyp2e1 | mRNA | RT-PCR | Kidney | Rat | Wistar | Male | 8 W | ZT12 | - | - | - | [91] |
| Cyp2e1 | Protein | Western blot | Kidney | Rat | Wistar | Male | 8 W | ZT21 | ZT6 | - | p < 0.05 | [91] |
| Cyp2e1 | Activity | Hydroxylation/HPLC | Kidney | Rat | Wistar | Male | 8 W | ZT21 | ZT6 | - | p < 0.05 | [91] |
| Cyp2e1 | mRNA | RT-PCR | Liver | Mouse | Kunming | Male | 8 W | ZT10 | ZT22 | p < 0.05 | - | [75] |
| Cyp2e1 | mRNA | RT-PCR | Liver | Mouse | Kunming | Female | 8 W | ZT10 | ZT22 | p < 0.05 | - | [75] |
| Cyp2e1 | Activity | p-Nitrophenol hydroxylation | Liver | Mouse-AD | ICR | Male | 7 W | ZT14-18 | ZT2-6 | - | p < 0.01 | [92] |
| Cyp2e1 | Activity | p-Nitrophenol hydroxylation | Liver | Mouse-TRF | ICR | Male | 7 W | ZT22-2 | ZT14 | - | p < 0.01 | [92] |
| Cyp2e1 | mRNA | Branched DNA assay | Liver | Mouse | C57BL/6 | Male | 9 W | ZT17 | ZT5 | - | - | [60] |
| Cyp3a | Activity | EDA | Liver | Rat | Wistar | Male | 10 W | ZT19 | ZT6 | - | - | [93] |
| Cyp3a | Activity | EDA | Liver | Rat | Wistar | Male | 22 W | ZT19 | ZT8 | - | - | [93] |
| Cyp3a11 | mRNA | RT-PCR | Small intestine | Mouse | FVB | Female | 8–12 W | ZT16 | ZT4 | p < 0.0172 | - | [89] |
| Cyp3a11 | mRNA | RT-PCR | Liver | Mouse | ICR | Male | 6 W | ZT10 | ZT22 | - | - | [85] |
| Cyp3a11 | mRNA | RT-PCR | Liver | Mouse | Kunming | Male | 8 W | ZT2 | ZT14-16 | p < 0.05 | - | [75] |
| Cyp3a11 | mRNA | RT-PCR | Liver | Mouse | Kunming | Female | 8 W | ZT2 | ZT14-22 | p < 0.05 | - | [75] |
| Cyp3a11 | mRNA | Branched DNA assay | Liver | Mouse | C57BL/6 | Male | 9 W | ZT21 | ZT1 | - | - | [60] |
| Cyp3a25 | mRNA | RT-PCR | Liver | Mouse | Kunming | Male | 8 W | ZT2 | ZT14-16 | p < 0.05 | - | [75] |
| Cyp3a25 | mRNA | RT-PCR | Liver | Mouse | Kunming | Female | 8 W | ZT2 | ZT14-22 | p < 0.05 | - | [75] |
| Cyp4a10 | mRNA | RT-PCR | Liver | Mouse | Kunming | Male | 8 W | ZT10 | ZT22 | p < 0.05 | - | [75] |
| Cyp4a10 | mRNA | RT-PCR | Liver | Mouse | Kunming | Female | 8 W | ZT18 | ZT6-10 | p < 0.05 | - | [75] |
| Cyp4a14 | mRNA | RT-PCR | Liver | Mouse | Kunming | Male | 8 W | ZT10 | ZT22 | p < 0.05 | - | [75] |
| Cyp4a14 | mRNA | RT-PCR | Liver | Mouse | Kunming | Female | 8 W | ZT10 | ZT22 | p < 0.05 | - | [75] |
| Cyp4a14 | mRNA | Branched DNA assay | Liver | Mouse | C57BL/6 | Male | 9 W | ZT13 | ZT21 | - | - | [60] |
| Cyp4x1 | mRNA | RT-PCR | Hippocampus | Rat | Wistar | Male | - | ZT8.24 | - | p = 0.0016 | [88] | |
| Cyp4x1 | mRNA | RT-PCR | Inferior vena cava | Rat | Wistar | Male | - | ZT18.58 | - | p = 0.0003 | [88] | |
