1. Introduction
Birch pollen allergy affects over a hundred million people worldwide. In Europe, approximately 20% of allergic patients are allergic to birch pollen [
1]. Bet v 1 and Bet v 2 are the two clinically most relevant allergens in birch pollen allergy, recognized by more than 95% and up to 40% of the birch pollen allergic patients, respectively, depending on geographical regions [
2,
3]. Bet v 2 is a plant profilin panallergen, i.e., it is ubiquitously distributed throughout nature and shares a highly conserved amino acid sequence of up to 75% even between distantly-related organisms [
4]. This sequence conservation translates into a high similarity of the structural fold and function within other members of the profilin family [
5]. Conversely, it also results in a broad IgE cross-reactivity of profilin allergic patients to other inhalant and nutritive allergen sources [
6]. However, their sensitizing capabilities remain unclear.
Physiologically, profilins are known to play a role in cell elongation, cell shape maintenance, the growth of pollen tubes and root hairs in plants [
7]. Additionally to its ability to bind actin, profilins also interact with poly-
l-proline and phosphoinositides [
4]. Due to the high similarity among profilin family members, properties obtained from a particular profilin are often transferred by similarity to other members. As an example, detailed analyses of the profilin sequences and structures suggested the presence of an intra-molecular disulfide bridge in several members including Bet v 2 [
8]. A recent study showed that rubber tree profilin, Hev b 8, was able to homodimerize via an inter-molecular disulfide bridge [
9]. Another study also observed an intra-disulfide bond between Cys95 and Cys117 in the three cysteines containing Art v 4 crystal structure, however, it is not observed in all the molecules [
10]. It needs to be pointed out that the cysteine 95 is not conserved in profilin. By contrast, Bet v 2 contains only two conserved cysteines (Cys13 and Cys117), which were expected to form a disulfide bridge. The available Bet v 2 crystal structure (pdb entry 1cqa) did not confirm this conclusion, although the structure was obtained under reducing conditions. The existence of such post-translational modifications could influence the antigen stability toward endo-lysosomal proteases, which ultimately may impact the allergenicity of Bet v 2.
In this study, we present crystal structures of Bet v 2 in the reduced (without disulfide bridge) and the oxidized (with disulfide bridge) states at a resolution of 2.0 and 1.7 Å respectively. Furthermore, we examined the relevance of the existence of the intra-molecular disulfide bond with respect to structural, biochemical and immunological characteristics. Importantly, the observed differences are independent of the presence of reducing agents, reflecting the conformational differences of Bet v 2 in the presence and the absence of the disulfide bridge.
3. Discussion
A broad variety of post-translational modifications are known to regulate the stability and function of proteins, including glycosylation, phosphorylation, and disulfide formation [
16]. While the endo-lysosome is equipped with an array of hydrolases to remove many of these modifications, the removal is often incomplete. Indeed, several of these modifications persist during antigen processing, thereby influencing the recognition by and stimulation of immune cells [
16]. This is particularly true for disulfide bond formation, which has been previously shown to have the capacity to modulate T-cell recognition [
17,
18].
The correct post-translational modification is dependent on the host organism, which explains why the authentic modifications are sometimes not obtained in recombinantly-produced proteins, and are therefore often under-appreciated. As an example, heterologous expression in
E. coli cells will typically produce proteins without disulfides formed, because the preferred cytosolic expression has a reducing environment. However, bioinformatic analysis and strict sequence conservation suggested that Bet v 2 should contain a disulfide bridge (
Figure S1a) [
8]. By contrast, the reported crystal structure of Bet v 2 lacked a disulfide bridge (PDB entry 1cqa), leaving open the question of whether a disulfide bridge might have formed under non-reducing protein production and crystallization protocols, i.e., whether Bet v 2 has the intrinsic property to form a disulfide bond.
These expectations were further reinforced by the strict conservation of the cysteine residues of the proposed disulfide in related plant profilins (
Figure S1a). Furthermore, Thr113 was suggested to be phosphorylated in Bet v 2 by sequence similarity, albeit not strictly conserved, and also not supported by a birch pollen proteome analysis [
11]. Therefore, additional experimental data were critical to evaluate the possible Cys13-Cys117 disulfide bond and its possible relevance.
