NADPH Oxidase-Related Pathophysiology in Experimental Models of Stroke
Abstract
:1. Introduction
2. Experimental Models of Stroke
2.1. Distal Middle Cerebral Artery Occlusion
2.2. Proximal MCAO
2.3. Intraluminal Suture Occlusion
2.4. Photothrombotic MCAO in Spontaneously Hypertensive Rats
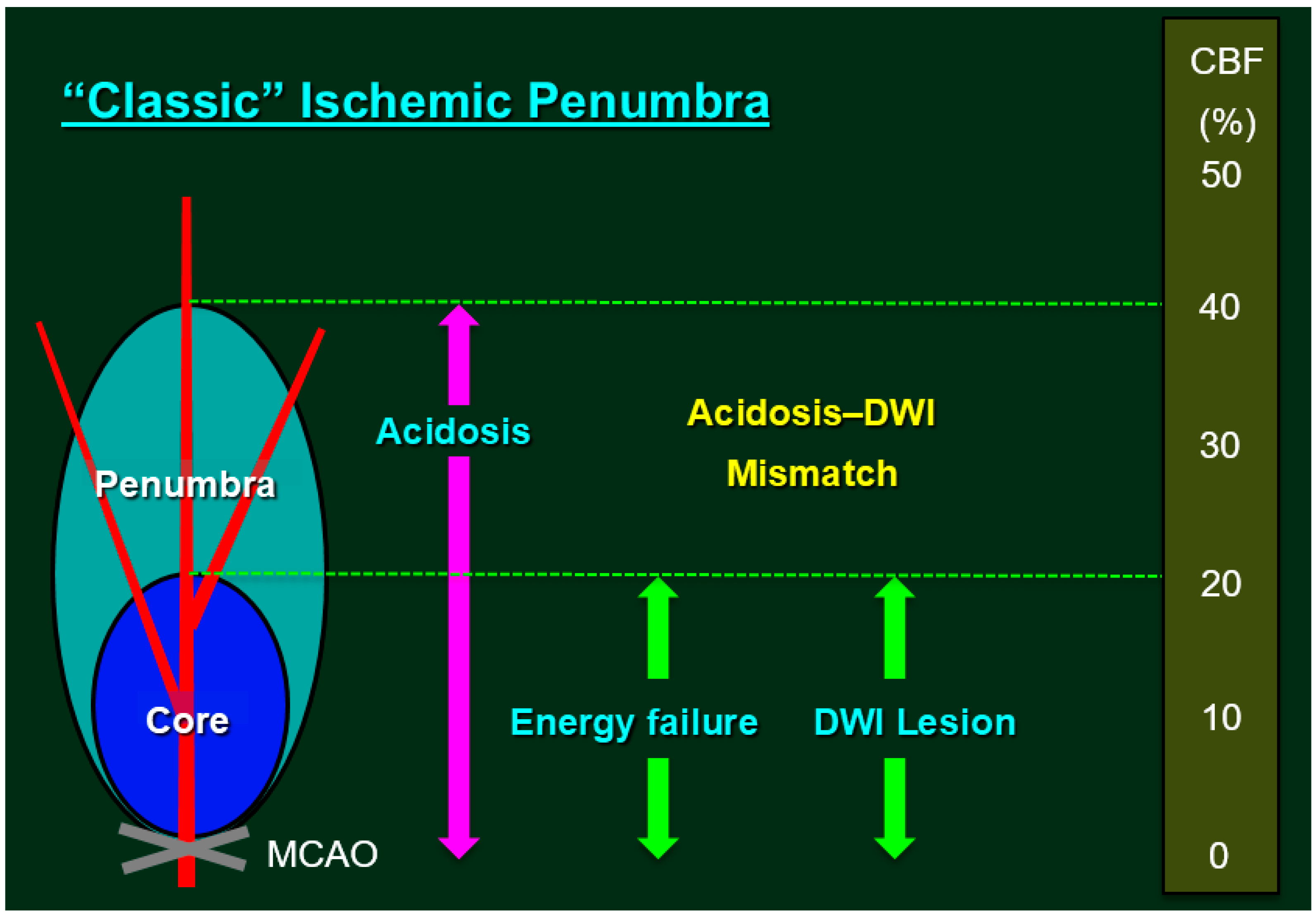
2.5. Ischemic Penumbra
2.6. Nox in Experimental Models of Stroke
2.7. Nox2
2.8. Nox1
2.9. Nox4
2.10. Pericyte Nox4 in Focal Brain Ischemia
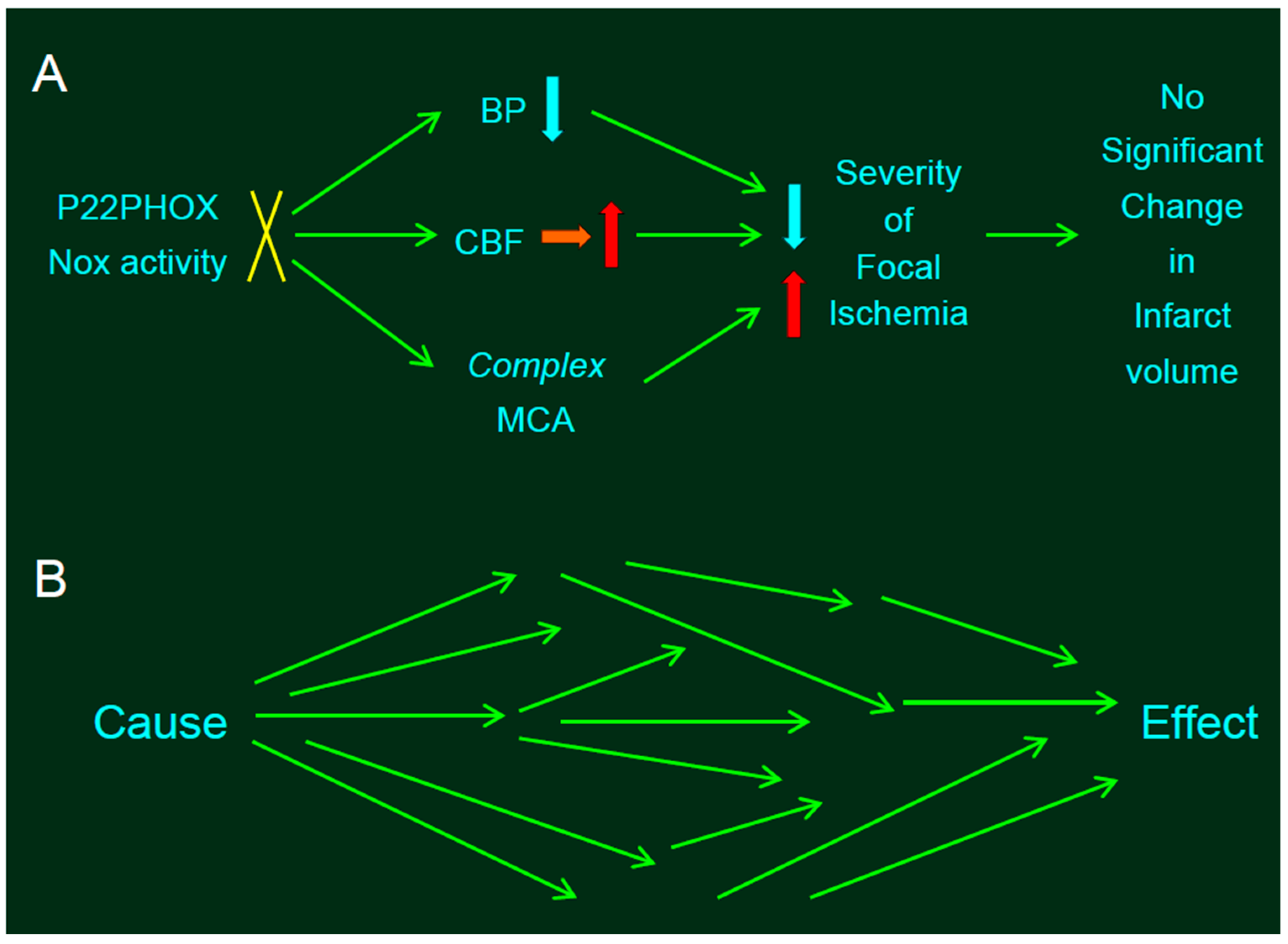
2.11. Stroke-Prone Spontaneously Hypertensive Rats (SHRSP) with Loss-of-Function in Nox
2.12. Nox and Branching Morphogenesis
2.13. Effects of Nox on Blood Pressure
3. Perspectives—Nox Knockout in Rats
4. Concluding Comments
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Sorce, S.; Stocker, R.; Seredenina, T.; Holmdahl, R.; Aguzzi, A.; Chio, A.; Depaulis, A.; Heitz, F.; Olofsson, P.; Olsson, T.; et al. NADPH oxidases as drug targets and biomarkers in neurodegenerative diseases: What is the evidence? Free Radic. Biol. Med. 2017, 112, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Amanso, A.M.; Griendling, K.K. Differential roles of NADPH oxidases in vascular physiology and pathophysiology. Front. Biosci. 2012, 4, 1044–1064. [Google Scholar]
- Miller, A.A.; Drummond, G.R.; Schmidt, H.H.; Sobey, C.G. NADPH oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ. Res. 2005, 97, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Ago, T.; Kitazono, T.; Ooboshi, H.; Iyama, T.; Han, Y.H.; Takada, J.; Wakisaka, M.; Ibayashi, S.; Utsumi, H.; Iida, M. Nox4 as the major catalytic component of an endothelial NADPH oxidase. Circulation 2004, 109, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ago, T.; Kitazono, T.; Kuroda, J.; Kumai, Y.; Kamouchi, M.; Ooboshi, H.; Wakisaka, M.; Kawahara, T.; Rokutan, K.; Ibayashi, S.; et al. NADPH oxidases in rat basilar arterial endothelial cells. Stroke 2005, 36, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Paravicini, T.M.; Chrissobolis, S.; Drummond, G.R.; Sobey, C.G. Increased NADPH-oxidase activity and Nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke 2004, 35, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Sorby-Adams, A.J.; Marcoionni, A.M.; Dempsey, E.R.; Woenig, J.A.; Turner, R.J. The role of neurogenic inflammation in blood-brain barrier disruption and development of cerebral oedema following acute central nervous system (CNS) Injury. Int. J. Mol. Sci. 2017, 18, 1788. [Google Scholar] [CrossRef] [PubMed]
- Radermacher, K.A.; Wingler, K.; Kleikers, P.; Altenhöfer, S., Jr.; Hermans, J.; Kleinschnitz, C.; Schmidt, H.H.H.W. The 1027th target candidate in stroke: Will NADPH oxidase hold up? Exp. Transl. Stroke Med. 2012, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Astrup, J.; Siesjö, B.K.; Symon, L. Thresholds in cerebral ischemia—The ischemic penumbra. Stroke 1981, 12, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Astrup, J.; Symon, L.; Branston, N.M.; Lassen, N.A. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke 1977, 8, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Belayev, L.; Ginsberg, M.D. Transient middle cerebral artery occlusion by intraluminal suture: II. Neurological deficits, and pixel-based correlation of histopathology with local blood flow and glucose utilization. J. Cereb. Blood Flow Metab. 1997, 17, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Hossmann, K.A. Viability thresholds and the penumbra of focal ischemia. Ann. Neurol. 1994, 36, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.Z.; Zhou, J.; Sun, W.; Huang, J.; van Zijl, P.C. Detection of the ischemic penumbra using pH-weighted MRI. J. Cereb. Blood Flow Metab. 2007, 27, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Warach, S.J.; Luby, M.; Albers, G.W.; Bammer, R.; Bivard, A.; Campbell, B.C.; Derdeyn, C.; Heit, J.J.; Khatri, P.; Lansberg, M.G.; et al. Acute Stroke Imaging Research Roadmap III Imaging Selection and Outcomes in Acute Stroke Reperfusion Clinical Trials. Stroke 2016, 47, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Kudo, K.; Christensen, S.; Yamashita, F.; Goodwin, J.; Higuchi, S.; Ogawa, A. Penumbral imaging by using perfusion computed tomography and perfusion-weighted magnetic resonance imaging: Current concepts. J. Stroke Cerebrovasc. Dis. 2013, 22, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; Purushotham, A.; Christensen, S.; Desmond, P.M.; Nagakane, Y.; Parsons, M.W.; Lansberg, M.G.; Mlynash, M.; Straka, M.; de Silva, D.A.; et al. The infarct core is well represented by the acute diffusion lesion: Sustained reversal is infrequent. J. Cereb. Blood Flow Metab. 2012, 32, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Nabika, T. Standards and pitfalls of focal ischemia models in spontaneously hypertensive rats: With a systematic review of recent articles. J. Transl. Med. 2012, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.; Jokelainen, P.T. Differential outcome to middle cerebral artery occlusion in spontaneously hypertensive stroke-prone rats (SHRSP) and Wistar Kyoto (WKY) rats. Stroke 1983, 14, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Graham, D.I.; McCulloch, J.; Teasdale, G.M. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 1981, 1, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Duverger, D.; MacKenzie, E.T. The quantification of cerebral infarction following focal ischemia in the rat: Influence of strain, arterial pressure, blood glucose concentration, and age. J. Cereb. Blood Flow Metab. 1988, 8, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, J.; Yoshida, Y.; Nakazawa, T.; Ooneda, G. Experimental studies of ischemic brain edema. I: A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Jpn. J. Stroke 1986, 8, 1–8. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Howells, D.W.; Porritt, M.J.; Rewell, S.S.; O’Collins, V.; Sena, E.S.; van der Worp, H.B.; Traystman, R.J.; Macleod, M.R. Different strokes for different folks: The rich diversity of animal models of focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2010, 30, 1412–1431. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, H.; Nakagomi, T.; Tamura, A.; Tsuchiya, T.; Kono, G.; Sano, K. Differences in the extent of primary ischemic damage between middle cerebral artery coagulation and intraluminal occlusion models. J. Cereb. Blood Flow Metab. 2002, 22, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Watson, B.D.; Dietrich, W.D.; Busto, R.; Wachtel, M.S.; Ginsberg, M.D. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann. Neurol. 1985, 17, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Watson, B.D.; Dietrich, W.D.; Prado, R.; Nakayama, H.; Kanemitsu, H.; Futrell, N.; Yao, H.; Markgraf, C.G.; Wester, P. Concepts and techniques of experimental stroke induced by cerebrovascular photothrombosis. In Central Nervous System Trauma: Laboratory Techniques and Recent Advancement; Ohnishi, S.T., Ohnishi, T., Eds.; CRC Press: Boca Raton, FL, USA, 1995; pp. 169–194. [Google Scholar]
- Prado, R.; Watson, B.D.; Zhao, W.; Yao, H.; Busto, R.; Dietrich, W.D.; Ginsberg, M.D. l-arginine does not improve cortical perfusion or histopathological outcome in spontaneously hypertensive rats subjected to distal middle cerebral artery photothrombotic occlusion. J. Cereb. Blood Flow Metab. 1996, 16, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Sugimori, H.; Fukuda, K.; Takada, J.; Ooboshi, H.; Kitazono, T.; Ibayashi, S.; Iida, M. Photothrombotic middle cerebral artery occlusion and reperfusion laser system in spontaneously hypertensive rats. Stroke 2003, 34, 2716–2721. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Ibayashi, S.; Sugimori, H.; Fujii, K.; Fujishima, M. Simplified model of krypton laser-induced thrombotic distal middle cerebral artery occlusion in spontaneously hypertensive rats. Stroke 1996, 27, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yao, H.; Ibayashi, S.; Uchimura, H.; Fujishima, M. Photothrombotic middle cerebral artery occlusion in spontaneously hypertensive rats: Influence of substrain, gender, and distal middle cerebral artery patterns on infarct size. Stroke 1998, 29, 1982–1986. [Google Scholar] [CrossRef] [PubMed]
- Watson, B.D.; Prado, R.; Veloso, A.; Brunschwig, J.P.; Dietrich, W.D. Cerebral blood flow restoration and reperfusion injury after ultraviolet laser-facilitated middle cerebral artery recanalization in rat thrombotic stroke. Stroke 2002, 33, 428–434. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.A.; Brekenfeld, C.; Ebinger, M.; Christensen, S.; Barber, P.A.; Butcher, K.S.; Levi, C.R.; Parsons, M.W.; Bladin, C.F.; Donnan, G.A.; et al. Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) Investigators: The benefits of intravenous thrombolysis relate to the site of baseline arterial occlusion in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET). Stroke 2010, 41, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Okada, Y.; Ibayashi, S. Therapeutic time window for YAG laser-induced reperfusion of thrombotic stroke in hypertensive rats. Neuroreport 2002, 13, 1005–1008. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Yoshii, N.; Akira, T.; Nakahara, T. Reperfusion-induced temporary appearance of therapeutic window in penumbra after 2 h of photothrombotic middle cerebral artery occlusion in rats. J. Cereb. Blood Flow Metab. 