| Cyp51 | mRNA | RT-PCR | Adrenal gland | Mouse | WT | - | ZT16 | ZT4 | - | - | [81] | |
| Cyp51 | mRNA | Northern blot | Liver | Rat | Wistar | Male | 8 W | ZT14-22 | ZT10 | - | - | [94] |
| Cyp7a | mRNA | in situ hybridization | Liver | Rat | Fischer | Male | - | ZT16 | ZT4 | - | - | [95] |
| Cyp7a | mRNA | RT-PCR | Liver | Rat | Wistar | Male | 8 W | ZT16 | ZT4 | - | - | [96] |
| Cyp7a | mRNA | Northern blot | Liver | Rat | Wistar | Male | 8 W | ZT18 | ZT6 | - | - | [94] |
| Cyp7a | Activity | 7α-hydroxylase | Liver | Rat-AD | Wistar | Male | - | ZT14 | ZT6 | - | p < 0.05 | [97] |
| Cyp7a | Activity | 7α-hydroxylase | Liver | Rat-TRF | Wistar | Male | - | ZT7 | ZT19 | - | p < 0.05 | [97] |
| Cyp7a | mRNA | Northern blot | Liver | Rat-AD | Wistar | Male | - | ZT14 | ZT7 | - | p < 0.01 | [97] |
| Cyp7a | mRNA | Northern blot | Liver | Rat-TRF | Wistar | Male | - | ZT7 | ZT14 | - | p < 0.01 | [97] |
| Cyp7a1 | Activity | Enzymatic | Liver | Rat | Wistar | Male | - | ZT16 | ZT0-4 | - | - | [98] |
| Cyp7a1 | mRNA | RT-PCR | Liver | Rat-AD | F344/DuCrj | Male | 7 W | ZT18 | ZT6 | - | p < 0.01 | [99] |
| Cyp7a1 | mRNA | RT-PCR | Liver | Rat-TRF | F344/DuCrj | Male | 7 W | ZT6 | ZT18 | - | p < 0.01 | [99] |
| Cyp7a1 | mRNA | RPA | Liver | Rat | Lewis | - | - | ZT16 | ZT4 | - | - | [100] |
| Cyp7a1 | mRNA | RT-PCR | Liver | Mouse | Kunming | Male | 8 W | ZT10 | ZT22 | p < 0.05 | - | [75] |
| Cyp7a1 | mRNA | RT-PCR | Liver | Mouse | Kunming | Female | 8 W | ZT10 | ZT22 | p < 0.05 | - | [75] |
| Cyp7a1 | mRNA | Northern blot | Liver | Rat | Wistar | Male | - | 10 p.m. | 10 a.m. | - | - | [101] |
| Cyp7a1 | Protein | Western blot | Liver | Rat | Wistar | Male | - | 10 p.m. | 10 a.m. | - | - | [101] |
| Cyp7a1 | Activity | Hydroxylase | Liver | Rat | Wistar | Male | - | 10 p.m. | 10 a.m. | - | - | [101] |
| Cyp7b1 | mRNA | RT-PCR | Liver | Mouse | Kunming | Female | 8 W | ZT6 | ZT22 | p < 0.05 | - | [75] |
| Cyp8b | mRNA | RT-PCR | Liver | Rat | Wistar | Male | 8 W | ZT7-10 | ZT19 | - | - | [96] |
| Cyp8b | mRNA | Northern blot | Liver | Rat | Wistar | Male | 8 W | ZT10 | ZT22 | - | - | [94] |
| Cyp8b | Activity | 12α-hydroxylase | Liver | Rat-AD | Wistar | Male | - | ZT14 | ZT6 | - | p < 0.05 | [97] |
| Cyp8b | Activity | 12α-hydroxylase | Liver | Rat-TRF | Wistar | Male | - | ZT7 | ZT19 | - | p < 0.05 | [97] |
| Cyp8b | mRNA | Northern blot | Liver | Rat-AD | Wistar | Male | - | ZT7 | ZT19 | - | p < 0.01 | [97] |
| Cyp8b | mRNA | Northern blot | Liver | Rat-TRF | Wistar | Male | - | ZT19 | ZT14 | - | p < 0.05 | [97] |
