The Immunogenicity of Branded and Biosimilar Infliximab in Rheumatoid Arthritis According to Th9-Related Responses
Abstract
:1. Introduction
2. Results
2.1. Baseline Demographic and Clinical Assessment
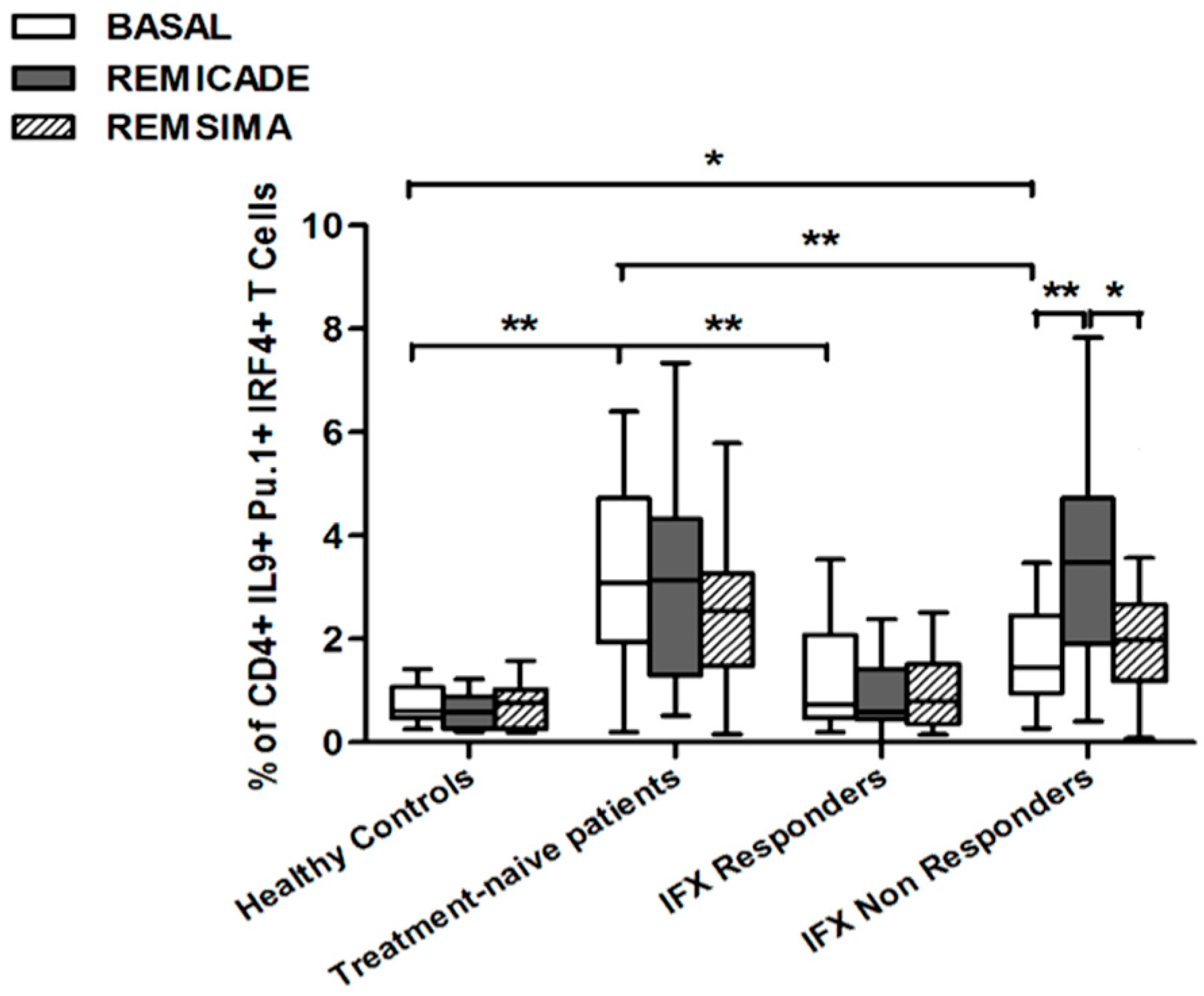
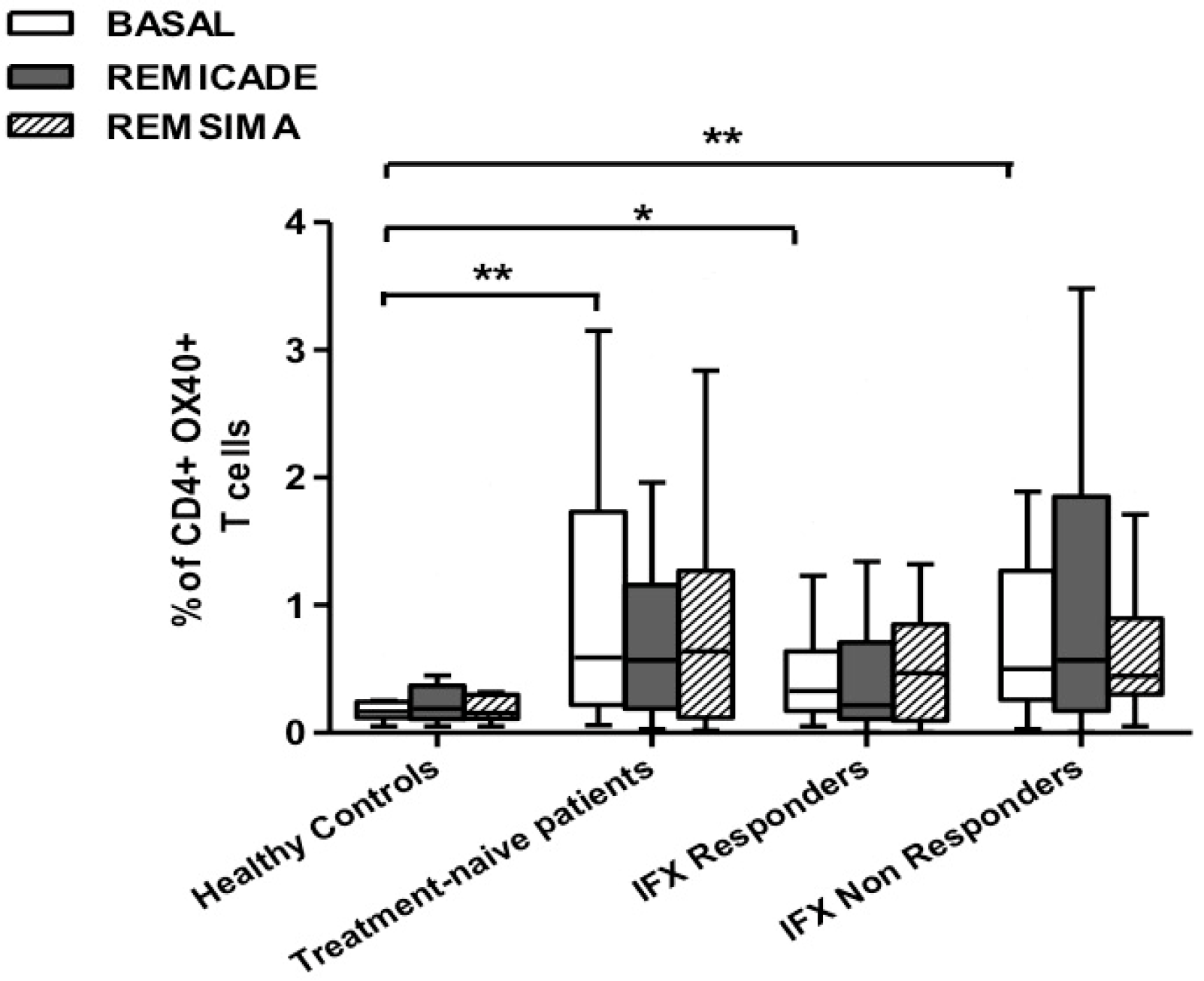
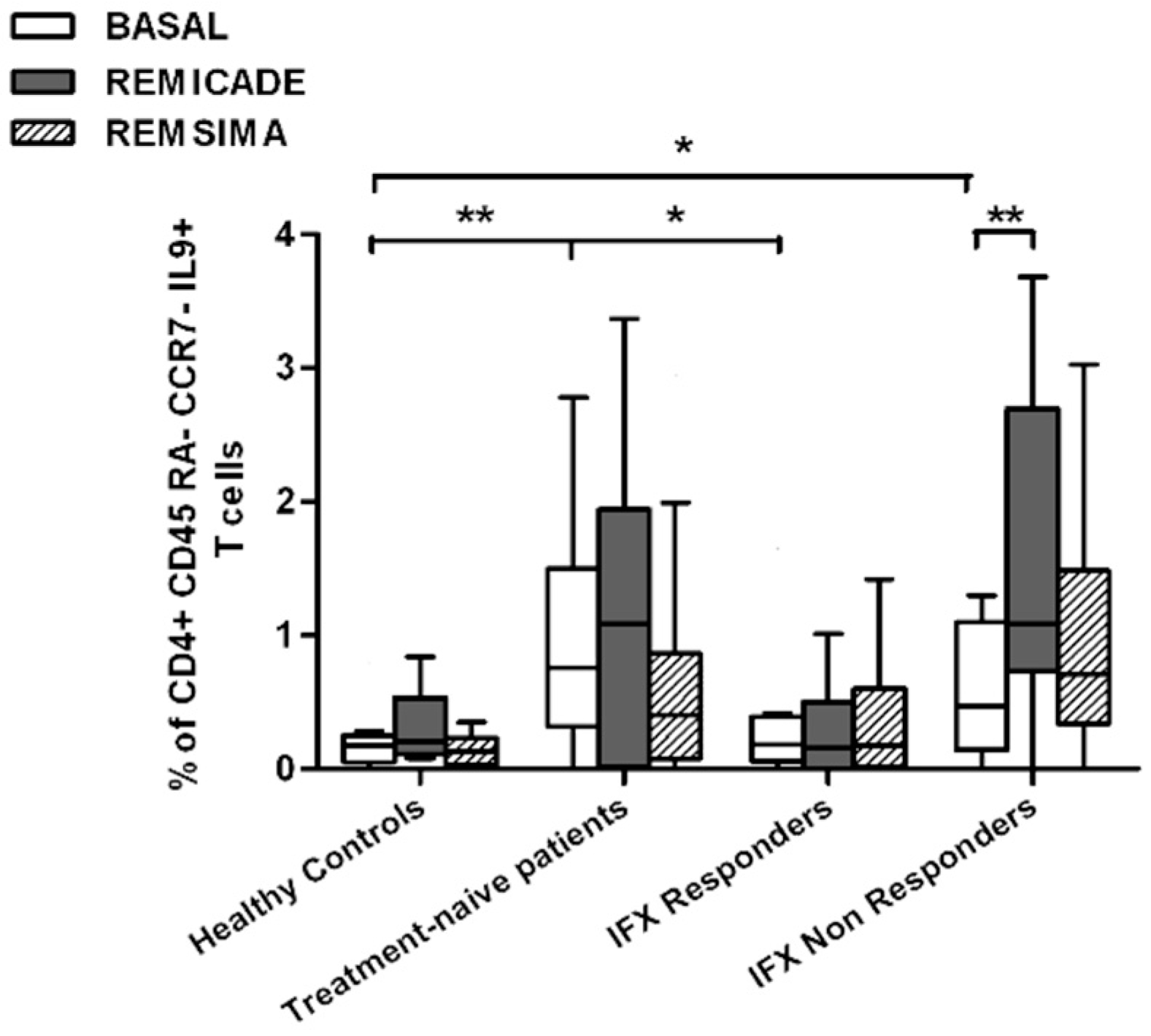
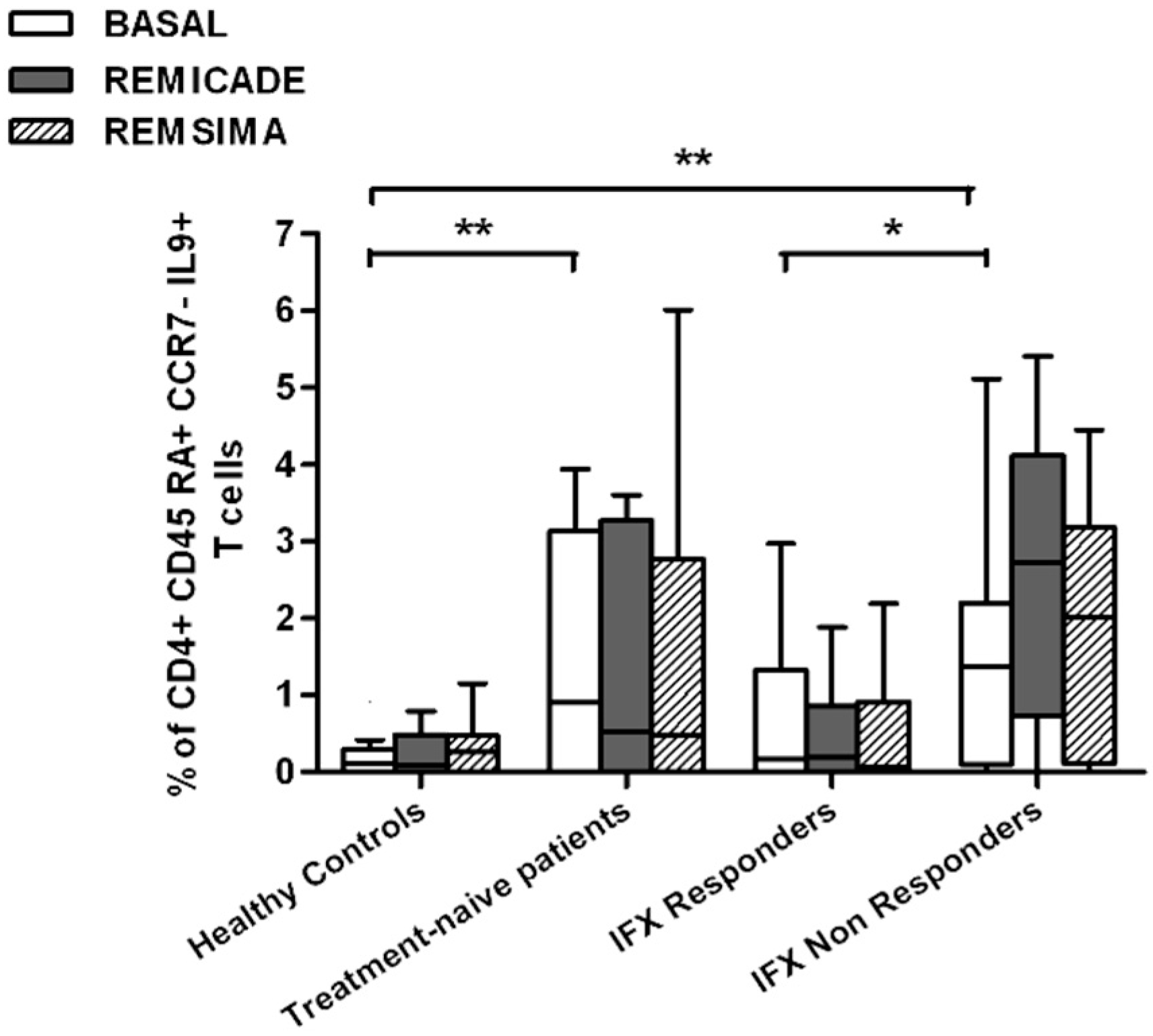
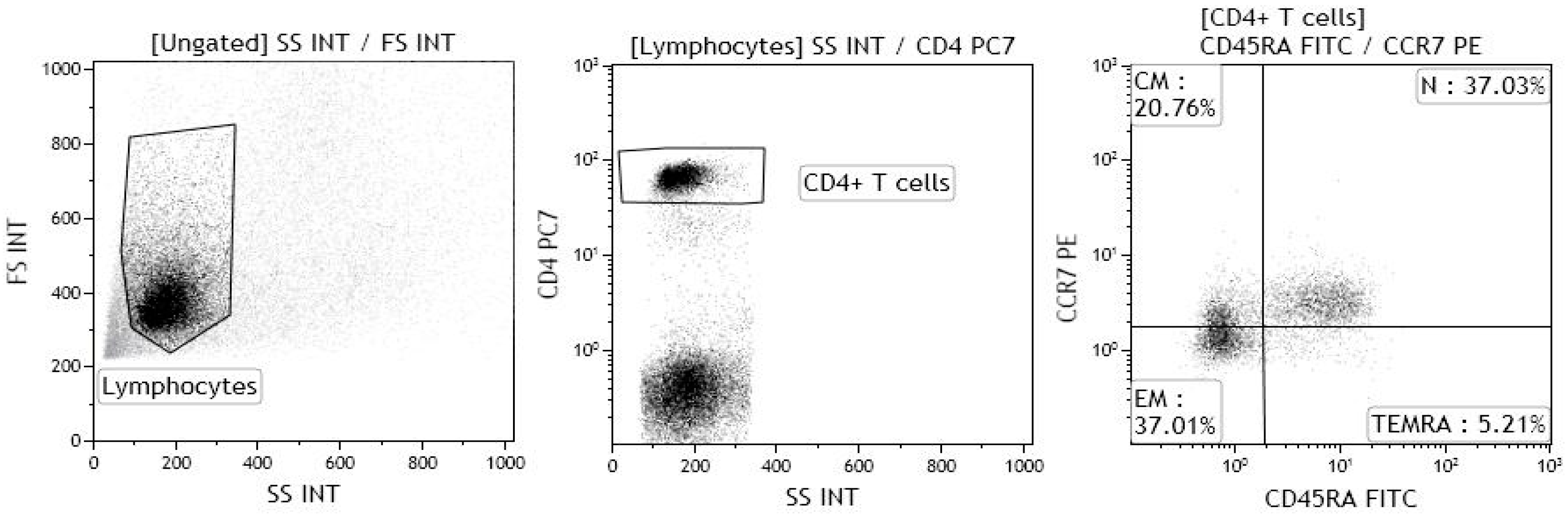
2.2. T helper 9 Cells at Baseline
2.3. Effects of Infliximab (Remicade®) on T Helper 9 Cells
2.4. Comparison of the Effects of Remicade® and Remsima® on T Helper 9 Cells
3. Discussion
4. Materials and Methods
4.1. Population
4.2. Immunological Analyses
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Kaplan, M.H.; Hufford, M.M.; Olson, M.R. The development and in vivo function of T helper 9 cells. Nat. Rev. Immunol. 2015, 15, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Balasubramanian, S.; Liu, W.; Chu, X.; Wang, H.; Taparowsky, E.J.; Fu, Y.X.; Choi, Y.; Walsh, M.C.; Li, X.C. OX40 signaling favors the induction of Th 9 cells and airway inflammation. Nat. Immunol. 