2009, 29, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Walder, C.E.; Green, S.P.; Darbonne, W.C.; Mathias, J.; Rae, J.; Dinauer, M.C.; Curnutte, J.T.; Thomas, G.R. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke 1997, 28, 2252–2258. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Song, Y.S.; Chan, P.H. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J. Cereb. Blood Flow Metab. 2009, 29, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kim, G.S.; Okami, N.; Narasimhan, P.; Chan, P.H. NADPH oxidase is involved in post-ischemic brain inflammation. Neurobiol. Dis. 2011, 42, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Jackman, K.A.; Miller, A.A.; de Silva, T.M.; Crack, P.J.; Drummond, G.R.; Sobey, C.G. Reduction of cerebral infarct volume by apocynin requires pretreatment and is absent in Nox2-deficient mice. Br. J. Pharmacol. 2009, 156, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Brait, V.H.; Lee, S.; de Silva, T.M.; Diep, H.; Eisenhardt, A.; Drummond, G.R.; Sobey, C.G. Brain infarct volume after permanent focal ischemia is not dependent on Nox2 expression. Brain Res. 2012, 1483, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Brait, V.H.; Jackman, K.A.; Walduck, A.K.; Selemidis, S.; Diep, H.; Mast, A.E.; Guida, E.; Broughton, B.R.; Drummond, G.R.; Sobey, C.G. Mechanisms contributing to cerebral infarct size after stroke: Gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J. Cereb. Blood Flow Metab. 2010, 30, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- De Silva, T.M.; Brait, V.H.; Drummond, G.R.; Sobey, C.G.; Miller, A.A. Nox2 oxidase activity accounts for the oxidative stress and vasomotor dysfunction in mouse cerebral arteries following ischemic stroke. PLoS ONE 2011, 6, e28393. [Google Scholar] [CrossRef] [PubMed]
- Kahles, T.; Luedike, P.; Endres, M.; Galla, H.J.; Steinmetz, H.; Busse, R.; Neumann-Haefelin, T.; Brandes, R.P. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke 2007, 38, 3000–3006. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.K.; Dusting, G.J.; Roulston, C.L. Nox2 knockout delays infarct progression and increases vascular recovery through angiogenesis in mice following ischaemic stroke with reperfusion. PLoS ONE 2014, 9, e110602. [Google Scholar] [CrossRef] [PubMed]
- Jackman, K.A.; Miller, A.A.; Drummond, G.R.; Sobey, C.G. Importance of NOX1 for angiotensin II-induced cerebrovascular superoxide production and cortical infarct volume following ischemic stroke. Brain Res. 2009, 1286, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Kahles, T.; Kohnen, A.; Heumueller, S.; Rappert, A.; Bechmann, I.; Liebner, S.; Wittko, I.M.; Neumann-Haefelin, T.; Steinmetz, H.; Schroeder, K.; et al. NADPH oxidase Nox1 contributes to ischemic injury in experimental stroke in mice. Neurobiol. Dis. 2010, 40, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Kim, J.H.; Lee, K.H.; Kim, H.Y.; Kim, Y.S.; Choi, W.S.; Lee, J. Role of neuronal NADPH oxidase 1 in the peri-infarct regions after stroke. PLoS ONE 2015, 10, e0116814. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Grund, H.; Wingler, K.; Armitage, M.E.; Jones, E.; Mittal, M.; Barit, D.; Schwarz, T.; Geis, C.; Kraft, P.; et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010, 8, e1000479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schröder, K.; Zhang, M.; Benkhoff, S.; Mieth, A.; Pliquett, R.; Kosowski, J.; Kruse, C.; Luedike, P.; Michaelis, U.R.; Weissmann, N.; et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012, 110, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Schröder, K.; Weissmann, N.; Brandes, R.P. Organizers and activators: Cytosolic Nox proteins impacting on vascular function. Free Radic. Biol. Med. 2017, 109, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genove, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Mishra, A.; Hall, C.N.; O’Farrell, F.M.; Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. 2016, 36, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, J.; Ago, T.; Nishimura, A.; Nakamura, K.; Matsuo, R.; Wakisaka, Y.; Kamouchi, M.; Kitazono, T. Nox4 is a major source of superoxide production in human brain pericytes. J. Vasc. Res. 2014, 51, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Ago, T.; Kuroda, J.; Kamouchi, M.; Sadoshima, J.; Kitazono, T. Pathophysiological roles of NADPH oxidase/nox family proteins in the vascular system.-Review and perspective. Circ. J. 2011, 75, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Bonello, S.; Zahringer, C.; BelAiba, R.S.; Djordjevic, T.; Hess, J.; Michiels, C.; Kietzmann, T.; Gorlach, A. Reactive oxygen species activate the HIF-1α promoter via a functional NFκB site. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Diebold, I.; Petry, A.; Hess, J.; Gorlach, A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol. Biol. Cell 2010, 21, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Ago, T.; Kuroda, J.; Arimura, K.; Tachibana, M.; Nakamura, K.; Wakisaka, Y.; Sadoshima, J.; Iihara, K.; Kitazono, T. Detrimental role of pericyte Nox4 in the acute phase of brain ischemia. J. Cereb. Blood Flow Metab. 2016, 36, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Irani, K. Oxidant Signaling in Vascular Cell Growth, Death, and Survival: A Review of the Roles of Reactive Oxygen Species in Smooth Muscle and Endothelial Cell Mitogenic and Apoptotic Signaling. Circ. Res. 2000, 87, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Shiose, A.; Kuroda, J.; Tsuruya, K.; Hirai, M.; Hirakata, H.; Naito, S.; Hattori, M.; Sakaki, Y.; Sumimoto, H. A novel superoxide-producing NADPH oxidase in kidney. J. Biol. Chem. 2001, 276, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Yemisci, M.; Gursoy-Ozdemir, Y.; Vural, A.; Can, A.; Topalkara, K.; Dalkara, T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 2009, 15, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Jickling, G.C.; Liu, D.; Stamova, B.; Ander, B.P.; Zhan, X.; Lu, A.; Sharp, F.R. Hemorrhagic transformation after ischemic stroke in animals and humans. J. Cereb. Blood Flow Metab. 2014, 34, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Craige, S.M.; Chen, K.; Pei, Y.; Li, C.; Huang, X.; Chen, C.; Shibata, R.; Sato, K.; Walsh, K.; Keaney, J.F., Jr. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 2011, 124, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Goritz, C.; Dias, D.O.; Tomilin, N.; Barbacid, M.; Shupliakov, O.; Frisen, J. A pericyte origin of spinal cord scar tissue. Science 2011, 333, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Makihara, N.; Arimura, K.; Ago, T.; Tachibana, M.; Nishimura, A.; Nakamura, K.; Matsuo, R.; Wakisaka, Y.; Kuroda, J.; Sugimori, H.; et al. Involvement of platelet-derived growth factor receptor beta in fibrosis through extracellular matrix protein production after ischemic stroke. Exp. Neurol. 2015, 264, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Ago, T.; Wakisaka, Y.; Kuroda, J.; Shijo, M.; Yoshikawa, Y.; Komori, M.; Nishimura, A.; Makihara, N.; Nakamura, K.; et al. Early reperfusion after brain ischemia has beneficial effects beyond rescuing neurons. Stroke 2017, 48, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Hecker, L.; Vittal, R.; Jones, T.; Jagirdar, R.; Luckhardt, T.R.; Horowitz, J.C.; Pennathur, S.; Martinez, F.J.; Thannickal, V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009, 15, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; Bruggen, N.; Chopp, M. VEGF enhances angiogenesis and promotes blood–brain barrier leakage in the ischemic brain. J. Clin. Investig. 2000, 106, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Cui, Z.H.; Masuda, J.; Nabika, T. Congenic removal of a QTL for blood pressure attenuates infarct size produced by middle cerebral artery occlusion in hypertensive rats. Physiol. Genom. 2007, 30, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Weno, B.L.; Baumbach, G.L.; Heistad, D.D. Effect of antihypertensive treatment on focal cerebral infarction. Hypertension 1992, 19, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Li, G.; Hashimoto, M.; Nishio, A.; Tomozawa, H.; Suzuki, N.; Usami, S.; Higuchi, K.; Matsumoto, K. Pivotal Advance: Eosinophilia in the MES rat strain is caused by a loss-of-function mutation in the gene for cytochrome b(-245), alpha polypeptide (Cyba). J. Leukoc. Biol. 2009, 86, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Ferdaus, M.Z.; Zahid, H.M.; Ohara, H.; Nakahara, T.; Nabika, T. Focal ischemic injury with complex middle cerebral artery in stroke-prone spontaneously hypertensive rats with with loss-of-function in NADPH Oxidases. PLoS ONE 2015, 10, e0138551. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Simons, M. Branching morphogenesis. Circ. Res. 2009, 104, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Mettouchi, A. The role of extracellular matrix in vascular branching morphogenesis. Cell Adhes. Migr. 2012, 6, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Dikalova, A.; Clempus, R.; Lassègue, B.; Cheng, G.; McCoy, J.; Dikalov, S.; San Martin, A.; Lyle, A.; Weber, D.S.; Weiss, D.; et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 2005, 112, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Clempus, R.E.; Sorescu, D.; Dikalova, A.E.; Pounkova, L.; Jo, P.; Sorescu, G.P.; Schmidt, H.H.; Lassègue, B.; Griendling, K.K. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Lipsitz, L.A.; Goldberger, A.L. Loss of “complexity” and aging. Potential applications of fractals and chaos theory to senescence. JAMA 1992, 267, 1806–1809. [Google Scholar] [CrossRef] [PubMed]