| P450 | Protein | from [102] | Liver | Rat-AD | F344 | - | 10 W | ZT3 | ZT19 | - | p < 0.01 | [103] |
| P450 | Protein | from [102] | Liver | Rat-fasted | F344 | - | 10 W | ZT19-23 | ZT7 | - | p < 0.01 | [103] |
| P450 | Activity | 7ACoD | Liver | Rat | F344 | - | 10 W | ZT19-23 | ZT7 | - | p < 0.01 | [103] |
| P450 | Activity | 7ACoD | Liver | Rat-AD | F344/DuCrj | Male | 7 W | ZT18 | ZT6 | - | p < 0.01 | [99] |
| P450 | Activity | 7ACoD | Liver | Rat-TRF | F344/DuCrj | Male | 7 W | ZT6 | ZT18 | - | p < 0.01 | [99] |
| Transporter | Parameter | Method | Tissue/Cell Line | Species | Strain | Sex | Age | Peak | Trough | Cosinor | ANOVA | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abcb1 | mRNA | RT-PCR | Small intestine | Monkey | Cynomolgus | Male | 4–7 Y | ZT3 | ZT9 | - | NS | [108] |
| Abcb1 | mRNA | RT-PCR | Liver | Monkey | Cynomolgus | Male | 4–7 Y | ZT21 | ZT15 | - | NS | [108] |
| Abcb1a | mRNA | RT-PCR | Liver | Mouse | C57BL/6 | Male | 10 W | ZT12-16 | ZT0 | - | p < 0.01 | [109] |
| Abcb1a | mRNA | RT-PCR | Jejunum | Mouse | C57BL/6 | Male | 10 W | ZT12 | ZT0 | - | p < 0.01 | [109] |
| Abcb1a | mRNA | RT-PCR | Kidney | Mouse | C57BL/6 | Male | 10 W | ZT12 | ZT0 | - | NS | [109] |
| Abcb1a | mRNA | RT-PCR | Ileum | Mouse | B6D2F1 | Female | 10 W | ZT15 | ZT3 | - | NS | [33] |
| Abcb1a | mRNA | RT-PCR | Jejunum (proximal) | Rat-AD | Wistar | Male | 8 W | ZT12 | ZT0 | - | p < 0.05 | [105] |
| Abcb1a | mRNA | RT-PCR | Jejunum (proximal) | Rat-TRF | Wistar | Male | 8 W | ZT0 | ZT12 | - | p < 0.01 | [105] |
| Abcb1a | Activity | Digoxin conc./intestinal perfusion | Jejunum (proximal) | Rat-AD | Wistar | Male | 8 W | ZT18 | ZT6 | - | p < 0.05 | [105] |
| Abcb1a | Activity | Digoxin AUC/intestinal perfusion | Jejunum (proximal) | Rat-AD | Wistar | Male | 8 W | ZT18 | ZT6 | - | p < 0.01 | [105] |
| Abcb1a | Activity | Digoxin conc. | Jejunum (proximal) | Rat-TRF | Wistar | Male | 8 W | ZT6 | ZT18 | - | p < 0.05 | [105] |
| Abcb1a | Activity | Digoxin AUC | Jejunum (proximal) | Rat-TRF | Wistar | Male | 8 W | ZT6 | ZT18 | - | p < 0.05 | [105] |
| Abcb1a | mRNA | RT-PCR | Ileum | Mouse | C57BL/6 | Male | - | ZT10 | ZT2 | - | - | [67] |
| Abcb1a | mRNA | RT-PCR | Liver | Mouse | C57BL/6 | - | 8 W | ZT13 | ZT1 | p = 0.016 | p = 0.029 | [111] |
| Abcb1a/1b | mRNA | RT-PCR | Jejunal mucosa | Rat | SD | Male | - | ZT6 | ZT18 | p < 0.05 | p < 0.0001 | [107] |
| Abcb1a/1b | mRNA | Branched DNA assay | Liver | Mouse | C57BL76 | Male | 9 W | ZT16 | ZT0 | - | - | [60] |
| Abcb1b | mRNA | RT-PCR | Liver | Mouse | C57BL/6 | Male | 10 W | ZT16 | ZT0 | - | NS | [109] |