2012, 13, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielinski, C.E.; Corti, D.; Mele, F.; Pinto, D.; Lanzavecchia, A.; Sallusto, F. Dissecting the human immunologic memory for pathogens. Immunol. Rev. 2011, 240, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Harari, A.; Vallelian, F.; Pantaleo, G. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur. J. Immunol. 2004, 34, 3525–3533. [Google Scholar] [CrossRef] [PubMed]
- Jahn, E.M.; Schneider, C.K. How to systematically evaluate immunogenicity of therapeutic proteins—Regulatory considerations. New Biotecnhnol. 2009, 25, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, F.R.; Valesini, G. Immunogenicity of anti-tumor necrosis factor drugs in rheumatic diseases. Clin. Exp. Rheumatol. 2013, 31, 954–963. [Google Scholar] [PubMed]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R.B. The immunogenicity of humanised and fully human antibodies. Residual immunogenicity resides in the CDR regions. mAbs 2010, 2, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Talotta, R.; Salaffi, F.; Cassinotti, A.; Varisco, V.; Battellino, M.; Ardizzone, S.; Pace, F.; Sarzi-Puttini, P. Immunogenicity and autoimmunity during anti-TNF therapy. Autoimmun. Rev. 2013, 12, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, N.E.; de Carvalho, J.F.; Silva, C.A.; Bonfà, E. Immunogenicity of anti-TNF-α agents in autoimmune diseases. Clinic Rev. Allerg. Immunol. 