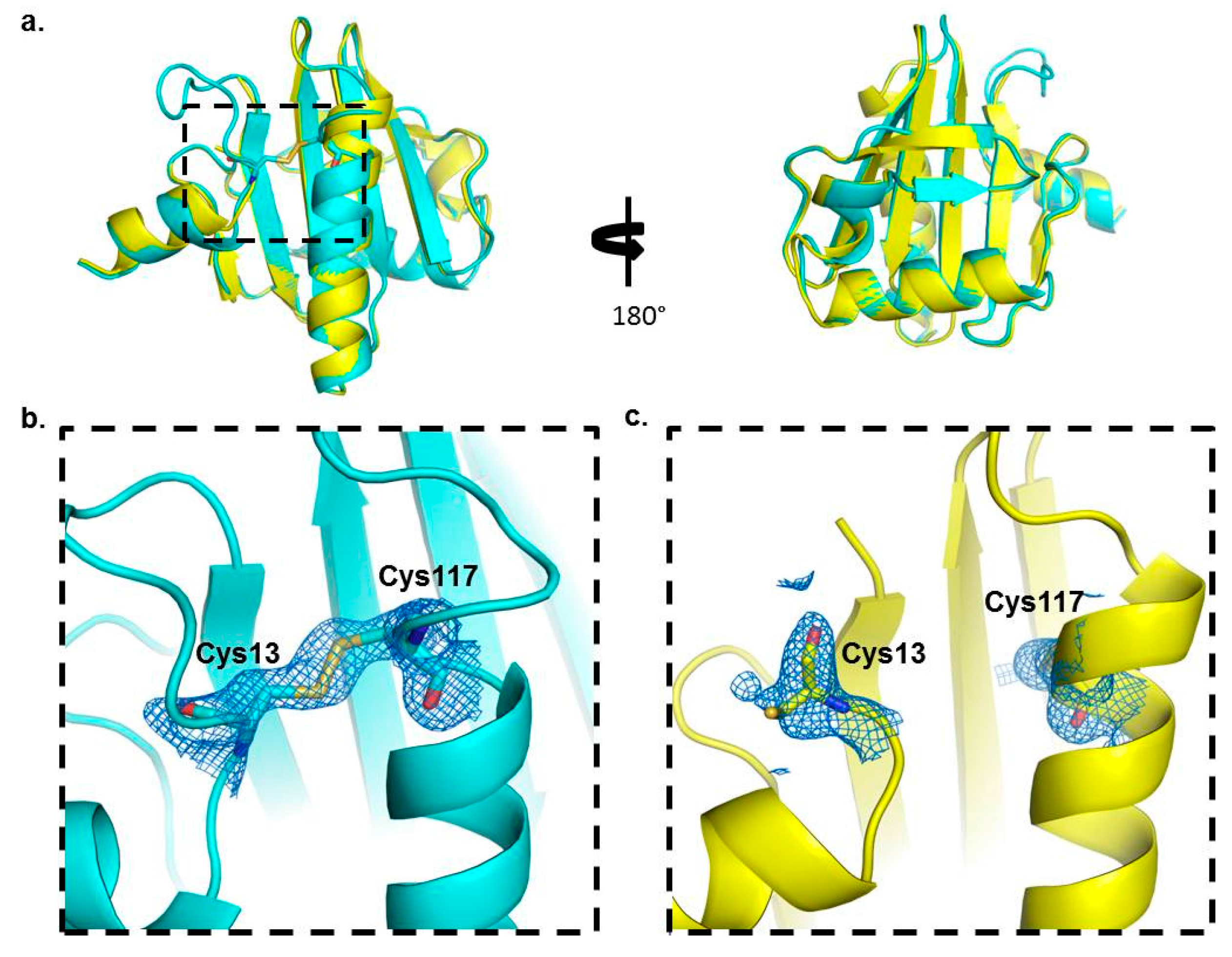
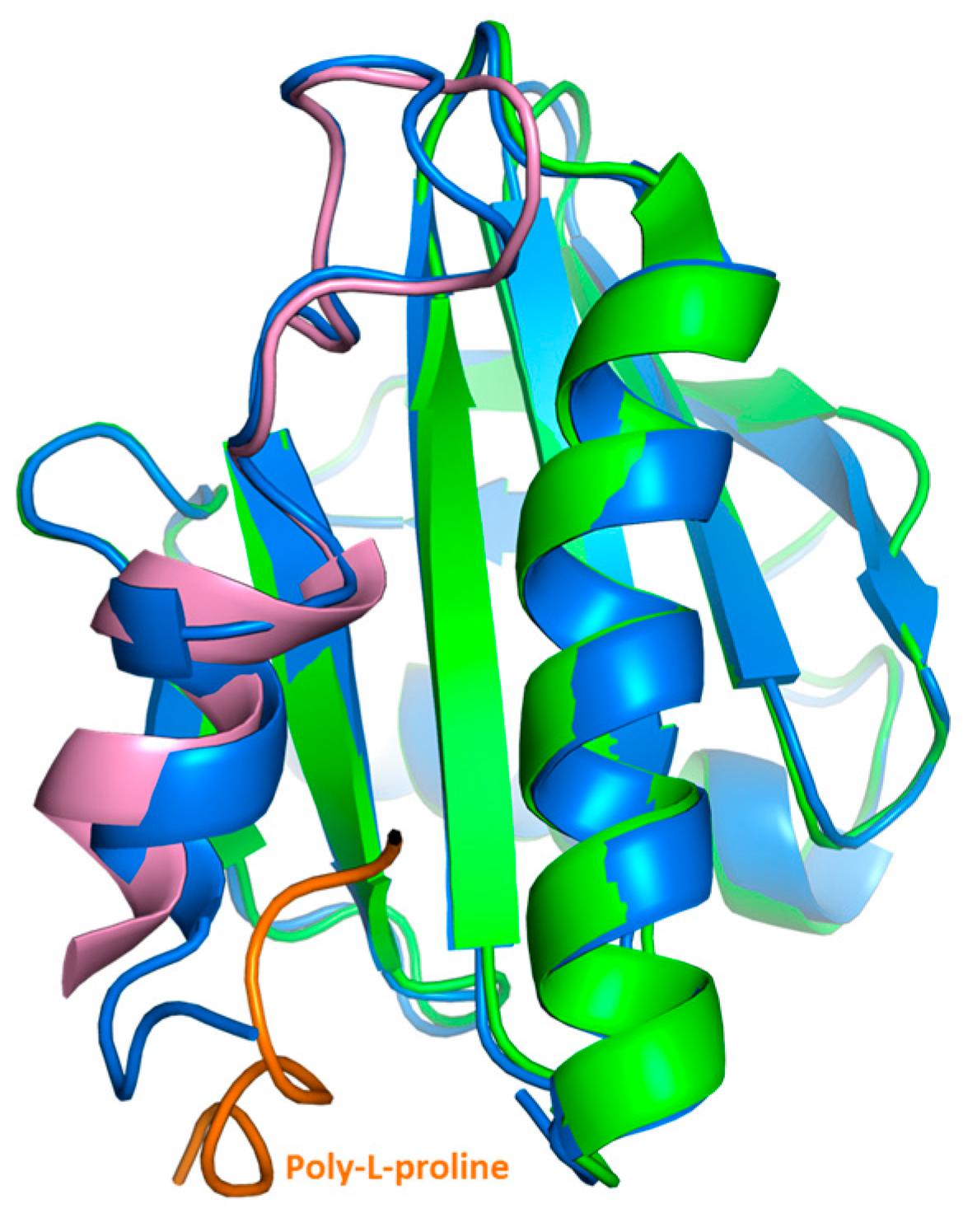
Our crystal structure analysis revealed that Bet v 2 can indeed adopt two distinct conformations, representing the reduced and the oxidized, i.e., disulfide-bridged form. While the overall structure was conserved between these two forms, the crystal structures revealed marked differences near the N- and C-terminal segments, which are linked by the disulfide bridge Cys13-Cys117 (
Figure 2). These differences were also manifested in solution by a significantly higher binding affinity of the oxidized (disulfide-bonded) than the reduced form towards poly-
l-proline resin, as evidenced by a preferential elution of the reduced Bet v 2 form. Recently, a crystal structure of the complex of latex profilin with poly-
l-proline has revealed the direct involvement of the terminal helices in ligand binding [
10]. The poly-
l-proline binding sites of Bet v 2 map onto the immediate neighborhood of the Cys13-Cys117 disulfide bond, providing a direct link between the differences in proline binding with the differences in the crystal structures of both Bet v 2 forms (
Figure 5). Hence, the apparent higher binding ability to poly-
l-proline of the oxidized Bet v 2 can be explained by the conformational selection principle: the likelihood for the ligand to find the receptor in a conformation suitable for binding is increased if the conformational space of the receptor is restricted by a disulfide bridge.