| Abcb1b | mRNA | RT-PCR | Jejunum | Mouse | C57BL/6 | Male | 10 W | ZT20 | ZT0 | - | NS | [109] |
| Abcb1b | mRNA | RT-PCR | Kidney | Mouse | C57BL/6 | Male | 10 W | ZT20 | ZT0 | - | NS | [109] |
| Abcb1b | mRNA | RT-PCR | Ileum | Mouse | B6D2F1 | Female | 10 W | ZT15 | ZT3 | - | p = 0.03 | [33] |
| ABCB1 | mRNA | RT-PCR | Caco-2 | Human | - | - | - | ZT16 | ZT4 | p = 0.013 | - | [112] |
| ABCB1 | mRNA | RT-PCR | Caco-2 | Human | - | - | - | ZT16 | ZT4 | - | - | [113] |
| abcb1a | mRNA | RT-PCR | AdenoCA colon 26 | Mouse | - | - | - | ZT0 | ZT12 | - | - | [67] |
| P-gp | Protein | Western blot | Liver | Mouse | C57BL/6 | - | 8 W | ZT20 | ZT0 | NS | - | [111] |
| P-gp | Protein | Western blot | Liver | Mouse | C57BL/6 | Male | 10 W | ZT8 | ZT20 | - | NS | [109] |
| P-gp | Protein | Western blot | Kidney | Mouse | C57BL/6 | Male | 10 W | ZT0 | ZT16 | - | NS | [109] |
| P-gp | Protein | Western blot | Jejunum | Mouse | C57BL/6 | Male | 10 W | ZT8 | ZT0 | - | p = 0.04 | [109] |
| P-gp | Activity | Digoxin accumulation | Jejunum | Mouse | C57BL/6 | Male | 10 W | ZT12 | ZT0 | - | p < 0.05 | [109] |
| P-gp | Protein | Western blot | Jejunum | Monkey | Cynomolgus | Male | 4–7 Y | ZT21 | ZT9 | - | NS | [108] |
| P-gp | Activity | Quinidine conc. | Brain homogenate | Rat | Wistar | Male | - | ZT8 | ZT20 | - | p < 0.05 | [114] |
| P-gp | Activity | Unbound quinidine in CSF | Microdialysis | Rat | Wistar | Male | - | ZT8 | ZT20 | - | p < 0.05 | [114] |
| P-gp | Protein | Western blot | Ileum | Mouse | C57BL/6 | Male | - | ZT10 | ZT2 | - | [67] | |
| P-gp | Activity | Talinolol intestinal perfusion | Jejunum | Rat-fasted | Wistar | Male | - | ZT13-15 | ZT1-3 | - | p < 0.05 | [115] |
| P-gp | Activity | Talinolol intestinal perfusion | Ileum | Rat-fasted | Wistar | Male | - | ZT13-15 | ZT1-3 | - | p < 0.05 | [115] |
| P-gp | Activity | Losartan intestinal perfusion | Jejunum | Rat-fasted | Wistar | Male | - | ZT13-15 | ZT1-3 | - | p < 0.05 | [115] |
| P-gp | Activity | Losartan intestinal perfusion | Ileum | Rat-fasted | Wistar | Male | - | ZT13-15 | ZT1-3 | - | p < 0.05 | [115] |
| P-gp | Activity | [18F]MC225 PET | Whole brain | Rat | SD | Male | 14–16 W | ZT3-9 | ZT15 | - | p < 0.001 | [116] |
| P-gp | Activity | [18F]MC225 PET | Cortex | Rat | SD | Male | 14–16 W | ZT3-9 | ZT15 | - | p < 0.001 | [116] |
| P-gp | Activity | [18F]MC225 PET | Striatum | Rat | SD | Male | 14–16 W | ZT3 | ZT15 | - | p < 0.01 | [116] |
| P-gp | Activity | [18F]MC225 PET | Hippocampus | Rat | SD | Male | 14–16 W | ZT3 | ZT15 | - | p < 0.01 | [116] |