2010, 38, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Vultaggio, A.; Matucci, A.; Nencini, F.; Pratesi, S.; Parronchi, P.; Rossi, O.; Romagnani, S.; Maggi, E. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy 2010, 65, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Baert, F.; Noman, M.; Vermeire, S.; Van Assche, G.; D’Haens, G.; Carbonez, A.; Rutgeerts, P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N. Engl. J. Med. 2003, 348, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Noman, M.; Van Assche, G.; Baert, F.; D’Haens, G.; Rutgeerts, P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut 2007, 56, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Talotta, R.; Berzi, A.; Atzeni, F.; Batticciotto, A.; Clerici, M.; Sarzi-Puttini, P.; Trabattoni, D. Paradoxical Expansion of Th1 and Th17 Lymphocytes in Rheumatoid Arthritis Following Infliximab Treatment: A Possible Explanation for a Lack of Clinical Response. J. Clin. Immunol. 2015, 35, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Chaves, P.; Doña, I.; Blanca-López, N.; Canto, G.; Mayorga, C.; Blanca, M. T-cell involvement in delayed-type hypersensitivity reactions to infliximab. J. Allergy Clin. Immunol. 2011, 128, 1365–1367. [Google Scholar] [CrossRef] [PubMed]
- Dardalhon, V.; Awasthi, A.; Kwon, H.; Galileos, G.; Gao, W.; Sobel, R.A.; Mitsdoerffer, M.; Strom, T.B.; Elyaman, W.; Ho, I.C.; et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nat. Immunol. 2008, 9, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; Klein, M.; Bopp, T. Th9 cells, new players in adaptive immunity. Trends Immunol. 2014, 35, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Elyaman, W.; Bradshaw, E.M.; Uyttenhove, C.; Dardalhon, V.; Awasthi, A.; Imitola, J.; Bettelli, E.; Oukka, M.; van Snick, J.; Renauld, J.C.; et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc. Natl. Acad. Sci. USA. 2009, 106, 12885–12890. [Google Scholar] [CrossRef] [PubMed]
- Leng, R.X.; Pan, H.F.; Ye, D.Q.; Xu, Y. Potential role of IL-9 in the pathogenesis of systemic lupus erythematosus. Am. J. Clin. Exp. Immunol. 2012, 1, 28–32. [Google Scholar] [PubMed]
- Nalleweg, N.; Chiriac, M.T.; Podstawa, E.; Lehmann, C.; Rau, T.T.; Atreya, R.; Krauss, E.; Hundorfean, G.; Fichtner-Feigl, S.; Hartmann, A.; et al. IL-9 and its receptor are predominantly involved in the pathogenesis of UC. Gut 2015, 64, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Yoshizaki, A.; Asano, Y.; Kadono, T.; Sato, S. Serum