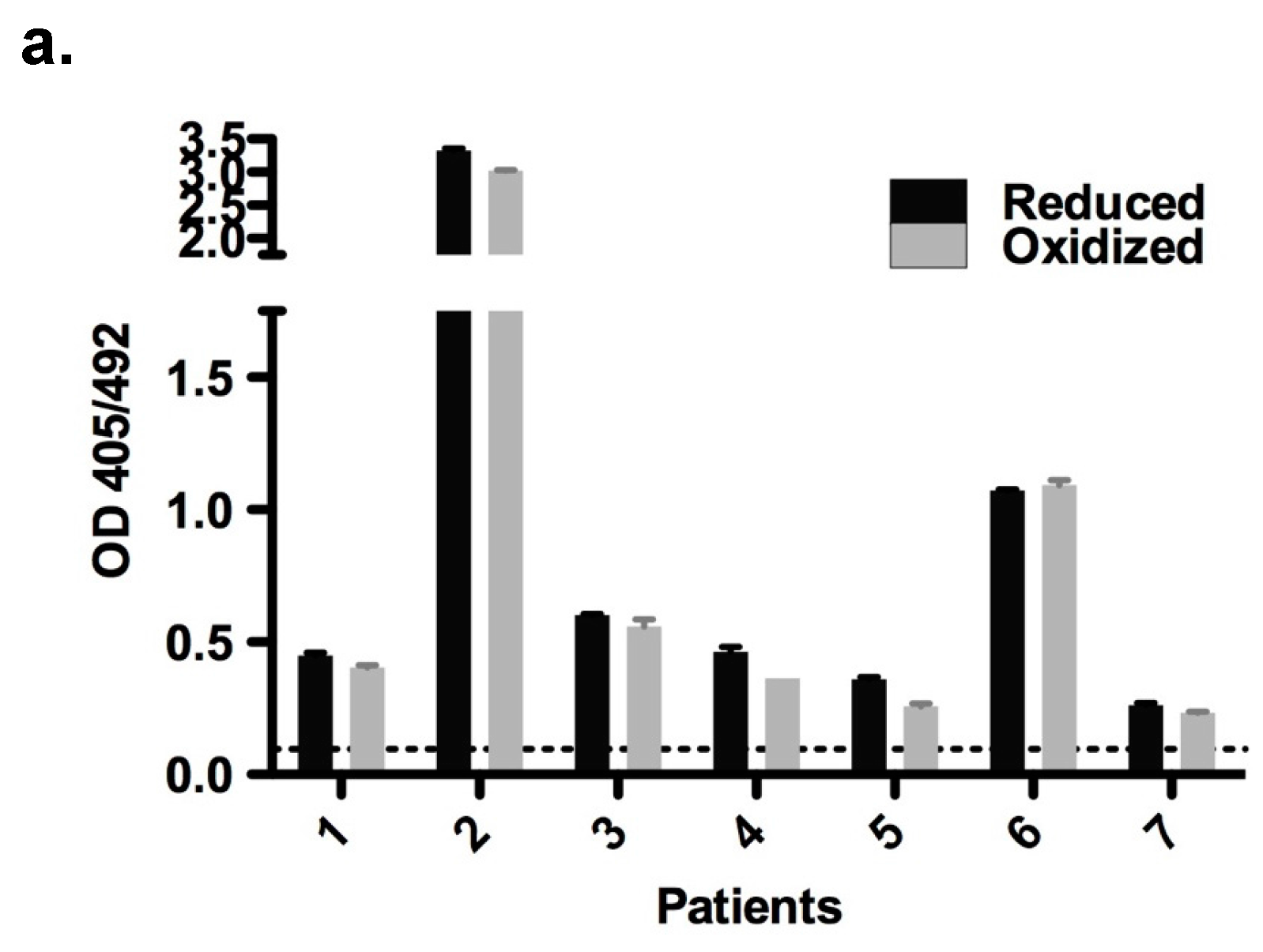
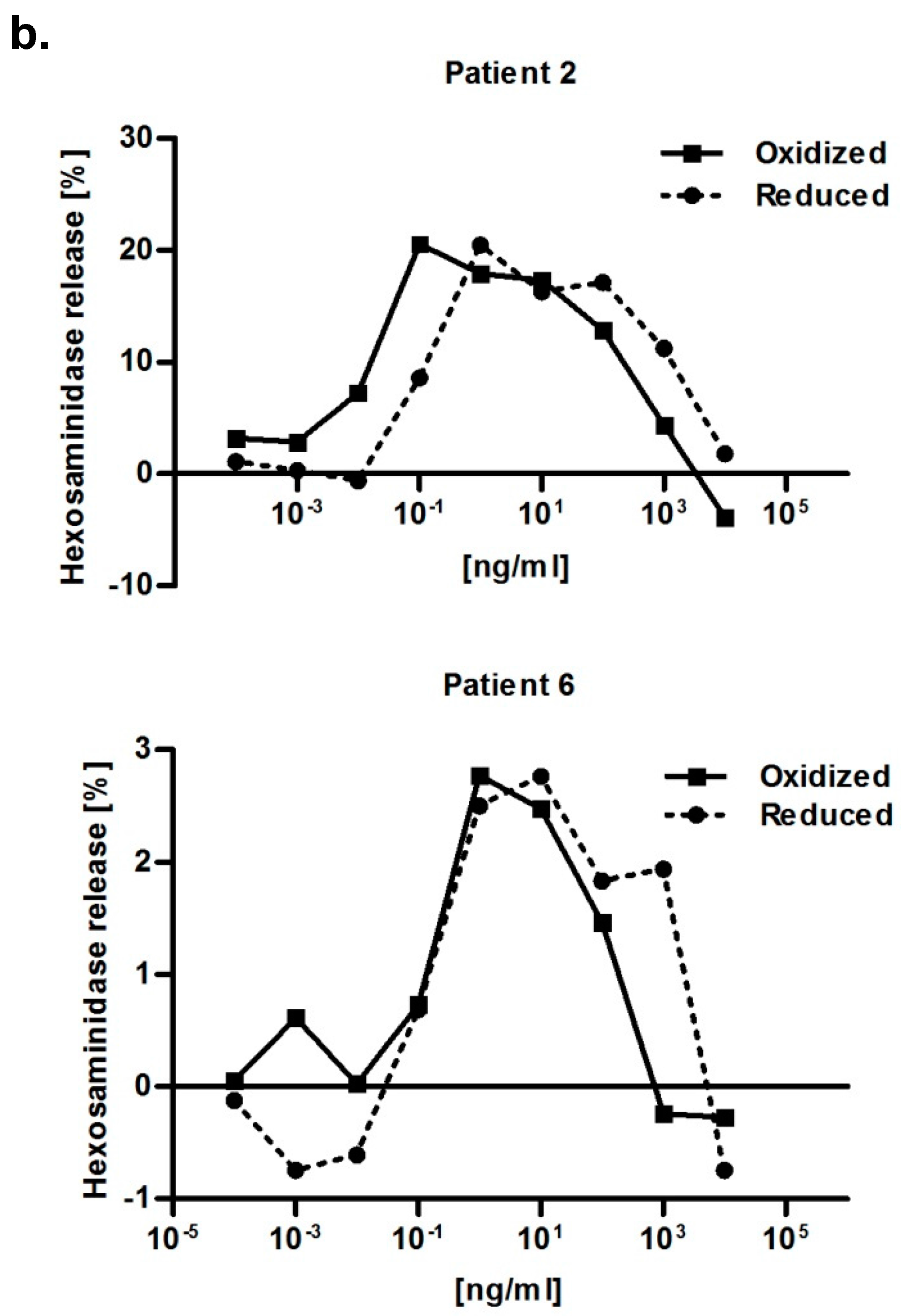
Both redox forms exhibited comparable allergenic reactivity with respect to IgE binding and histamine release (
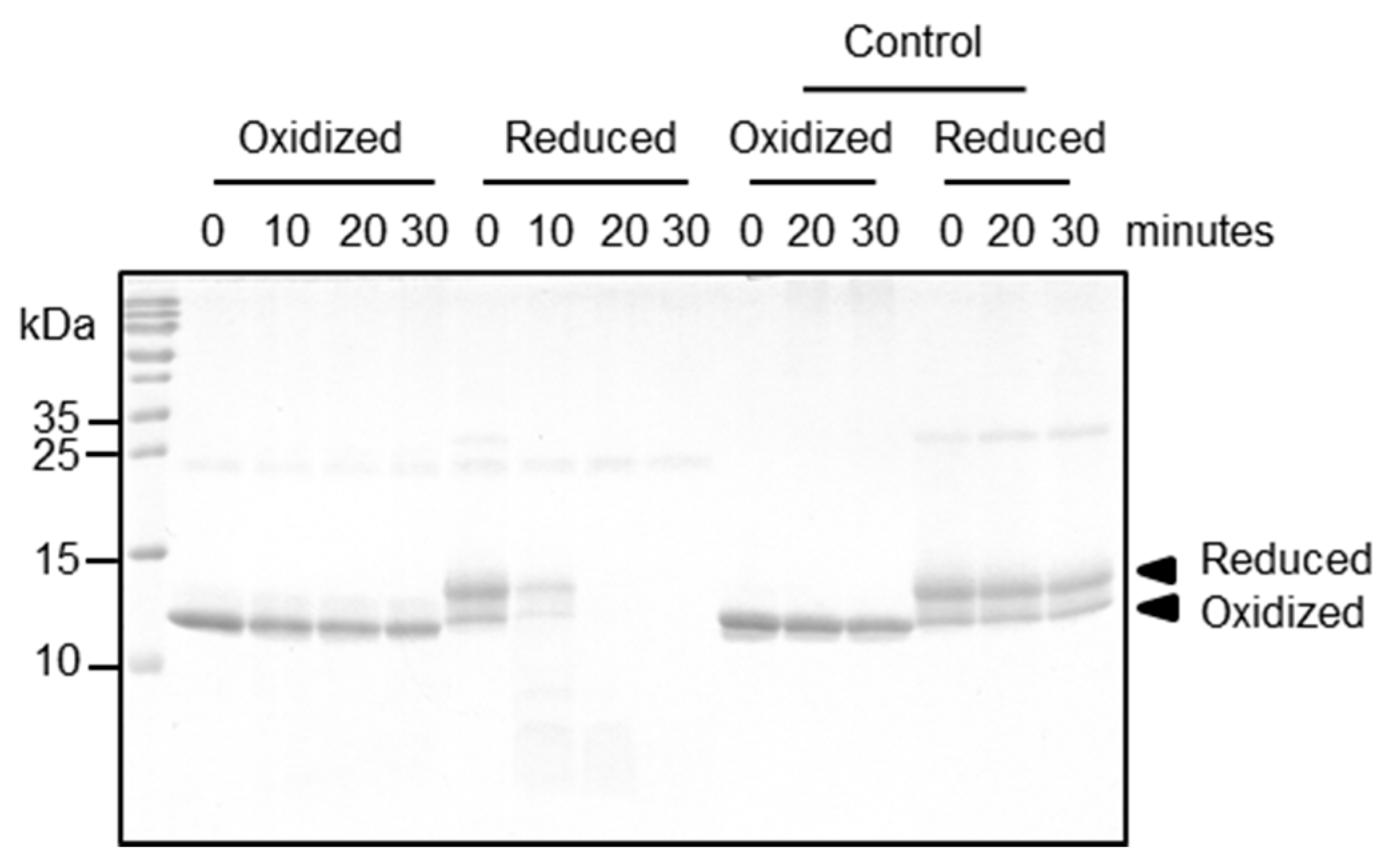
Figure 4). Further immunological conclusions would require a broader data base, i.e., a larger patient cohort—which is intrinsically difficult when working with a minor allergen. Importantly, however, the two redox forms differed strongly with respect to their proteolytic resistance, which can have major impacts on the immunologic properties of Bet v 2 for several reasons: firstly, given the long storage times of birch pollen proteins before the contact with a patient, the preferential degradation of the reduced Bet v 2 will accumulate the oxidized Bet v 2 form, resulting in a preferential exposure of patients with the more stable oxidized Bet v 2 form. Secondly, the process of allergic sensitization is intimately linked to the proteolytic processing of the allergen preceding the MHCII presentation. In this work, we showed that cathepsin S degrades the reduced form of Bet v 2 rapidly, whereas the processing of the oxidized form is sufficiently retarded. Cathepsin S has been shown to be responsible in the generation of most Bet v 1 peptide repertoire [
19]. In previous studies we showed that Bet v 1.0101 was more resistant towards cathepsin S as compared to Bet v 1.0104. This resistance in Bet v 1.0101 resulted in fewer peptides available for MHCII presentation and correlated with a stronger Th2 polarization as compared to Bet v 1.0104 [
20]. These results were supported and refined by in vivo studies, demonstrating that an increase in the fold stability of Bet v 1.0101 enhances its allergenicity and immunogenicity [