- Doubal, F.N.; MacGillivray, T.J.; Patton, N.; Dhillon, B.; Dennis, M.S.; Wardlaw, J.M. Fractal analysis of retinal vessels suggests that a distinct vasculopathy causes lacunar stroke. Neurology 2010, 74, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.; Brosnan, M.J.; McIntyre, M.; Reid, J.L.; Dominiczak, A.F.; Hamilton, C.A. Superoxide anion production is increased in a model of genetic hypertension: Role of the endothelium. Hypertension 1999, 33, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Lassègue, B.; San Martín, A.; Griendling, K.K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012, 110, 1364–1390. [Google Scholar]
- Matsuno, K.; Yamada, H.; Iwata, K.; Jin, D.; Katsuyama, M.; Matsuki, M.; Takai, S.; Yamanishi, K.; Miyazaki, M.; Matsubara, H.; et al. Nox1 is involved in angiotensin II-mediated hypertension: A study in Nox1-deficient mice. Circulation 2005, 112, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Cai, H.; Dikalov, S.; McCann, L.; Hwang, J.; Jo, H.; Holland, S.M.; Harrison, D.G. Role of p47phox in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 2002, 40, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Xu, S.; Johns, D.G.; Du, Y.; Quinn, M.T.; Cayatte, A.J.; Cohen, R.A. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ. Res. 2001, 88, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Mercure, C.; He, Y.; Javeshghani, D.; Yao, G.; Callera, G.E.; Yogi, A.; Lochard, N.; Reudelhuber, T.L. Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NADPH oxidase. Hypertension 2005, 45, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Murdoch, C.E.; Wang, M.; Santos, C.X.; Zhang, M.; Alom-Ruiz, S.; Anilkumar, N.; Ouattara, A.; Cave, A.C.; Walker, S.J.; et al. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.M.; Ferdaus, M.Z.; Ohara, H.; Isomura, M.; Nabika, T. Effect of p22phox depletion on sympathetic regulation of blood pressure in SHRSP: Evaluation in a new congenic strain. Sci. Rep. 2016, 6, 36739. [Google Scholar] [CrossRef] [PubMed]
- Geurts, A.M.; Moreno, C. Zinc-finger nucleases: New strategies to target the rat genome. Clin. Sci. 2010, 119, 303–311. [Google Scholar] [CrossRef] [PubMed]
| Models | Anatomic Sites of MCAO | Duration | Characteristics | Disadvantage |
|---|---|---|---|---|
| Distal MCAO | MCAO distal to rhinal fissure | P | Distal MCAO was studied for the first time in SHRSP, revealing increased stroke sensitivity by hypertension. | The infarct size and penumbra are too small to evaluate the effects of pharmacotherapeutic agents in normotensive rats. |
| Proximal MCAO | MCAO proximal to lenticulostriate arteries | P (T) | The subtemporal approach method have emerged as a standard method of proximal MCAO. | The procedure is surgically demanding and may induce local traumatic effects. |