interleukin-9 levels are increased in patients with systemic sclerosis: Association with lower frequency and severity of pulmonary fibrosis. J. Rheumatol. 2011, 38, 2193–2197. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.H.; Krishnan, V.V.; Ziman, M.; Janatpour, K.; Wun, T.; Luciw, P.A.; Tuscano, J. Comparison of multiplex suspension array large-panel kits for profiling cytokines and chemokines in rheumatoid arthritis patients. Clin. Cytometry 2009, 76, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Austin, J.M.; Deane, K.D.; Derber, L.A.; Kolfenbach, J.R.; Zerbe, G.O.; Sokolove, J.; Lahey, L.J.; Weisman, M.H.; Buckner, J.H.; Mikuls, T.R.; et al. Multiple cytokines and chemokines are associated with rheumatoid arthritis-related autoimmunity in first-degree relatives without rheumatoid arthritis: Studies of the Aetiology of Rheumatoid Arthritis (SERA). Ann. Rheum. Dis. 2013, 72, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, F.; Guggino, G.; Rizzo, A.; Manzo, A.; Vitolo, B.; La Manna, M.P.; Giardina, G.; Sireci, G.; Dieli, F.; Montecucco, C.M.; et al. Potential involvement of IL-9 and Th9 cells in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2015, 54, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Kundu-Raychaudhuri, S.; Abria, C.; Raychaudhuri, S.P. IL-9, a local growth factor for synovial T cells in inflammatory arthritis. Cytokine 2016, 79, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Talotta, R.; Berzi, A.; Atzeni, F.; Dell’Acqua, D.; Sarzi Puttini, P.; Trabattoni, D. Evaluation of Th9 lymphocytes in peripheral blood of rheumatoid arthritis patients and correlation with anti-tumor necrosis factor therapy: Results from an in vitro pivotal study. Reumatismo 2016, 68, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Paul, W.E. Heterogeneity and plasticity of T helper cells. Cell Res. 2010, 20, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Vultaggio, A.; Petroni, G.; Pratesi, S.; Nencini, F.; Cammelli, D.; Milla, M.; Prignano, F.; Annese, V.; Romagnani, S.; Maggi, E.; et al. ABIRISK Consortium. Circulating T cells to infliximab are detectable mainly in treated patients developing anti-drug antibodies and hypersensitivity reactions. Clin. Exp. Immunol. 2016, 186, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Remsima Assessment Report. EMA/CHMP/589317/2013. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002576/WC500151486.pdf (accessed on 27 June 2013).