12]. These studies support the notion that resistance to endolysosomal protease is an intrinsic feature of a sensitizing allergen. Thirdly, the disulfide-mediated stabilization of the N-terminal segment is likely to prevent or attenuate Bet v 2 cleavages at Tyr6-Val7 and Trp35-Ala36 by α-chymase, which gets released from mast cells. These cleavages were found to affect and destruct IgE epitopes [
15,
21]. These findings together suggest that the Cys13-Cys117 disulfide bridge serves as a reversible proteolytic stability switch that regulates the quantity and quality, i.e., fragmentation of the available Bet v 2. The proteolytic stability switch thus determines its immunogenicity and allergenicity.
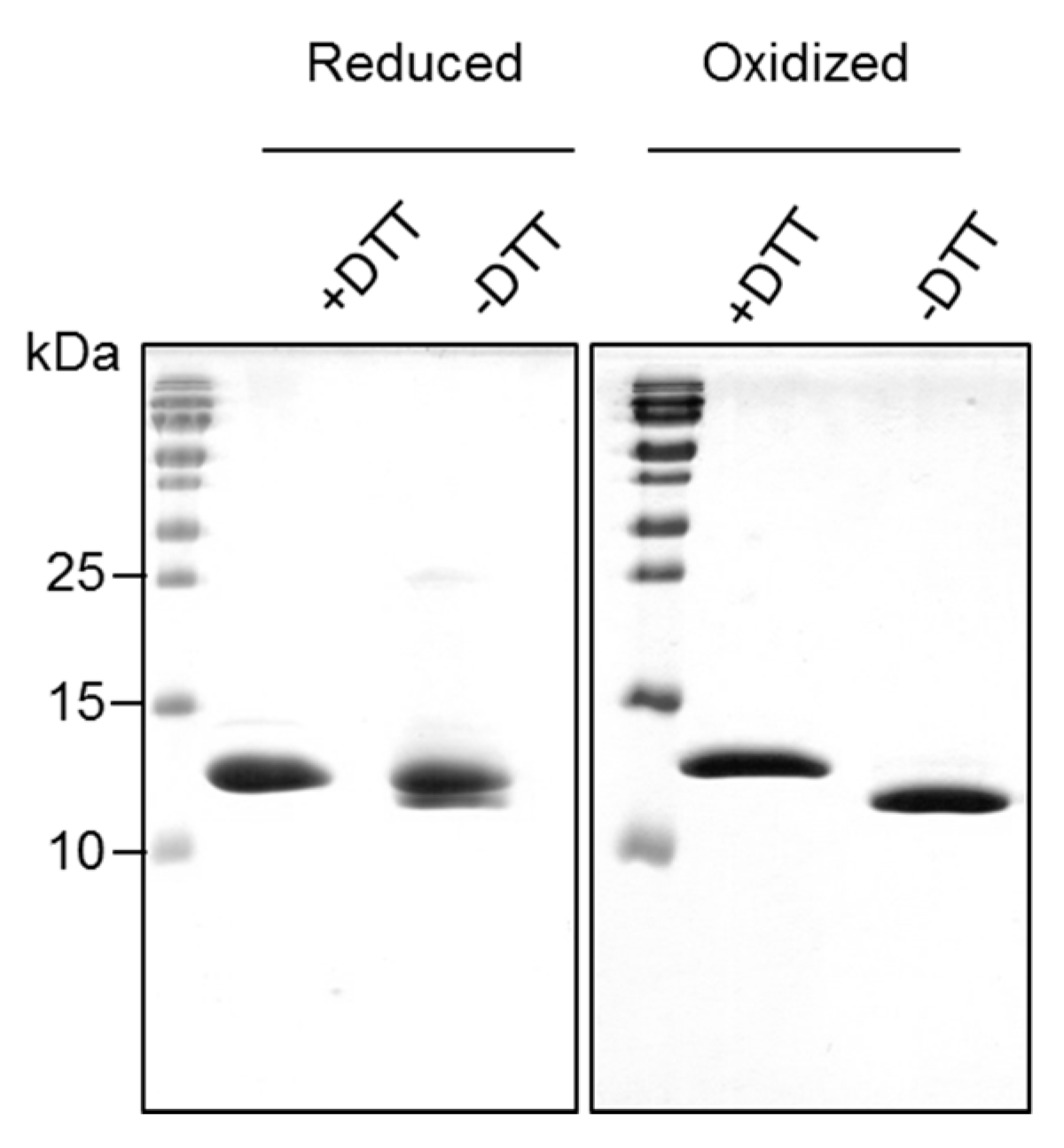
The reduced form of Bet v 2 was found prone to dimerization and higher oligomerization states under non-reducing conditions, whereas the oxidized form of Bet v 2 remained stable as a monomer. This can be explained by the exposed cysteine 13 in the reduced Bet v 2 crystal structure. It was also reported that recombinant Bet v 2 preparation easily form oligomers under non-reducing conditions, resulting in the low IgE response in monkeys, but with substantial IgG response [
22]. This oligomerization tendency of the reduced Bet v 2 form should be considered also in diagnostic tests such as the skin prick test, which could lead to false negative results.