| P-gp | Activity | [18F]MC225 PET | Cerebellum | Rat | SD | Male | 14–16 W | ZT3-9 | ZT15 | - | p < 0.01 | [116] |
| P-gp | Activity | [18F]MC225 PET | Pons | Rat | SD | Male | 14–16 W | ZT3-9 | ZT15 | - | p < 0.01 | [116] |
| Abcb4 | mRNA | RT-PCR | Liver | Mouse | C57BL/6 | Male | 10 W | ZT8 | ZT20 | - | p = 0.06 | [109] |
| Abcb4 | mRNA | RT-PCR | Jejunum | Mouse | C57BL/6 | Male | 10 W | ZT4 | ZT16 | - | NS | [109] |
| Abcb4 | mRNA | RT-PCR | Kidney | Mouse | C57BL/6 | Male | 10 W | ZT0 | ZT12 | - | NS | [109] |
| Abcb4 | mRNA | Northern blot | Liver | Mouse | Slc:ICR | Male | - | ZT0 | ZT16 | - | - | [106] |
| Abcb4 | mRNA | Branched DNA assay | Liver | Mouse | C57BL/6 | Male | 9 W | ZT4 | ZT16 | - | - | [60] |
| ABCC1 | mRNA | RT-PCR | Caco-2 | Human | - | - | - | ZT10 | ZT0 | - | - | [113] |
| ABCC2 | mRNA | RT-PCR | Caco-2 | Human | - | - | - | ZT12 | ZT0 | - | - | [113] |
| Abcc2 | mRNA | RT-PCR | Liver | Mouse | C57BL/6 | Male | 10 W | ZT12 | ZT0 | - | p < 0.01 | [109] |
| Abcc2 | mRNA | RT-PCR | Jejunum | Mouse | C57BL/6 | Male | 10 W | ZT8 | ZT20 | - | p < 0.01 | [109] |
| Abcc2 | mRNA | RT-PCR | Kidney | Mouse | C57BL/6 | Male | 10 W | ZT12 | ZT0 | - | p = 0.02 | [109] |
| Abcc2 | mRNA | RT-PCR | Liver | Mouse | C57BL/6 | - | 8 W | ZT7 | ZT19 | p = 0.032 | p = 0.045 | [111] |
| Abcc2 | Protein | Western blot | Liver | Mouse | C57BL/6 | - | 8 W | ZT16 | ZT4 | p < 0.05 | [111] | |
| Abcc2 | mRNA | RT-PCR | Ileum mucosa | Mouse | B6D2F1 | Male | - | ZT12 | ZT0 | p = 0.0023 | p = 0.04 | [31] |
| Abcc2 | mRNA | RT-PCR | Ileum mucosa | Mouse | B6D2F1 | Female | - | ZT9 | ZT0 | p = 0.0023 | p = 0.008 | [31] |
| Abcc2 | mRNA | RT-PCR | Ileum mucosa | Mouse | B6CBAF1 | Male | - | ZT12 | ZT0 | p = 0.00026 | p = 0.004 | [31] |
| Abcc2 | mRNA | RT-PCR | Ileum mucosa | Mouse | B6CBAF1 | Female | - | ZT9 | ZT0 | p = 0.00012 | p = 0.004 | [31] |
| Abcc2 | mRNA | RT-PCR | Ileum serosa | Mouse | B6D2F1 | Male | - | ZT4 | ZT20 | NS | NS | [31] |
| Abcc2 | Protein | IHC | Ileum mucosa | Mouse | B6D2F1 | Male | - | ZT12 | ZT15 | - | p < 0.001 | [31] |
| Abcc2 | Protein | IHC | Ileum mucosa | Mouse | B6D2F1 | Female | - | ZT12 | ZT3 | - | p < 0.001 | [31] |
| Abcc2 | Protein | IHC | Ileum mucosa | Mouse | B6CBAF1 | Male | - | ZT15 | ZT12 | - | p < 0.001 | [31] |
| Abcc2 | Protein | IHC | Ileum mucosa | Mouse | B6CBAF1 | Female | - | ZT0 | ZT15 | - | p < 0.001 | [31] |
| Abcc2 | mRNA | RT-PCR | Jejunal mucosa | Rat | SD | Male | - | ZT12 | ZT3 | p < 0.05 | p = 0.001 | [107] |