- Jung, S.K.; Lee, K.H.; Jeon, J.W.; Lee, J.W.; Kwon, B.O.; Kim, Y.J.; Bae, J.S.; Kim, D.I.; Lee, S.Y.; Chang, S.J. Physicochemical characterization of Remsima. mAbs 2014, 6, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Pierri, C.L.; Bossis, F.; Punzi, G.; De Grassi, A.; Cetrone, M.; Parisi, G.; Tricarico, D. Molecular modeling of antibodies for the treatment of TNFα-related immunological diseases. Pharmacol. Res. Perspect. 2016, 4, e00197. [Google Scholar] [CrossRef] [PubMed]
- Yamane-Ohnuki, N.; Satoh, M. Production of therapeutic antibodies with controlled fucosylation. mAbs 2009, 1, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Homann, A.; Röckendorf, N.; Kromminga, A.; Frey, A.; Jappe, U. B cell epitopes on infliximab identified by oligopeptide microarray with unprocessed patient sera. J. Transl. Med. 2015, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.H.; Hrycaj, P.; Miranda, P.; Ramiterre, E.; Piotrowski, M.; Shevchuk, S.; Kovalenko, V.; Prodanovic, N.; Abello-Banfi, M.; Gutierrez-Ureña, S.; et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: The PLANETRA study. Ann Rheum. Dis. 2013, 72, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Hrycaj, P.; Jeka, S.; Kovalenko, V.; Lysenko, G.; Miranda, P.; Mikazane, H.; Gutierrez-Ureña, S.; Lim, M.; Lee, Y.A.; et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: The PLANETAS study. Ann Rheum. Dis. 2013, 72, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Ben-Horin, S.; Yavzori, M.; Benhar, I.; Fudim, E.; Picard, O.; Ungar, B.; Lee, S.; Kim, S.; Eliakim, R.; Chowers, Y. Cross-immunogenicity: Antibodies to infliximab in Remicade-treated patients with IBD similarly recognise the biosimilar Remsima. Gut 2016, 65, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Smits, L.J.; Derikx, L.A.; de Jong, D.J.; Boshuizen, R.S.; van Esch, A.A.; Drenth, J.P.; Hoentjen, F. Clinical Outcomes Following a Switch from Remicade® to the Biosimilar CT-P13 in Inflammatory Bowel Disease Patients: A Prospective Observational Cohort Study. J. Crohns Colitis 2016, 10, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Buer, L.C.; Moum, B.A.; Cvancarova, M.; Warren, D.J.; Medhus, A.W.; Høivik, M.L. Switching from Remicade® to Remsima® is safe and feasible: A prospective, open-label study. J. Crohns Colitis 2016, 11, 297–304. [Google Scholar]
- Benucci, M.; Gobbi, F.L.; Bandinelli, F.; Damiani, A.; Infantino, M.; Grossi, V.; Manfredi, M.; Parisi, S.; Fusaro, E.; Batticciotto, A.; et al. Safety, efficacy and immunogenicity of switching from innovator to biosimilar infliximab in patients with spondyloarthritis: A 6-month real-life observational study. Immunol. Res. 2016, 65, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- García-Piñeres, A.; Hildesheim, A.; Dodd, L.; Kemp, T.J.; Williams, M.; Harro, C.; Lowy, D.R.; Schiller, J.T.; Pinto, L.A. Cytokine and chemokine profiles following vaccination with human papillomavirus type 16L1 virus-like particles. Clin. Vaccine Immunol. 2007, 14, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Purvis, S.; Asaad, R.; Valerio, I.; Sha, B.E.; Landay, A.L.; Lederman, M.M. Levels of proinflammatory cytokines in plasma after pneumoccoccal immunization in human immunodeficiency virus type 1-infected patients. Clin. Diagn. Lab. Immunol. 1999, 6, 427–428. [Google Scholar]