Here we presented how a disulfide bridge can critically impact the proteolytic processing and may in turn influence the immunogenicity of Bet v 2. However, we should emphasize that our finding serves as a paradigm that is more generally applicable. For instance glycosylation, lipidation, or phosphorylation may similarly affect proteolytic processing, MHCII loading and presentation. While there are endolysosomal hydrolases such as glycosidases, lipases and phosphatases, which remove most of such modifications, these reactions are never complete and may be further regulated by pH or inhibitors as found in distinct sub-compartments of the endolysosome. Our example thus nicely illustrates that even a minority fraction of post-translationally modified proteins may have drastically different proteolytic accessibility and may thus become the immuno-dominant protein species. We therefore propose that the post-translational modifications of allergens deserve much higher attention both for sensitization and also the IgE-mediated allergic reactions.
4. Materials and Methods
4.1. Cloning, Expression and Purification of Bet v 2
Total RNA was isolated from commercial birch pollen (Ängelholm, Sweden). Pollen (0.7 g) was ground and homogenized in 10 mL extraction buffer (4 M guanidine thiocyanate, 4 mM BES pH 7.2, 4 mM EDTA, 0.01% β-mercaptoethanol, 1% N-lauroylsarkosine, 0.008% n-butanol). Supernatant was obtained by centrifugation for 5 min at 900 g, 4 °C and used for total RNA isolation using the Macherey-Nagel NucleoSpin® RNA II kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany) according to the manufacturer’s instructions. Reverse transcription of the Bet v 2 gene was performed using the SuperScript III RTS One-Step RT-PCR Kit (Invitrogen, Grand Island, NY, USA) according to the manufacturer’s specifications. Bet v 2 was amplified with Bet v 2 specific primer pair (Forward 