| Abcc2 | mRNA | Branched DNA assay | Liver | Mouse | C57BL/6 | Male | 9 W | ZT4 | ZT16 | - | - | [60] |
| MRP-2 | Protein | Western blot | Jejunum | Monkey | Cynomolgus | Male | 4–7 Y | ZT21 | ZT9 | - | NS | [108] |
| Abcg2 | mRNA | RT-PCR | Liver | Mouse | Per1 and Per2 knockout | - | 8 W | ZT7 | ZT19 | p = 0.023 | p = 0,049 | [111] |
| Abcg2 | mRNA | RT-PCR | Jejunal mucosa | Rat | SD | Male | - | ZT3 | ZT15 | p < 0.05 | p = 0.04 | [107] |
| Abcg2 | mRNA | Branched DNA assay | Liver | Mouse | C57BL/6 | Male | 9 W | ZT16 | ZT4 | - | - | [60] |
| Abcg2 isoform B | mRNA | RT-PCR | Liver | Mouse | ICR | - | - | ZT6 | ZT18 | - | p < 0.05 | [104] |
| Abcg2 isoform B | mRNA | RT-PCR | Kidney | Mouse | ICR | - | - | ZT10 | ZT18 | - | p < 0.05 | [104] |
| Abcg2 isoform B | mRNA | RT-PCR | Small intestine | Mouse | ICR | - | - | ZT6 | ZT22 | - | p < 0.05 | [104] |
| ABCG2 | Protein | Western blot | Jejunum | Monkey | Cynomolgus | Male | 4–7 Y | ZT15-21 | ZT9 | - | NS | [108] |
| ABCG2 | mRNA | RT-PCR | Caco-2 | Human | - | - | - | ZT12 | ZT0 | - | - | [113] |
| abcg2 | mRNA | RT-PCR | aMoS7 | Mouse | - | - | - | ZT12 | ZT0 | - | - | [104] |
| Target | Parameter | Method | Tissue | Species | Strain | Sex | Peak | Trough | Cosinor | ANOVA | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BCL2 | Protein | Western blot | Bone marrow | Mouse | C3H/HeN | Male | ZT3 | ZT15 | p = 0.024 | - | [56] |
| BCL2 | Protein | Western blot | Bone marrow | MA13/C bearing mouse | C3H/HeN | Male | ZT7 | ZT23 | p = 0.001 | - | [56] |
| BCL2 | Protein | Western blot | Bone marrow | Mouse | B6D2F1 | Male | ZT4–7 | ZT1 | p = 0.025 | - | [56] |
| c-Myc | mRNA | Northern blot | Liver | Mouse | 129/C57BL6 | - | ZT14 | ZT10 | - | - | [133] |
| Cdk-4 | mRNA | Northern blot | Liver | Mouse | 129/C57BL6 | - | ZT18 | ZT10 | - | - | [133] |
| Cyclin D1 | mRNA | Northern blot | Liver | Mouse | 129/C57BL6 | - | ZT14 | ZT22 | - | - | [133] |
| Cyclin A (G2) | Protein | IHC | Oral mucosa | Human | - | Male | ZT20 | ZT4 | p < 0.001 | - | [134] |
| Cyclin B1 (M) | Protein | IHC | Oral mucosa | Human | - | Male | ZT20 | ZT12 | p = 0.016 | - | [134] |
| Cyclin E (G1/S) | Protein | IHC | Oral mucosa | Human | - | Male | ZT16 | ZT4 | p < 0.001 | - | [134] |
| p53 (G1) | Protein | IHC | Oral mucosa | Human | - | Male | ZT12 | ZT0 | p = 0.016 | - | [134] |
| TS | Activity | Tritium release assay | Oral mucosa | Human | - | Male | ZT16 | ZT4 | p = 0.008 | NS | [135] |
| TS | Activity | Tritium release assay | Bone marrow | Mouse | CD2F1 | Female | ZT18 | ZT2 | p < 0.001 | p < 0.001 | [136] |