- Weigmann, B.; Jarman, E.R.; Sudowe, S.; Bros, M.; Knop, J.; Reske-Kunz, A.B. Induction of regulatory T cells by leflunomide in a murine model of contact allergen sensitivity. J. Investig. Dermatol. 2006, 126, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Chong, A.S.; Ma, L.L.; Shen, J.; Blinder, L.; Yin, D.P.; Williams, J.W. Modification of humoral responses by the combination of leflunomide and cyclosporine in Lewis rats transplanted with hamster hearts. Transplantation 1997, 64, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Belmar, N.A.; Lombardo, J.R.; Chao, D.T.; Li, O.; Ma, X.; Pong-Afar, M.; Law, D.A.; Starling, G.C. Dissociation of the efficacy and cytokine release mediated by an Fc-modified anti-CD3 mAb in a chronic experimental autoimmune encephalomyelitis model. J. Neuroimmunol. 2009, 212, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Clair, E.W.; Wagner, C.L.; Fasanmade, A.A.; Wang, B.; Schaible, T.; Kavanaugh, A.; Keystone, E.C. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: Results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002, 46, 1451–1459. [Google Scholar] [CrossRef] [PubMed]


| Variables | Healthy Controls | Treatment-Naïve RA Patients | RA Patients Responding to IFX | RA Patients Non-Responding to IFX |
|---|---|---|---|---|
| Number of subjects | 10 | 15 | 20 | 20 |
| Mean age ± SD, years | 43.9 ± 8.3 | 54.8 ±16.2 | 61.3 ± 12.2 | 57.0 ± 12.2 |
| Mean disease duration ± SD, years | / | 2.3 ± 3.9 | 13.4 ± 7.2 | 18.1 ± 9.5 |
| Gender, F/M (number) | 4/6 | 12/3 | 16/4 | 15/5 |
| ACPAs+, (number) | / | 5 | 15 | 15 |
| RF+, (number) | / | 7 | 11 | 15 |
| ANAs+, (number) | / | 2 | 12 | 17 |
| Anti-dsDNA Ab, (number) | / | 0 | 3 | 2 |
| Anti-ENAs Ab+, (number) | / | 0 | 1 | 3 |
| ACLAs/LAC+, (number) | / | 0 | 1 | 2 |
| Mean CRP-DAS28 ± SD | / | 4.6 ± 1.0 | 2.5 ± 1.0 | 2.9 ± 0.8 |
| Prednisone (2.5–10 mg/day), (number) | / | / | 8 | 14 |
| Methotrexate (5–15 mg/week), (number) | / | / | 20 | 9 |
| Hydroxychloroquine (200–400 mg/day), (number) | / | / | 3 | 5 |
| NSAIDs, (number) | / | 14 | as needed | as needed |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talotta, R.; Berzi, A.; Doria, A.; Batticciotto, A.; Ditto, M.C.; Atzeni, F.; Sarzi-Puttini, P.; Trabattoni, D. The Immunogenicity of Branded and Biosimilar Infliximab in Rheumatoid Arthritis According to Th9-Related Responses. Int. J. Mol. Sci. 2017, 18, 2127. https://doi.org/10.3390/ijms18102127
Talotta R, Berzi A, Doria A, Batticciotto A, Ditto MC, Atzeni F, Sarzi-Puttini P, Trabattoni D. The Immunogenicity of Branded and Biosimilar Infliximab in Rheumatoid Arthritis According to Th9-Related Responses. International Journal of Molecular Sciences. 2017; 18(10):2127. https://doi.org/10.3390/ijms18102127
Chicago/Turabian StyleTalotta, Rossella, Angela Berzi, Andrea Doria, Alberto Batticciotto, Maria Chiara Ditto, Fabiola Atzeni, Piercarlo Sarzi-Puttini, and Daria Trabattoni. 2017. "The Immunogenicity of Branded and Biosimilar Infliximab in Rheumatoid Arthritis According to Th9-Related Responses" International Journal of Molecular Sciences 18, no. 10: 2127. https://doi.org/10.3390/ijms18102127