5′-GAGACATATG TCGTGGCAAACGTACGTG-3′; Reverse 5′-GAGAGAATTCCTACAGGCCCTGGTCAATAA-3′) and cloned into the pHis-Parallel2 vector using the NdeI and EcoRI restriction sites. The vector containing Bet v 2 was verified by DNA sequencing and transformed into BL21 Star (DE3) expression host.
Overexpression was induced with 0.5 mM IPTG when the cell concentration reached an OD 600 nm of 0.6 at 37 °C for 4 h. Cells were harvested by centrifugation and resuspended in TBS buffer (50 mM Tris-HCl pH 8.5, 150 mM NaCl). The cells were then lysed by ultra-sonication. Upon centrifugation at 4 °C, clear cell lysate was filtered and subjected to affinity purification using poly-
l-proline column. The column was prepared by coupling poly-
l-proline (Sigma, St. Louis, MO, USA) onto NHS-activated agarose beads (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer protocol. The column was first washed with two column volume of TBS buffer and further washed with five column volume using the same buffer (
Figure S5). The later fraction contained relatively pure Bet v 2 which we termed it as Bet v 2 reduced form. The remaining bound Bet v 2 was then eluted with five column volume of TBS buffer containing 6 M Urea. The eluted fraction was then dialyzed against TBS buffer at 4 °C for 72 h and we termed this fraction as Bet v 2 oxidized form. Both reduced and oxidized forms of Bet v 2 were subjected to final polishing with Superdex S75 10/300 GL (GE Healthcare, Milwaukee, WI, USA) size exclusion chromatography into storage buffer (10 mM Tris-HCl pH 7.5, 20 mM NaCl). The protein concentration was determined by absorption at wavelength 280 nm and extinction coefficient of 17,085 M
−1 cm
−1.