| Intraluminal suture occlusion | MCA origin and the proximal segment of ACA | T (P) | The procedure is easy to perform, minimally invasive, and does not require craniectomy. | This model has a wide ischemic zone, and the mortality rate is high in the case of PO. |
| Photothrombotic MCAO | Photochemical MCAO distal to rhinal fissure | P T | Photothrombotic MCAO in SHR yields a highly reproducible infarct volume, and does not requireopening of the dura. | Same as mentioned for distal MCAO. |
| Author Ref. | Year | Mice (WT: C57 Bl/6J) | T/P | BP | CBF | Nox Isoform | Outcome | Protection by KO | |
|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | ||||||||
| Walder [36] | 1997 | 8–10 wk | m | T | NA | NA | Nox2 | Infarct volume was reduced by 46% in KO mice compared with WT mice. | Yes |
| Kahles [43] | 2007 | 7–9 wk | m | T | NA | NA | Nox2 | BBB disruption and lesion volume were largely attenuated in KO mice. | Yes |
| Chen [37] | 2009 | NA | m | T | NA | NS | Nox2 | Mean infarct volume was 106.2 mm3 in WT mice, and 52.0 mm3 in KO mice. | Yes |
| Jackman [39] | 2009 | 5–9 wk WT 8–12 wk KO | m m | T | NA | NS | Nox2 | Protection by apocynin was found in WT mice but not in KO mice. | Yes |
| Brait [41] | 2010 | 6–8 wk | m+f | T/P | NA | NS | Nox2 | The larger infarction in male mice was dependent on both reperfusion and NOX2. | Yes (female) |
| De Silva [42] | 2011 | NA | m | T | NA | NS | Nox2 | Smaller infarct volume was observed in KO mice than in WT mice. | Yes |
| Chen [38] | 2011 | 12–16 wk | m | T | NA | NA | Nox2 | Brain infarction was 35–44% less in KO mice compared with WT mice. | Yes |
| Kim [40] | 2012 | 8–12 wk | m | P | NA | NS | Nox2 | No protection by KO was found in the absence of reperfusion. | NA |
| MaCann [44] | 2014 | 2–3 mo | NA | T | NA | NS | Nox2 | KO showed transient nature of protection and increased revascularization. | Yes |
| Jackman [45] | 2009 | 11–17 wk | m | T | NA | NS | Nox1 | Cortical but not total infarct was increased in KO mice. | No |
| Kahles [46] | 2010 | NA | m + f | T/P | NA | NA | Nox1 | Infarct volume was reduced by 44% after 1 h but not 2 h and pMCAO in KO mice. | Yes |
| Kleinschnitz [48] | 2010 | 6–8 wk | m | T | NA | NA | Nox1 | Deletion of NOX4 but not NOX1 or NOX2 prevented focal ischemic injury. | No |
| 6–8 wk | m | T | NA | NA | Nox2 | No | |||
| 6–8 wk | m + f | T/P | NA | NA | Nox4 | Yes | |||
| 18–20 wk | m | T | NA | NA | Nox4 | Yes | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, H.; Ago, T.; Kitazono, T.; Nabika, T. NADPH Oxidase-Related Pathophysiology in Experimental Models of Stroke. Int. J. Mol. Sci. 2017, 18, 2123. https://doi.org/10.3390/ijms18102123
Yao H, Ago T, Kitazono T, Nabika T. NADPH Oxidase-Related Pathophysiology in Experimental Models of Stroke. International Journal of Molecular Sciences. 2017; 18(10):2123. https://doi.org/10.3390/ijms18102123
Chicago/Turabian StyleYao, Hiroshi, Tetsuro Ago, Takanari Kitazono, and Toru Nabika. 2017. "NADPH Oxidase-Related Pathophysiology in Experimental Models of Stroke" International Journal of Molecular Sciences 18, no. 10: 2123. https://doi.org/10.3390/ijms18102123