| TS | Activity | Tritium release assay | Small intestine | Mouse | CD2F1 | Female | ZT6 | ZT18 | p < 0.001 | p < 0.001 | [136] |
| TS | Activity | Tritium release assay | Tumor | Mouse | CD2F1 | Female | ZT6 | ZT14 | p = 0.003 | p < 0.001 | [136] |
| TS | mRNA | PCR | Tumor | Mouse | CD2F1 | Female | ZT2 | ZT14 | NS | NS | [136] |
| TS | Protein | Western blot | Tumor | Mouse | CD2F1 | Female | ZT22 | ZT14 | p < 0.001 | p = 0.008 | [136] |
| Wee-1 | Protein | Western blot | Tumor | Mouse | CD2F1 | Female | ZT14 | ZT18 | p = 0.011 | p = 0.047 | [136] |
| Top-1 | mRNA | RT-PCR | Liver | Mouse | ICR | Male | ZT21 | ZT9 | - | - | [137] |
| Top-1 | mRNA | RT-PCR | Tumor | Mouse | ICR | Male | ZT21 | ZT9 | - | - | [137] |
| Top-1 | Activity | Relaxation of SCP DNA | Sarcoma 180 tumor | Tumor bearing mouse | ICR | Male | ZT5 | ZT13 | - | p < 0.05 | [137] |
| Anticancer Drug(s) | Cancer Type | Study Design | Dose/Chronomodulated Schedule | Main Pharmacological Findings | Main Clinical Findings | Reference |
|---|---|---|---|---|---|---|
| Pharmacokinetics/Pharmacodynamics | Efficacy/Toxicity/Adverse Effects | |||||
| Cisplatin (combined with Gemcitabine and Docetaxel) | Non-small cell lung cancer | Randomized controlled study, pharmacokinetic analysis | Cisplatin 30-min i.v. infusion at 06:00 (morning) and 18:00 (evening) | Total and unbound platin CL 18:00 > 06:00 | Leucopenia, neutropenia and nausea symptoms lower at 18:00 | [187] |
| Irinotecan + Oxaliplatin + 5-FU (combined with cetuximab) | Colorectal cancer with liver metastases | Pharmacokinetic study | Hepatic artery infusion (chronomudulated) Irinotecan (180 mg/m2)-6 h sinusidal infusion peak at 05:00 h, Oxaliplatin (85 mg/m2)-12 h sinusoidal infusion peak at 16:00, 5-FU (2800 mg/m2)-12 h sinusoidal infusion peak at 04:00 h | Good correlation between AUC of irinotecan, SN-38, ultrafiltrated Pt and leukopenia. The AUC and Cmax of ultrafiltrated Pt were significantly correlated with the severity of diarrhea | - | [183] |
| 5-FU, LV and oxaliplatin | Colon or rectum cancers with metastases | Meta-analysis of three Phase III trials | ChronoFLO = 5-FU-LV from 2215 to 09:45 h with a peak at 04:00 h, and oxaliplatin from 10:15 to 21:45 h with a peak at 1600 h. | - | Overall survival was higher in males on chronoFLO when compared with CONV (p = 0.009) | [188] |