4.2. Mass Spectrometry Analyses
For the determination of the intact mass of Bet v 2, 1 μg of protein either reduced or untreated (disulfide-bridged) protein was desalted with C18 ZipTips (EMD Millipore, Billerica, MA, USA). Using direct infusion at a flow rate of 1 μL/min, mass determination was done with a Q Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) at maximum resolution. Background peaks of the solvent were used for lock mass calibration, resulting in an accuracy of less than 2 ppm. For deconvolution of the raw data, the Xtract modul of Xcalibur (Thermo Fisher Scientific) was used. To determine intramolecular disulfide bridges, Bet v 2 were digested with the ProteoExtract All-in-One Trypsin Digestion Kit (EMD Millipore, Billerica, MA, USA) omitting the reduction/alkylation step. After the digest, samples were desalted using C18 ZipTips. Resulting peptides were separated by reverse-phase nano-HPLC (Dionex Ultimate 3000, Thermo Fisher Scientific, column: PepSwift Monolithic Nano Column, 100 μm × 25 cm, Dionex). The column was developed with an acetonitrile gradient (Solvent A: 0.1% (
v/
v) FA/0.01% (
v/
v) TFA; solvent B: 0.1% (
v/
v) FA/0.01% (
v/
v) TFA/90% (
v/
v) ACN; 5–45% B in 60 min) at a flow rate of 1 μL/min at 55 °C). The HPLC was directly coupled via nano electrospray to the Q Exactive mass spectrometer (Thermo Fisher Scientific). After deconvolution with Xtract, the mass list of uncharged signals was exported to xQuest [
23] and cross-linked peptides were matched to the Bet v 2 sequence with xBobcat. For sequence determination, tryptic peptides were HPLC-separated as described above and fragmented in the mass spectrometer with a top 12 method using a normalized fragmentation energy of 27%. Sequence analyses were performed with Peaks Studio 8 (Bioinformatics Solutions, Waterloo, ON, Canada).
4.3. Crystallization and Structure Determination
The purified oxidized Bet v 2 was concentrated to 10 mg/mL using an ultrafiltration unit with a 5 kDa cutoff membrane. To obtain a reduced form of Bet v 2, concentrated oxidized Bet v 2 was preincubated with 50 mM DTT. Hampton index screens (Hampton Research, Aliso Viejo, CA, USA) were set up to search for suitable crystallization conditions with a protein to drop ratio of 1:1 in a 96-well-plate (Art Robbins Instruments, Sunnyvale, CA, USA). Drops were equilibrated at 293 K. Bet v 2 oxidized form crystals were obtained in condition consisting of 0.1 M HEPES pH 7.5, 0.2 M magnesium chloride hexahydrate, 25% PEG3350, while the reduced form crystals were obtained in 0.1 M Tris pH 8.5, 2.0 M ammonium sulfate. The oxidized and reduced Bet v 2 crystals were cryo-protected with 35% PEG3350 and 20% ethylene glycol respectively and flash frozen in liquid nitrogen. Diffraction datasets were collected at the ESRF beamline ID30a3 and processed using XDS [
24]. The structure of both Bet v 2 oxidized and reduced forms were solved by molecular replacement using existing birch pollen profilin structure (PDB:1CQA). Molecular replacement was carried out using the CCP4 software suite [
25]. Structure models were built using COOT [
26] and refined using Phenix [
27]. Coordinates and data sets are deposited with the protein data bank (entries 5NZB and 5NZC). Since the crystal forms for the reduced and oxidized Bet v 2 differ significantly in their crystal lattice, including unit cell constants and solvent content, the two crystal lattices served to select a certain redox state, i.e., an oxidized Bet v 2 protein would be incompatible with the reduced crystal form and would, therefore, not be incorporated into the lattice, even if some oxidized Bet v 2 protein is present in solution.
4.4. Proteolytic Processing Assay
Human cathepsin S was overexpressed, purified and activated as described previously [
20]. Cathepsin S and Bet v 2 were incubated with a molar ratio of 1 to 20 in digestion buffer (50 mM Bis-Tris pH 5.5, 100 mM NaCl) at 37 °C for 10, 20 and 30 min. At the indicated time point, samples were collected and heated at 95 °C for 5 min. All samples were then analyzed on 18% SDS-PAGE under non-reducing conditions. As a control, Bet v 2 was incubated at the same conditions without the presence of cathepsin S. Processing of Bet v 2 by human legumain was performed in the same condition by replacing cathepsin S. Human legumain was prepared as described in [
28].
4.5. Patients and Sera
Upon case history, positive in vivo skin prick test and in vitro IgE detection (CAP system, Thermo Fisher Scientific, Phadia AB, Uppsala, Uppland, Sweden), birch pollen allergic patients reactive to Bet v 2 were selected (n = 7). Informed consent was obtained from all subjects included in the study and experiments with patients’ sera were approved by the ethics committee of the Medical University of Vienna (no. EK028/2006).
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
MaxiSorp™ flat-bottom plates (Nunc, Roskilde, Zealand, Denmark) were coated with purified oxidized and reduced forms of rBet v 2 (100 ng/well in 50 μL PBS) overnight at 4 °C. Following a blocking step, plates were incubated with 50 μL/well patients’ sera (n = 7) diluted 1:10 overnight at 4 °C. Detection of bound human IgE was performed with alkaline phosphatase-conjugated monoclonal anti-human IgE antibody (BD Biosciences, San Jose, CA, USA) and measured at 405/492 nm.
4.7. RBL Mediator Release Assay
Mediator release assays were performed according to a protocol adapted from [
29]. RBL-2H3 rat basophilic leukemia cells transfected with the human IgE receptor Fcε type-1 were passively sensitized with Bet v 2-allergic patient sera by overnight incubation at 37 °C, 7% CO
2. Every patient’s serum was previously pre-incubated 1:10 in a AG-8 cell (ATCC, Germany) suspension resuspended in tissue culture medium (MEM) with Earle´s salts (PAN-Biotech, Aidenbach, Germany) supplemented with 5% inactivated FCS (PAN-Biotech, Germany), 4 mM L-glutamine (PAN-Biotech, Germany) and the antibiotic geneticin (diluted 1:100 in medium) (Sigma, St. Louis, MO, USA) in order to deplete complement factors. After washing the cells with Tyrode’s buffer (containing 9.5 g/L Tyrode’s salts (Sigma), 1 g/L sodium bicarbonate and 0.1% (
w/
v) BSA), serial 100 μL/well dilutions of the antigens (each in nine dilution steps starting with 10,000 ng/mL until 0.0001 ng/mL as well as a 0 ng/mL control) in Tyrode’s D2O (Tyrode’s buffer diluted 1:1 in deuterium oxide (Sigma)) were added to the cells, resulting in an antigen-dependent β-hexosaminidase release into the supernatant. The release was measured by enzymatic cleavage of the fluorogenic substrate 4-methylumbelliferyl-
N-acetyl-β-glucosaminide (Sigma, USA) after 1 h of incubation at 37 °C and the addition of 100 μL/well of 0.2 M glycine solution (pH 10.7) at an excitation wavelength of 360 nm and an emission of 440 nm. The release was expressed as percentage of release from cells sensitized with patient’s serum corrected for spontaneous release (just buffer, no serum) divided through total release. The total release of RBL cells was obtained by lysing the cells with 10% Triton X-100 (Amresco, Solon, OH, USA). Afterwards, curves were normalized, meaning the 0 ng/mL control value of each serum was subtracted from the measurement values. Serum obtained from a Bet v 2-non allergic individual was used as a negative control for IgE-mediated degranulation.
4.8. NMR One-Dimensional Spectrum
NMR spectra were recorded at 298 K on 600 MHz Avance III HD Bruker spectrometer equipped with a QXI probe and four channels for 1H/13C/15N/31P. The Bet v 2 samples at 0.2 mM protein concentrations were measured in a buffer consisting of 10 mM Tris pH 7.5 and 20 mM NaCl containing 7% D2O in 5 mm Shigemi tubes. Standard 1D 1H spectra with a WATERGATE (WATER suppression by GrAdient Tailored Excitation) scheme for solvent suppression using a 3-9-19 pulse train were recorded with 32 scans and an interscan delay of 2 s. All spectra are referenced to 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS) using the 2 mM Sucrose/0.5 mM DSS standard sample as an external reference. All spectra were processed and analyzed with Topspin 3.1 (Bruker, Billerica, MA, USA).
4.9. Circular Dichroism (CD) Spectroscopy
Secondary structure elements were determined using CD spectroscopy. The measurement was performed using a JASCO J-815 spectropolarimeter fitted with a PTC-423S Peltier-type single position cell holder (Jasco, Japan). Spectra were recorded ranging from a wavelength of 190 to 260 nm and presented as mean residue molar ellipticity. The samples were suspended in a 10 mM potassium phosphate buffer pH 7 at a concentration of 0.1 mg/mL. The background signal (buffer only) was recorded and subtracted from each spectrum. Secondary structure contents were calculated using CDNN CD Spectra Deconvolution software version 2.1 (Applied Photophysics, Leatherhead, UK).
4.10. Fourier Transform Infrared (FTIR) Spectroscopy
The proteins at a concentration of 0.5 to 1.5 mg/mL were analyzed at a constant temperature of 25 °C using an AquaSpec transmission cell adapted to a Tensor II FTIR system (Bruker Optics Inc., Billerica, MA, USA). Amide bands were recorded at a wavenumber ranging from 1500 to 700 cm−1. Recorded spectra were analyzed with the OPUS spectroscopy software 6.0 (Bruker Optics Inc., USA). The 2nd derivative of amide I bands was calculated by applying the Savitzky–Golay algorithm on the vector normalized spectra (25 smoothing points). The secondary structure elements were quantified via a Quant2 method of the OPUS software.
4.11. Thermal Shift Assay
The thermal stability of Bet v 2 was accessed through incubation with SYPRO Orange dye (Thermo Fisher Scientific, USA) and recorded using an Applied Biosystems 7500 RT-PCR thermocycler (Thermo Fisher Scientific, USA). The protein concentration was 0.3 mg/mL in assay buffer (50 mM Tris, 100 mM NaCl, pH 7.4) with or without 10 mM of DTT or 0.3 mg/mL of poly-l-proline (molecular weight from 1–10 kDa, Sigma-Aldrich, USA). The reaction was then filtered (0.2 μm centrifugation device, Pall Life Sciences, Port Washington, NY, USA) prior to mixing with a final concentration of 2× SYPRO Orange dye (excitation, 280 and 450 nm; emission, 610 nm). Each reaction was carried out in a volume of 25 μL in triplicates and heated in an Applied Biosystems 7500 RT-PCR thermocycler from 20 to 95 °C in 1 °C increments for 1 min. The results were analyzed using GraphPad Prism v5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
4.12. Sequence Alignment and Other Computational Analysis
Multiple amino acid sequence alignment was done using CLUSTAL O (version 1.2.4) (
http://www.uniprot.org/align/) multiple sequence alignment tool. Program Superpose of CCP4 suite was used to calculate Cα-rmsd.5. Statistical analyses were performed using GraphPad Prism 5 (version 5.0a).