| Capecitabine (combined with radiotherapy) | Rectal cancer | Prospective single-center, single-arm Phase II study | Oral Capecitabine (1650 mg/m2) 50% dose at 8:00 (morning) and 50% dose at 12:00 (noon)-BRUNCH | - | No Grade 2–3 toxicity of hand-foot syndrome, thrombocytopenia, diarrhea and mucositis. There were no grade IV toxicities | [189] |
| Capecitabine + Oxaliplatin | Treatment-naïve colorectal cancer patients with metastatic disease | Prospective single-center, single-arm, Phase II study | Oral Capecitabine (2000 mg/m2) 50% dose at 8:00 (morning) and 50% dose at 12:00 (noon)-BRUNCH | AUC0–4 h of Capecitabine 08:00 > 12:00 Cmax of Capecitabine 08:00 > 12:00 | Lower incidence of hand-foot syndrome | [190] |
| Tamoxifen | Breast cancer | Pharmacokinetic cross-over study | Oral 20 or 40 mg once a day at 8:00 or 13:00 or 20:00 | AUC0–8 h and Cmax of tamoxifen 08:00 > 20:00 (20%) tamoxifen tmax 08:00 < 20:00 Systemic exposure (AUC0–24 h) to endoxifen 08:00 > 20:00 (15%) | - | [89] |
| Everolimus (combined with exemastan and tamoxifen) | Metastatic breast cancers | - | Oral morning or evening administration | - | Morning administration of everolimus minimize metabolic alteration and fatigues. No pneumonitis after morning administration | [191] |
| Sunitinib | Advanced clear cell renal cell carcinoma, Pancreatic neuro-endocrine tumors | Prospective randomized crossover study | 8:00. (morning), 13:00 (noon) and 18:00. (evening) | Ctrough 13:00. or 18:00 p.m. > Ctrough 8:00 p = 0.006 | - | [176] |
| Linifanib | Advanced or metastatic solid tumors and refractory to standard therapy | Phase I, open-label, randomized, crossover study | 0.25 mg/kg (maximum 17.5 mg) morning or evening | Cmax morning > evening (p < 0.01) | - | [192] |
| Radiotherapy | Bone metastases | Cohort study | Treatment times are 08:00–11:00, 11:01–14:00 or 14:01–17:00 | - | Females in the 11:01 to 14:00 cohort exhibited higher response rate | [193] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozturk, N.; Ozturk, D.; Kavakli, I.H.; Okyar, A. Molecular Aspects of Circadian Pharmacology and Relevance for Cancer Chronotherapy. Int. J. Mol. Sci. 2017, 18, 2168. https://doi.org/10.3390/ijms18102168
Ozturk N, Ozturk D, Kavakli IH, Okyar A. Molecular Aspects of Circadian Pharmacology and Relevance for Cancer Chronotherapy. International Journal of Molecular Sciences. 2017; 18(10):2168. https://doi.org/10.3390/ijms18102168
Chicago/Turabian StyleOzturk, Narin, Dilek Ozturk, Ibrahim Halil Kavakli, and Alper Okyar. 2017. "Molecular Aspects of Circadian Pharmacology and Relevance for Cancer Chronotherapy" International Journal of Molecular Sciences 18, no. 10: 2168. https://doi.org/10.3390/ijms18102168




